Abstract
Bevacizumab has been reported to be effective for recurrent glioblastoma. In our hospital, ifosfamide, carboplatin, etoposide (ICE) is the second-line chemotherapy for first recurrence of glioblastoma after temozolomide failure. In the present analysis, we retrospectively investigated the feasibility and effectiveness of bevacizumab combined with ICE in patients with glioblastoma at second relapse during ICE treatment. Between 2010 and 2012, tumor progressions were diagnosed in consecutive 8 patients who were treated with ICE for the first recurrence of glioblastoma. These patients were administered 3 cycles of 10 mg/kg bevacizumab every two weeks in combination with ICE treatment. The objective response rate of bevacizumab combination was 75% in Neuro-Oncology Working Group (RANO criteria), including complete response and partial response. Median progression free survival (PFS) and median overall survival (OS) after second relapse were 3.7 months (95% confidence interval [CI], 2.5–18.5 months) and 6.0 months (95% CI, 3.2–19.7 months), respectively. The 6-month PFS rates were 25% (95% CI, 0–55.0%). The median OS after initial diagnosis was 23.3 months (95% CI, 16.2–55.8 months). The grade 2 or 3 hematologic adverse events were identified in 7 of 8 patients, most of which might be due to ICE chemotherapy. The results of our retrospective analysis suggest that combination treatment with bevacizumab and ICE may be safe and beneficial in patients with recurrent glioblastoma.
Keywords: recurrent glioblastoma, bevacizumab, ifosfamide, carboplatin, etoposide (ICE), second recurrence
Introduction
Glioblastomas are primary malignant brain tumors causing poor morbidity and mortality.17) Current standard treatment in newly diagnosed glioblastoma includes radiotherapy with concomitant and adjuvant temozolomide following surgery. The median survival for patients with glioblastoma remains 14.6 months.17) The biological nature of glioblastoma is extremely refractory and relapsing. However, there is no consensus on the optimal practice for patients with recurrent glioblastoma. In the literatures, there are many retrospective studies and prospective trials to treat recurrent glioblastoma. An alternative dosing schedule of temozolomide is a reasonable option in patients with glioblastoma who experience progression after conventional 150 or 200 mg/m2 5/28 dosing schedule.9,10,24) The RESCUE study showed clinical benefit with 6-month progression free survival (PFS) rates (PFS-6) of 17% and 23.9% with continuous dose-intense temozolomide 50 mg/m2/d in recurrent glioblastoma.9) The study of the “week on/week off” dosing schedule of temozolomide at a dose of 150 mg/m2/day demonstrated clinical benefit with a PFS-6 of 43.8% in recurrent glioblastoma.24)
Based on the highly angiogenic nature of glioblastoma, anti-angiogenic targeted agents have been applied to a treatment approach. Bevacizumab is a humanized monoclonal antibody against the vascular endothelial growth factor.22) First phase II study of bevacizumab and irinotecan in patients with recurrent malignant glioma showed clinical benefit with a PFS-6 of 38%.16,19) Following studies showed the efficacy with a PFS-6 of 29–42.6% of single-agent bevacizumab in patients with recurrent glioblastoma who were treated with conventional management with temozolomide.4,6) Japanese phase II study of single-agent bevacizumab in patients with recurrent malignant glioma also demonstrated a PFS-6 of 33.9%.8) However, bevacizumab responses are rarely durable.8,19,20)
Phase II study of ifosfamide, carboplatin, and etoposide (ICE) for recurrent glioblastoma showed a PFS-6 of 35% and mild adverse events.1) In our institute, ICE is used as second-line chemotherapy in patients with first relapsing glioblastoma treated with conventional management with temozolomide. Bevacizumab has generally been used in combination with cytotoxic agents in the management of solid malignancies. Retrospective studies have shown that regimens containing bevacizumab and carboplatin were effective on recurrent glioblastoma.3,7,11,12) Therefore, for patients with re-recurrent glioblastoma treated with ICE, we use another chemotherapeutic agents containing bevacizumab combination with ICE. Retrospectively, we investigated the feasibility and effectiveness of bevacizumab combined with ICE in patients with second recurrence of glioblastoma during ICE treatment following temozolomide failure.
Materials and Methods
Patient’s demographics, clinical data, radiological, and histopathological findings, type of chemotherapy, number of chemotherapy cycles, and survival data were obtained retrospectively from our hospital medical records. We reviewed consecutive 8 patients diagnosed as second relapse of glioblastoma resistant to ICE, who were treated with bevacizumab in combination with ICE between 2010 and 2012. All patients had undergone previous surgery and were diagnosed histologically with glioblastoma. This retrospective analysis is in compliance with the Declaration of Helsinki (Sixth Revision, 2008). All data were collected retrospectively and in accordance with institutional ethical policies.
Patients were evaluated with magnetic resonance imaging (MRI) every 1 to 2 months or according to clinical symptoms after the initial treatment. Tumor recurrence was diagnosed by MRI and positron emission tomography imaging with 18F-fluorode-oxyglucose (18F-FDG). In the case with suspicious pseudo-progression, the adjuvant chemotherapy was continued.
The initial treatment following the first surgery were three types: radiotherapy with concomitant and adjuvant temozolomide,17) radiotherapy concomitant and adjuvant nimustine, carboplatin, vincristine, and interferon-beta (VACferon) followed by adjuvant temozolomide, radiotherapy with concomitant and adjuvant temozolomide and interferon-beta.21) All patients were diagnosed with first recurrence of glioblastoma and received ICE chemotherapy following surgical resection or/and stereotactic radiosurgery (35 Gy, 5 fractions) or no treatment. ICE regimen consisted of ifosfamide (750 mg/m2/day on day 1, 2, and 3), carboplatin (75 mg/m2/day on day 1, 2, and 3), and etoposide (75 mg/m2/day on day 1, 2, and 3) in every 4–6 weeks.1) All patients were diagnosed with second recurrence of glioblastoma refractory to ICE and received 3 cycles of 10 mg/kg bevacizumab, every two weeks, in combination with the same regimen of ICE as before.
The objective response rate (ORR) to treatment was assessed using the Response Assessment in Neuro-Oncology Working Group (RANO criteria).23) We evaluated contrast-enhanced T1-weighted images and fluid attenuated inversion recovery (FLAIR) images at the second relapse and after 3 cycles of bevacizumab. Both complete and partial responses were considered objective responses. Toxicity was evaluated after 3 cycles of bevacizumab according to the National Cancer Institute Common Toxicity Criteria (CTCAE) version 4.0. PFS was measured from the date of image diagnosis to the date of disease progression or death. Patients alive and progression free at last contact are treated as censored in the PFS analysis. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death or last contact. The Kaplan–Meier method was used to estimate survival, which was measured from the time of diagnosis to the date of death. Statistical analyses were with PRISM version 5.0 (GraphPad Software Inc., La Jolla, California, USA).
Results
The characteristic features of 8 patients analyzed in this study are summarized in Table 1. There were 6 males and 2 females, and the median age was 53 years. Six patients received radiotherapy (60 Gy, 30 fractions) with concomitant and adjuvant temozolomide as the initial treatment. Exceptionally, one patient, Case 1, was received ACNU regimen of VACferon with radiotherapy followed by adjuvant temozolomide. Another patient, Case 5, was received interferon-beta in combination with the conventional management with radiotherapy and temozolomide. After the first recurrences were recognized during temozolomide treatment, three patients (Cases 2–4) had tumor resection, two (Cases 5 and 8) had tumor resection followed by stereotactic surgery for the residual tumor (35 Gy, 5 fractions), and one patient (Case 7) had only stereotactic surgery (35 Gy, 5 fractions). All patients were treated with ICE chemotherapy after temozolomide failure. At second relapse diagnosed during ICE treatment, 3 cycles of 10 mg/kg bevacizumab in every two weeks were administered. The median values of Karnofsky Performance status were 60 (50–80). The cycle numbers of ICE were 2–12 before the second relapse of glioblastoma. Only one patient (Case 2) had tumor resection before administration of bevacizumab.
Table 1.
Demographic characteristics of patients With second recurrence of glioblastoma
| Case | Age | Sex | First chemotherapy | Radiation (Gy) | First chemotherapy (cycle number) | Salvage treatments at first relapse | ICE cycle number at second relaspe | Salvage treatments at second relapse | KPS at second relapse (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | VACferon | 59.4 | VACferon (6), TMZ (27) | 6 | 60 | ||
| 2 | 27 | F | TMZ | 60 | TMZ (12) | Surgery | 12 | Surgery | 80 |
| 3 | 52 | M | TMZ | 60 | TMZ (11) | Surgery | 8 | 50 | |
| 4 | 47 | M | TMZ | 60 | TMZ (6) | Surgery | 3 | 70 | |
| 5 | 67 | F | TMZ | 60 | TMZ (7) | Surgery, SRT 35Gy | 2 | 60 | |
| 6 | 64 | M | TMZ+IFN | 60 | TMZ+IFN (5) | 2 | 50 | ||
| 7 | 62 | M | TMZ | 60 | TMZ (16) | SRT 35Gy | 4 | 70 | |
| 8 | 51 | M | TMZ | 60 | TMZ (4) | Surgery, SRT 35Gy | 4 | 60 |
F: female, KPS: Karnofsky performance status, M: male, SRT: stereotactic radiosurgery, TMZ: temozolomide, VACferon: nimustine, carboplatin, vincristine, interferon-beta.
Clinical results in 3 cycles of bevacizumab combination in patients with second recurrence of glioblastoma resistant to ICE are summarized in Table 2. The ORR of bevacizumab including complete response and partial response was 75% in RANO criteria (Fig. 1, Case 2). The median PFS and OS after bevacizumab in combination with ICE were 3.7 months (95% confidence interval [CI], 2.5–18.5 months) and 6.0 months (95% CI, 3.2–19.7 months), respectively. The PFS-6 rates were 25% (95% CI, 0–55.0%). The median OS after onset was 23.3 months (95% CI, 16.2–55.8 months). Two patients (Cases 2 and 7) were treated with additional bevacizumab at third relapse of glioblastoma.
Table 2.
Results of bevacizumab in combination with ICE in patients with second recurrence of glioblastoma
| Case | Bevacizumab cycles | ICE cycle number after second relaspe | RANO criteria | OS from the initial diagnosis | OS from ICE (months) | OS from bevacizumab (months) | Current status |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 2 | PR | 55.2 | 8.4 | 1.6 | Dead |
| 2 | 3 + 4 | 10 | PR | 44.2 | 30.2 | 18.7 | Dead |
| 3 | 3 | 17 | PR | 55.8 | 45.2 | 33.0 | Alive |
| 4 | 3 | 6 | PR | 20.2 | 11.1 | 8.0 | Dead |
| 5 | 3 | 1 | SD | 16.6 | 7.6 | 3.0 | Dead |
| 6 | 3 | 2 | PR | 16.2 | 8.4 | 4.8 | Dead |
| 7 | 3 + 2 | 3 | PR | 26.3 | 9.3 | 6.3 | Dead |
| 8 | 3 | 3 | SD | 16.4 | 9.6 | 5.6 | Dead |
ICE: ifosfamide, carboplatin, and etoposide, OS: overall survival, PR: partial response, RANO criteria: Response Assessment in Neuro-Oncology Working Group, SD: stable disease.
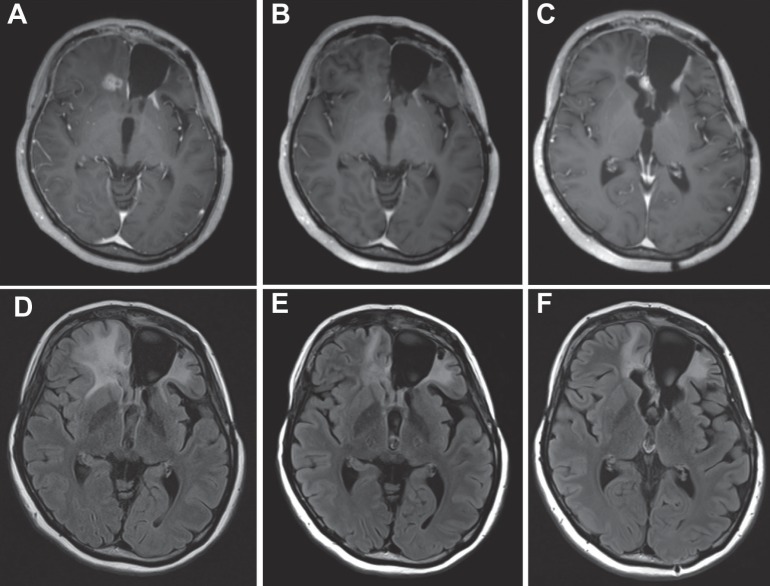
Fig. 1.
Illustrative Case 2, 27-year-old female. A, D: Glioblastoma of the patient had rapidly regrown after the third surgery for her second relapsing tumor resistant to ICE. B, E: Her lesion was decreased after 3 cycles of 10 mg/kg bevacizumab combined with ICE. C, F: Her lesion recurred after 3 cycles of ICE following bevacizumab. A, B, C: contrast-enhanced T1-weighted images. D, E, F: fluid attenuated inversion recovery images. ICE: ifosfamide, carboplatin, etoposide.
Bevacizumab in combination with ICE did not produce any acute toxic events. Hematologic and nonhematologic grade 2 or 3 adverse events are showed in Table 3. There was no grade 4 or higher adverse events except in one patient (Case 1). The death of the patient (Case 1) was not related to chemotherapy. Hematologic toxicities were identified in 7 of 8 patients and comprised 60% of the grade 2 or 3 adverse events. These adverse events were thought to be attributed to ICE chemotherapy. Cerebral hemorrhage, hypertension, proteinuria, and venous thromboembolism more than grade 3 were not identified in this series.
Table 3.
Number of patients who experienced adverse events according to CCTAE grade
| Toxicity | Grade 2 | Grade 3 | Total |
|---|---|---|---|
| Anemia | 1 | 0 | 1 |
| Lymphopenia | 3 | 3 | 6 |
| Platepenia | 2 | 0 | 2 |
| Hypoalbuminiemia | 1 | 0 | 1 |
| Constipation | 5 | 0 | 5 |
| Total | 12 | 3 | 15 |
CCTAE: Common Terminology Criteria for Adverse Events
Discussion
This is the first report to evaluate combined administration with bevacizumab and ICE in patients with second recurrence of glioblastoma during ICE treatment, although it is retrospective analysis in small number cases. These results indicated that bevacizumab combined with ICE improved clinical deterioration in 6 of 8 patients with glioblastoma at second relapse. Furthermore, this combination therapy did not cause any severe adverse events, which means that bevacizumab is well tolerated although during ICE chemotherapy.
Bevacizumab is widely used in recurrent glioblastoma, alone or in combination with other agents. In the meta-analysis of bevacizumab effect for recurrent glioblastoma using 15 studies published from 2005 to 2009, PFS-6 was 45%. The median OS was 9.3 months. The response rate analysis demonstrated 6% complete response, 49% partial response, and 29% stable disease.25) Japanese phase II study of single-agent bevacizumab showed that PFS-6 was 33.9% and median OS was 3.3 months in 29 patients with recurrent glioblastoma.8) In our analysis of bevacizumab in combination with ICE chemotherapy, PFS-6 was 25% and median OS was 6.0 months. Regarding the survival endpoints, our results seem to be worse than previously published data. It was a primary factor that the subjects were patients with second recurrence of glioblastoma in our analysis. This is compatible with previous reports that the PFS-6 and ORR were numerically higher in patients experiencing first relapse compared to those experiencing second relapse.4,8)
The phase II study to evaluate effect of bevacizumab-alone and bevacizumab-plus-irinotecan for recurrent glioblastoma demonstrated no significant difference of survival endpoints, median OS times were 9.2 months and 8.7 months, respectively. However, our analysis showed that in two patients (Cases 2 and 3) who received more than 8 cycles of ICE, bevacizumab improved their disease progressions refractory to ICE chemotherapy. Many previous reports also have implied that bevacizumab may have potential to affect tumor in combination with another chemotherapeutic agent.7,18,19) A possible mechanism is that antiangiogenic therapy affects tumor vascular structure and blood perfusion. The study to assess tumor blood perfusion in recurrent glioblastoma treated with cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, demonstrated that tumor blood perfusion increased in 7 of 30 patients. Increase of tumor blood perfusion was associated with longer survival. Antiangiogenic therapy induced-vascular normalization probably changes the efficacy of the combination drugs.15)
Recently, two phase III studies, AVAglio and RTOG 0825, to evaluate the addition of bevacizumab to standard temozolomide management in patients with newly diagnosed glioblastoma were performed.2,5) These studies showed that the addition of bevacizumab did not improve OS but did improve PFS. Based on these results, it is a controversial matter whether bevacizumab is combined with the standard temozolomide management as the initial treatment. And there are clinical questions to resolve. First, what is the factor to bring effect of bevacizumab? Bevacizumab-plus-irinotecan also resulted in high ORR and an increased PFS-6 value, but showed no improvement in OS. Some patients with recurrent glioblastoma and well respond to bevacizumab have survived significantly longer than non-responders.19) In our analysis, salvage effects of additional bevacizumab tend to be prominent in ICE responders. Second, how do we use bevacizumab to be more effective and less harmful, for example, continuation or short-period administration similar to steroid? The retrospective study demonstrated that bevacizumab continuation beyond initial progression was associated with modestly improved outcome compared with nonbevacizumab therapy.13) Third, no difference was seen in bevacizumab dose-response benefit between 5 mg/kg and 10 mg/kg to 15 mg/kg. The lack of a dose-response effect would require confirmation in a prospectively conducted clinical trial. A model for the potential therapeutic benefits of low-dose antiangiogenic therapy was introduced.22) Antiangiogenic therapy is perspective tool in association with tumor vascularity and drug delivery.
There is no established standard salvage chemo-therapy for recurrent glioblastoma after the failure of standard management with temozolomide. Phase II studies of ICE chemotherapy in patients with recurrent glioblastoma showed clinical benefit with a PFS-6 of 35%.1) In our hospital, we use dose-reduction regimen of ICE as second-line chemotherapy for first relapsing glioblastoma. A Germany retrospective study, which was reported by Schäfer et al., showed that ICE was not effective in patients with recurrent high-grade glioma if applied at second or third relapse.14) In our analysis, PFS-6 was 37.5% in patients treated with ICE chemotherapy at the first relapse of glioblastoma. Retrospective studies of chemotherapy containing bevacizumab and carboplatin have also shown favorable effect that PFS-6 rates were 22–50% in recurrent glioblastoma.3,7,11,12) These suppose that the regimen containing carboplatin has potency to be active in malignant glioma, and that the efficacy of regimen combined with bevacizumab and ICE in patients with first relapse of glioblastoma should be addressed.
In conclusion, we consider that the combination of bevacizumab and ICE is well tolerated and may derive some clinical benefits in recurrent glioblastoma patients, in spite of the limitations of our analysis. Bevacizumab seems to be more active with in patients with first recurrence of glioblastoma compared those with its second recurrence. The dose intensity and schedule of bevacizumab and ICE need be optimized in future studies.
Acknowledgments
This work was supported by grants from a Grant-in-Aid for Specially Promoted Research from the Ministry for Education, Culture, Sports, Science and Technology of Japan (Arakawa, Mizowaki, Kikuchi, Kunieda, Takahashi, Takagi, Miyamoto).
Referrences
- 1). Aoki T, Mizutani T, Nojima K, Takagi T, Okumura R, Yuba Y, Ueba T, Takahashi JA, Miyatake S, Nozaki K, Taki W, Matsutani M: Phase II study of ifosfamide, carboplatin, and etoposide in patients with a first recurrence of glioblastoma multiforme. J Neurosurg 112: 50– 56, 2010. [DOI] [PubMed] [Google Scholar]
- 2). Chinot O, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Hilton M, Abrey L, Cloughesy T: Phase III trail of bevacizumab added to standard radiotherapy and temozolomide for newly-diagnosed glioblastoma: mature progeression-free surveival and prelonimnary over survival results in AVAglio. Neuro-Oncol 14 ( Suppl 6): 1– 164, 2012. [Google Scholar]
- 3). Francesconi AB, Dupre S, Matos M, Martin D, Hughes BG, Wyld DK, Lickliter JD: Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci 17: 970– 974, 2010. [DOI] [PubMed] [Google Scholar]
- 4). Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T: Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27: 4733– 4740, 2009. [DOI] [PubMed] [Google Scholar]
- 5). Gilbert MR: RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J Clin Oncol 31 ( Suppl; abstr 1), 2013. [Google Scholar]
- 6). Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA: Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27: 740– 745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Mrugala MM, Crew LK, Fink JR, Spence AM: Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett 4: 1082– 1086, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, Shinoura N, Aoki T, Sugiyama K, Kuratsu J, Muragaki Y, Sawamura Y, Matsutani M: Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol 42: 887– 895, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, Thiessen B, Forsyth P, Pouliot JF: Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28: 2051– 2057, 2010. [DOI] [PubMed] [Google Scholar]
- 10). Perry JR, Rizek P, Cashman R, Morrison M, Morrison T: Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer 113: 2152– 2157, 2008. [DOI] [PubMed] [Google Scholar]
- 11). Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, Herndon JE, Bulusu A, Threatt S, Friedman AH, Vredenburgh JJ, Friedman HS: Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J Neurooncol 107: 155– 164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, McLendon RE, Herndon JE, Coan A, Threatt S, Friedman AH, Friedman HS: Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer 117: 5351– 5358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Reardon DA, Herndon JE, Peters KB, Desjardins A, Coan A, Lou E, Sumrall AL, Turner S, Lipp ES, Sathornsumetee S, Rich JN, Sampson JH, Friedman AH, Boulton ST, Bigner DD, Friedman HS, Vredenburgh JJ: Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer 107: 1481– 1487, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Schäfer N, Tichy J, Thanendrarajan S, Kim Y, Stuplich M, Mack F, Rieger J, Simon M, Scheffler B, Boström J, Steinbach JP, Herrlinger U, Glas M: Ifosfamide, carboplatin and etoposide in recurrent malignant glioma. Oncology 80: 330– 332, 2011. [DOI] [PubMed] [Google Scholar]
- 15). Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK: Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res 72: 402– 407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Stark-Vance V: Bevacizumab and CPT-11 in the Treatment of Relapsed Malignant Glioma. Neuro-Oncol 7 ( Suppl; abstr 342): 369, 2005. [Google Scholar]
- 17). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 18). Thompson EM, Dosa E, Kraemer DF, Neuwelt EA: Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery 67: 87– 93, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS: Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13: 1253– 1259, 2007. [DOI] [PubMed] [Google Scholar]
- 20). Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS: Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25: 4722– 4729, 2007. [DOI] [PubMed] [Google Scholar]
- 21). Wakabayashi T, Kayama T, Nishikawa R, Takahashi H, Yoshimine T, Hashimoto N, Aoki T, Kurisu K, Natsume A, Ogura M, Yoshida J: A multicenter phase I trial of interferon-beta and temozolomide combination therapy for high-grade gliomas (INTEGRA Study). Jpn J Clin Oncol 38: 715– 718, 2008. [DOI] [PubMed] [Google Scholar]
- 22). Wang Y, Fei D, Vanderlaan M, Song A: Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 7: 335– 345, 2004. [DOI] [PubMed] [Google Scholar]
- 23). Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM: Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28: 1963– 1972, 2010. [DOI] [PubMed] [Google Scholar]
- 24). Wick A, Pascher C, Wick W, Jauch T, Weller M, Bogdahn U, Hau P: Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 256: 734– 741, 2009. [DOI] [PubMed] [Google Scholar]
- 25). Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S: Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 9: 403– 407, 2011. [DOI] [PubMed] [Google Scholar]