Abstract
There have been few clinical studies in the area of cervical spine that focused on surgery for treating degenerative lumbar disease in patients with rheumatoid arthritis (RA). High rates of wound complications and instrumentation failure have been reported more for RA than for non-RA patients, although clinical outcomes are similar between the two groups. Lumbar canal stenosis in RA is caused not only by degeneration but also by RA-related spondylitis, which includes facet arthritis and inflammation around the vertebral endplate. The pitfalls in surgical management of lumbar canal stenosis in RA patients are highlighted in this study. The study reviewed 12 patients with RA,who were surgically treated for lumbar canal stenosis. Two out of five patients with pulmonary fibrosis died of worsened pulmonary condition, even though there were no perioperative pulmonary complications. Two patients with pedicle screw fixation showed no instrumentation failure, but two patients with spinous process fixation needed re-operation or vertebral fracture. Surgical treatment for lumbar canal stenosis in RA patients needs to be individually adjusted. Preoperative assessments and treatments of pulmonary fibrosis and osteopenia are essential. Surgery for lumbar canal stenosis with RA should be deferred for patients with advanced pulmonary fibrosis because of its potential life-threatening risk. Fusion surgery is indicated only in patients with kyphosis or severe symptoms caused by intervertebral instability. Pedicle screw fixation with hydroxyapatite granules or sublaminar tape is recommended. Closer follow-up after surgery is necessary because of possible delayed wound infection, instrumentation failure, pathological fracture, and respiratory deterioration.
Keywords: lumbar canal stenosis, rheumatoid arthritis, surgery
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown cause. It is characterized by persistent synovitis that affects hands and feet, although any joint lined by a synovial membrane may be involved. Extra-articular involvement of organs such as skin, heart, lungs, and eyes can be significant.24) Although cervical lesions are more common as spinal involvement of RA, lumbar lesions are not as infrequent as previously reported. Lumbar lesions of RA involve not only the facet, which is a synovial joint, but also tissues around the vertebral endplate.2,3,7,13,14,16,19,23,25) Compared with the attention paid to the cervical lesions, there have been relatively few reports in English about surgical treatment for degenerative lumbar lesions with RA.3,11,16) Surgery for lumbar canal stenosis relieves leg pain and neurogenic claudication adequately in RA as well as non-RA patients. However, the rate of perioperative complications, such as wound infection or instrumentation failure, has been reported more often in RA patients than in non-RA patients.3,11) In this study, we describe the pitfalls in surgical management of lumbar canal stenosis with RA.
Materials and Methods
A total of 12 patients (9 male and 3 female, age range: 61–79 years, mean age at the time of surgery: 73.0 years) with RA were surgically treated for lumbar canal stenosis at Kameda Medical Center in Japan between January 2007 and December 2009 (Table 1). The mean duration of RA was 9.8 years (range: 1–20 years) and the mean follow-up period was 20.6 months (range: 3–39 months). Eight patients had developed RA after the age of 60 years. All patients had severe leg pain, although they had received conservative treatments for several months. RA progression was classified by radio-graphic findings and soft-tissue findings at the most affected joint,26) followed by Steinblocker RA stages: I (early, osteoporosis) in two patents, II (moderate, osteoporosis with slight destruction of cartilage and bone) in six patients, III (severe, osteoporosis with destruction of cartilage and bone) in one patient, and IV (terminal, bony ankylosis) in three patients. Eleven patients took oral prednisolone (range 2–9 mg/day, mean 5 mg/day) and seven patients took methotrexate (range 8–10 mg/week, mean 8.9 mg/week) at the time of surgery. Three patients had received biological disease-modifying antirheumatic drugs, and total hip arthroplasty had been performed in three patients. All patients had checked their bone mineral density and were treated with medication according to the current existing guidelines.21) Five patients had complicating pulmonary fibrosis, and three of them had received biological drugs. Three patients presented with asymptomatic atlantoaxial subluxation, and one patient underwent anterior cervical decompression and fusion for cervical spondylotic myelopathy.
Table 1.
Demographics and radiological findings of patients
| Case No. | Age (years), sex | Duration of RA (years) | Steinbrocker stage | Cervical lesion | PF | PSL (mg) | MTX (mg) | THA | Instability | Scoliosis | Kyphosis | Disc wedge | Facet erosion | Wavy image |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69, M | 4 | I | – | + | 2 | 0 | − | − | + | − | + | − | − |
| 2 | 73, F | 16 | IV | – | − | 9 | 0 | + | − | + | − | + | − | + |
| 3 | 63, F | 1 | I | – | − | 5 | 8 | − | + | + | − | + | − | + |
| 4 | 68, M | 3 | III | – | − | 2 | 8 | − | − | − | − | − | + | + |
| 5 | 73, M | 4 | II | – | − | 2 | 0 | − | + | + | − | + | + | − |
| 6 | 72, M | 7 | II | – | + | 9 | 0 | − | + | − | − | + | + | − |
| 7 | 61, M | 20 | II | CSM | + | 5 | 10 | − | + | − | − | − | − | − |
| 8 | 71, M | 10 | II | – | − | 3 | 10 | + | + | − | − | − | − | − |
| 9 | 63, F | 18 | II | – | − | 8 | 8 | + | − | + | + | − | − | + |
| 10 | 75, M | 15 | IV | AAS | + | 0 | 10 | − | + | − | − | + | + | − |
| 11 | 72, M | 1 | II | AAS | + | 8 | 8 | − | − | − | − | − | − | − |
| 12 | 74, M | 19 | IV | AAS | − | 7.5 | 0 | − | − | − | − | − | − | − |
AAS: atlantaxial subluxation, CSM: cervical spondylotic myelopathy, MTX: methotrexate, PF: pulmonary fibrosis, PSL: prednisolone, RA: rheumatoid arthritis, THA: total hip arthoplasty.
The diagnostic protocols consisted of plain radiography, myelography, computed tomography (CT) scans after myelography, and magnetic resonance imaging (MRI). The clinical presentation and treatment outcomeswere assessed using the Japanese Orthopaedic Association scale (JOA score) for low-back pain (Table 2).18) The maximum score on the JOA is 29. The recovery rate was calculated using the Hirabayashi method (recovery rate = [postoperative JOA score–preoperative JOA score] ×100/(29–preoperative mJOA score)8) and was defined as excellent (100–75%), good (74–50%), fair (49–25%), unchanged (24–0%), or deteriorated (score < 0%). Surgical procedures and postoperative complications were also analyzed. In principle, we performed decompression surgery, laminectomy or laminotomy, to release the compression of neural structure in all cases. Additional fusion or fixation surgery was performed based on a risk-benefit analysis and where found necessary.
Table 2.
The JOA scoring system for low-back pain18)
| Definition and description | Score |
|---|---|
| Subjective symptoms (9 points) | |
| Low-back pain | |
| None | 3 |
| Occasional mild pain | 2 |
| Frequent mild or occasional severe pain | 1 |
| Frequent or continuous severe pain | 0 |
| Leg pain and/or tingling | |
| None | 3 |
| Occasional mild pain | 2 |
| Frequent mild or occasional severe pain | 1 |
| Frequent or continuous severe pain | 0 |
| Gait | |
| Normal | 3 |
| Able to walk > 500m, without pain, | 2 |
| tingling, and/or Muscle weakness | |
| Unable to walk > 500m, due to leg pain, tingling, and or Muscle weakness | 1 |
| Unable to walk > 100m, due to leg pain, tingling, and/or Muscle weakness | 0 |
| Clinical signs (6 points) | |
| Straight leg-raising test | |
| Normal | 2 |
| 30°–70° | 1 |
| < 30° | 0 |
| Sensory disturbance | |
| None | 2 |
| Slight disturbance | 1 |
| Marked disturbance | 0 |
| Motor disturbance (MMT) | |
| None (Grade 5) | 2 |
| Slight weakness (Grade 4) | 1 |
| Marked weakness (Grades 3–0) | 0 |
| Restriction of ADLs (14 points)† | |
| Turning over while lying down | 0–2 |
| Standing | 0–2 |
| Washing face | 0–2 |
| Leaning forward | 0–2 |
| Sitting (1 hr) | 0–2 |
| Lifting or holding | 0–2 |
| Walking | 0–2 |
| Urinary bladder function (–6 points) | |
| Normal | 0 |
| Mild dysuria | –3 |
| Severe dysuria | –6 |
A score of 0 indicates a severe restriction; a score of 1, moderate restriction; and a score of 2, no restriction. ADL: activities of daily living, JOA: Japanese Orthopaedic Association, MMT: manual muscle testing.
Results
I. Radiological findings
Wedging of the intervertebral space (tilting angle > 5°) was present in five patients. Lumbar scoliosis (Cobb angle > 10°) was observed in six patients, and one of them showed also kyphosis. Intervertebral instability (> 4 mm in translation or > 10° in sagittal rotation) was evident in six patients. Among them, three patients showed instability in translation and three showed instability in rotation. Facet erosion on CT scans was present in four patients (Fig. 1). An irregular lesion around the endplate was evident in four patients. This lesion showed low intensity both on T1- and T2-weighted MRI images with a wavy line and spreading from the discovertebral junction to vertebral body (Fig. 2). Pathological fracture caused lumbar canal stenosis in Case 12, in which the patient had suffered from low-back pain in standingup from sitting position.
Fig. 1.
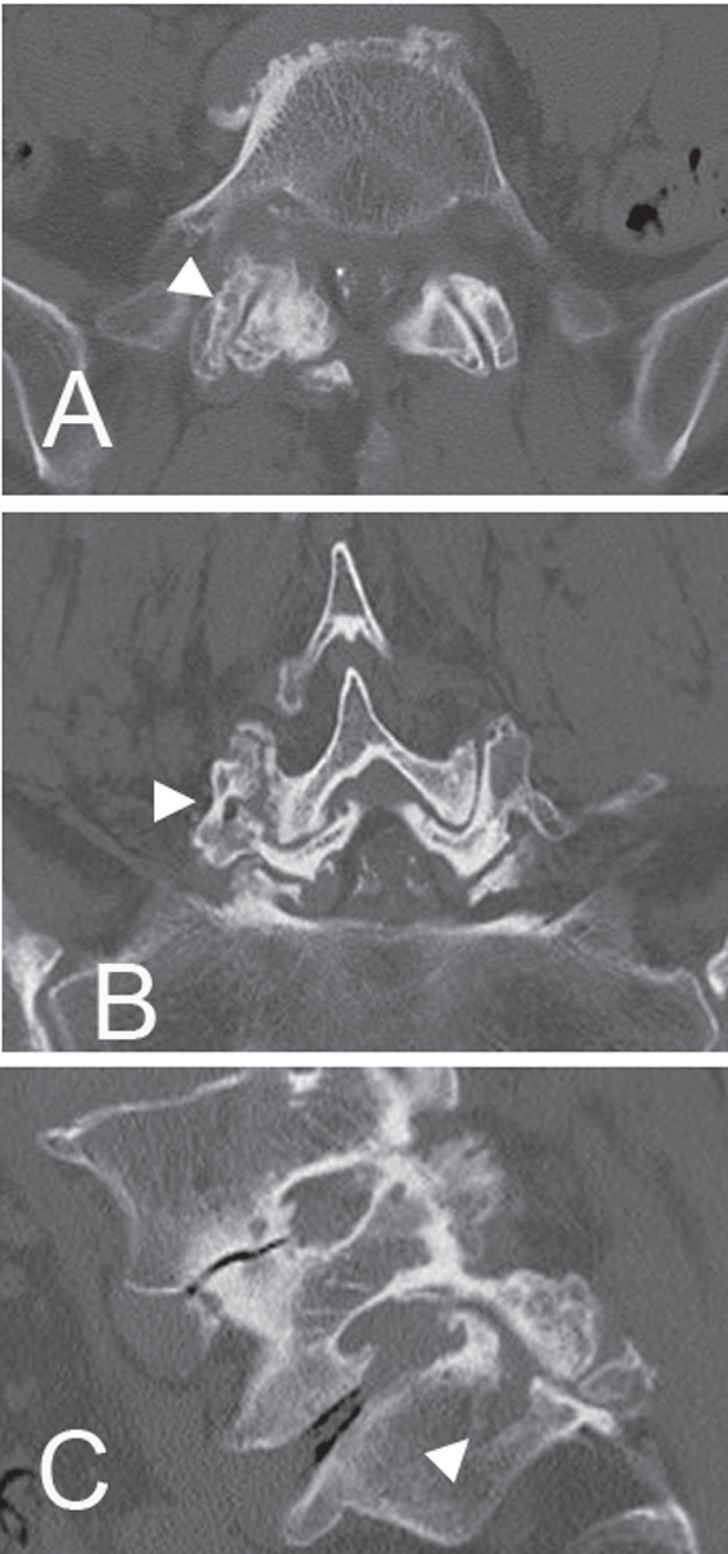
Case 5. Computed tomography (CT) scans after myelography showing bone erosion (arrowheads) at the right L5/S1 facet. A: axial image, B: coronal reconstruction image, C: sagittal reconstruction image.
Fig. 2.
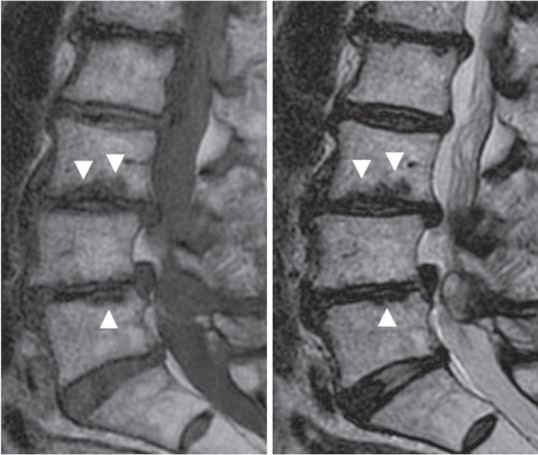
Case 2. Sagittal magnetic resonance images revealing wavy images (arrowheads) with low signal intensity on both T1 (left) and T2 (right)–weighted images at the endplate of the L3 and L5 vertebral bodies.
Fig. 2.
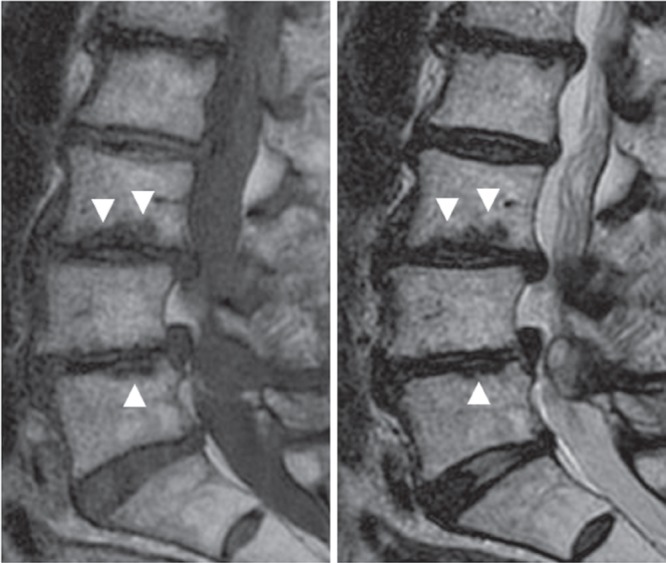
Case 2. Sagittal magnetic resonance (MR) images revealing wavy images (arrowheads) with low signal intensity on both T1 (left) and T2 (right)–weighted images at the endplate of the L3 and L5 vertebral bodies.
II. Surgical procedures and outcomes
Eight patients underwent only decompression surgery. Fusion surgery with a pedicle screw system was performed only in cases with severe instability or deformity. In Case 9, we performed posterolateral lumbar fusion for preventing the deformity progression because the patient had complained severe low-back pain with lumbar kyphosis as well as severe leg pain. In Case 7, the patient had severe lumbago and sciatica with walking and any body movement, and we performedtransforaminal lumbar intervertebral fusion for the existing severe intervertebral instability. Hydroxyapatite granules/sticks for augmentation were inserted in the pedicle holes as bioactive materials for enhancement of pedicle screw fixation. Both patients had their symptoms relieved without instrumentation failure after surgery (Fig. 3). We performed interspinous fixation surgery with S-plate (Kisco DIR Co. Ltd., Osaka) and without bone grafting for controlling the intervertebral motion in Cases 11 and 12. They were in apoor condition due to severe pulmonary fibrosis (Case 11) or were undergoing chemotherapy for colon carcinoma (Case 12). Both of them complained mainly leg pain and underwent surgery under general anesthesia in the same way as other cases. The extent of decompression was one intervertebral level in four patients, two levels in six patients, and three levels in two patients (see Table 3 for details).
Fig. 3.
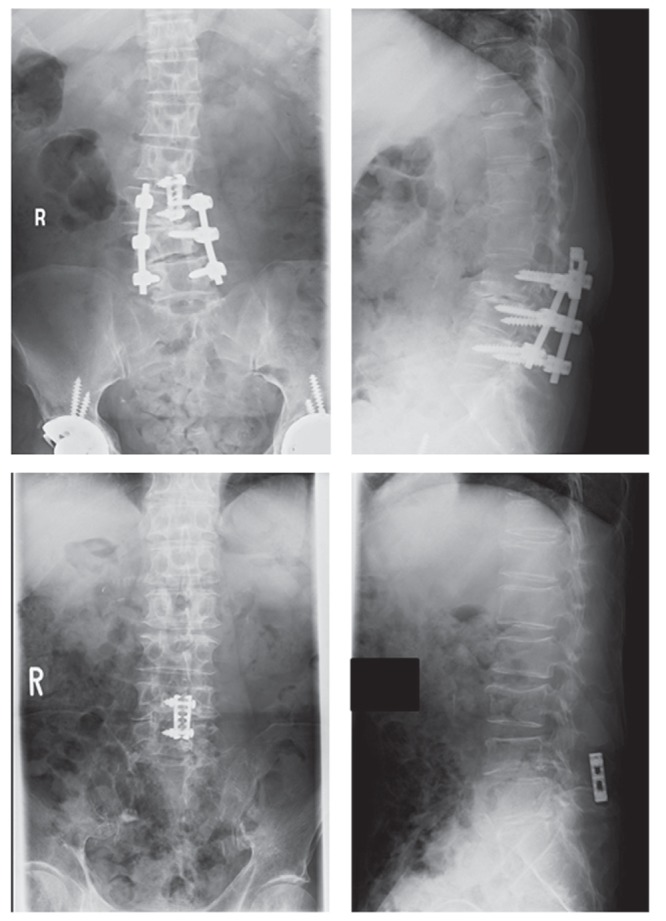
X-ray images 6 months after surgery of Case 9 showing posterolateral lumbar fusion with pedicle screws from L3 to L5 and plate fixation between L2 and L3 spinous processes without instrumentation failure (upper). X-ray images twelve months after surgery of Case 12 demonstrating the plate fixation between L4 and L5 spinous processes without instrumentation failure and consolidation of vertebral fractures of L3 and L4 (lower).
Table 3.
Surgical methods and outcomes
| Case No. | Operation | No. of levels treated | JOA score, preoperative | JOA score, postoperative | Recovery rate (%) | Follow-up (months) | Postoperative complication | Death from PF |
|---|---|---|---|---|---|---|---|---|
| 1 | Decomp. | 2 | 15 | 18 | 21.4 | 39 | ||
| 2 | Decomp. | 2 | 7 | 10 | 13.6 | 34 | ||
| 3 | Decomp. | 2 | 13 | 25 | 75 | 31 | ||
| 4 | Decomp. | 2 | 12 | 19 | 41.2 | 30 | Infection | |
| 5 | Decomp. | 3 | 12 | 19 | 41.2 | 30 | ||
| 6 | Decomp. | 1 | 13 | 24 | 68.8 | 12 | Post 13 months | |
| 7 | TLIF | 2 | 20 | 26 | 66.6 | 19 | ||
| 8 | Decomp. | 1 | 18 | 16 | −18.2 | 14 | ||
| 9 | PLF | 3 | −3 | 10 | 40.6 | 5 | ||
| 10 | Decomp. | 2 | 15 | 18 | 21.4 | 3 | Post 6 months | |
| 11 | SPF | 1 | 17 | 24 | 58.3 | 15 | JFG (reop.) | |
| 12 | SPF | 1 | 12 | 26 | 82.4 | 15 | Vertebral fx. |
Decomp.: only decompression surgery, fx.: fracture, JFG: juxtafacet granulation, JOA: Japanese Orthopaedic Association, PF: pulmonary fibrosis, PLF: posterolateral lumbar fusion, reop.: reoperation, SPF: interspinous fixation, TLIF: transforaminal lumbar interbody fusion.
All patients reported relief from preoperative symptoms after surgery and by the time of discharge from the hospital. The mean JOA scores were 12.6 (range: 3–20) points before surgery and 19.6 (range: 10–26) at the final follow-up, with a mean recovery rate was 42.7% (range: −8% to 75%). An excellent or good recovery rate (more than 50%) was obtained in five patients. On the other hand, an unchanged or deteriorated recovery rate (less than 25%) was observed in four patients.
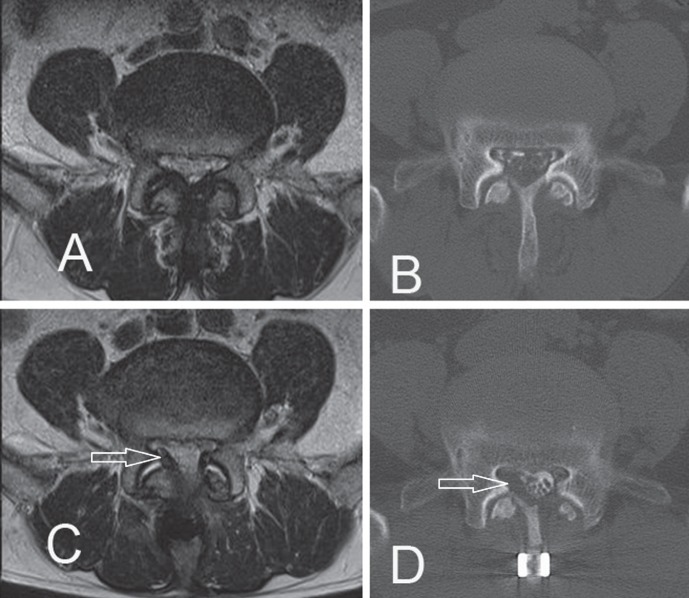
One patient (Case 11) underwent reoperation for a recurrence of leg pain 9 months after the initial-interspinous fixation surgery. CT scans and MRI before the second surgery revealed lateral recess stenosis by the hypertrophic granulation caused as a result of fixation failure (Fig. 4). We removed the granulation mass and performed facet screw fixation. One patient (Case 12) presented with another pathological fracture at an adjacent vertebral body at 1 month after the initial interspinous fixation surgery. It consolidated with conservative treatment (Fig. 3). Postoperative wound infection at 6 months after surgery was observed in one patient (Case 4) and resolved with conservative management.
Fig. 4.
Case 11. A preoperative axial magnetic resonance (MR) image (A) and a computed tomography (CT) scan after myelography (B) demonstrating severe stenosis at the L4/L5 level. An axial MR image (C) and a CT scan after myelography (D) at the L4/L5 level showing right juxtafacet granulation (arrows) compressing the nerve root 6 months after the first operation, which was composed of left hemilaminectomy with interspinous fixation between L4 and L5.
Two of the five patients who had a concomitant pulmonary fibrosis died of respiratory failure at 6 and 13 months after surgery, although they were discharged in good general condition without perioperative pulmonary complications.
Discussion
Although the cervical spine is frequently affected by RA, the frequency of lumbar lesions in RA is not considered to be as low as previously described.13,23) Compared with the attention paid to cervical lesions, there have been relatively few systematic reports on the surgical treatment of lumbar lesions in patients with RA.3,11,16) Several reports have described that a significant number of patients with RA have abnormal radiological findings in the lumbar spine, and some of them reported that the incidence in patients with RA is higher compared to that observed in patients without RA.6,13,23) On the other hand, Harzy et al. described radiological lumbar lesions, expect for vertebral fracture, were not more frequent among RA patients than among control populations.5) Introduction of biological drugs and early treatment by early diagnosis improve treatment results for RA.4,22,24) The number of severeRA-related cervical lesions, in which the main pathology is synovitis, is expected to decrease with early treatment and introduction of biological drugs. Furthermore, the number of lumbar lesions with RA may increase because of the following reasons: (1) The background pathology is degeneration and (2) improvement of RA treatment will prolong life expectancy, and as a result, the number of RA patients with age-related degeneration of spine will increase. Two major sites of pathology in RA-associated lumbar lesions are facet joints and vertebral endplates.2,3,7,13,14,16,19,23,25) Facet joints, also known as apophyseal joints, are synovial joints as well as peripheral joints.29) Synovitis probably starts in facet joints with erosion of cartilage and subchondral bone in exactly the same fashion as seen in peripheral joints.3,7,13,16) Chronic facet arthritis leads to erosion of facet joints, which causes, or enhances, intervertebral instability. In the present study, we observed distinct erosion of facet joints in the CT scans of four patients (25%), which is in agreement with the findings of Kawaguchi et al., who reported an affect rate of 20% in RApatients.13) Another lesion probably starts at the discovertebral junction as an enthesopathy with chronic inflammation around the vertebral end plates, leading to spondylodiscitis.2,3,7,13,14,16,17,19,23,25) The endplate consists of hyaline cartilage in which Type II collagen is predominant, and its biochemical structure is similar to that of the synovial joint.9) This inflammation at endplate leads to structural destruction of discovertebral junction, which causes or enhances disc wedging, intervertebral instability, and lumbar deformity. Kawaji et al. reported the pathognomonic findings around the endplate on MRI as a wavy image, which spreads from the discover-tebral junction to the vertebral body and shows low signal intensity on both T1- and T2-weighted MRI images in a wavelike fashion.14,19) It may indicate invasion of the pannus or rheumatoid nodule invasion around the endplate.14) This endplate lesion on MRIwas evident in four patients of this study (25%), a rate similar to that reported by Kawaguchi et al., −20%.13) On the other hand, RA can be treated with oral steroids to decrease the associated inflammation. Corticosteroids are known to be associated with osteopenia, in addition to that associated with RA.15) Degeneration of spine, in addition to the aforementioned pathological conditions, induces lumbar canal stenosis in patients with RA. These pathological changes tend to induceintervertebral instability and spinal deformity. We found instability in six patients (50%) and scoliosis in five patients (42%) in the present study. Kawaguchi et al. pointed out postural anomaly such as scoliosis in 28% and olisthesis in 23% in their study sample of 106 RA patients.13)
We performed lumbar fixation with pedicle screws only for those with severe instability or deformity. Crawford et al. reported more wound complications and implant complications in lumbar fusion of RA patients thanfor non-RA patients, although the clinical outcomes were similar between the two groups.3) These complications were likely a result of relative immunodeficiency and osteopeniacaused by RA itself or therapeutic drugs for RA, such as steroids and methotrexate. We inserted the pedicle screws using hydroxyapatite granules. Instrumentation surgery for patients with osteoporosis requires some measures for preventing instrumentation failure. Hydroxyapatite granules into each pedicle hole enhance pedicle screw fixation in osteoporotic pedicles and verte-brae in the early stages after surgery.10) Sublaminar wiring by ultra-high molecular-weight polyethylene tape provides a stiffer pedicle screw-rod construct than without augmentation by tape.27) Even with the aforementioned technical recommendations, fusion surgery for lumbar canal stenosis in RA is adopted only when it is absolutely necessary, given its perioperative risk.3,11) In this study, we performed interspinous fixation surgery for controlling the intervertebral motionin two patients with one-level lesion, given their poor general conditionrelated to severe pulmonary fibrosis (Case 11), or chemotherapy for colon carcinoma (Case 12). However, revision surgery was needed because of instrumentation failure and granulation aroundthe facet in Case 11 and an adjacent pathological fracture was observed in Case 12. An interspinous fixation system has been developed for short in situ fusions in select patients.12) But interspinous fixation surgeryis inadequate for lumbar canal stenosis with RA because of its diverse pathological affection and, therefore, unpredictable biomechanics.
All patients showed major improvement in their leg pain as mentioned in previous reports.3,11,16) However, recovery rate was less than 25% in four patients (33%) in the present study. Arthralgia due to RA, including low-back pain and joint pain, may have restricted the activities of daily living and led to this poor outcome. Three patients had undergone total hip arthroplasty (25%), and four patients had suffered from cervical lesion (33%). We have to evaluate separately leg pain from lumbar canal stenosis and knee or hip pain from RA. We had to also pay attention to prevent excessive neck motion under general anesthesia,since the patient should be kept immobilized with a cervical orthosis in case of atlantoaxial or subaxial subluxation.
Osteoporosis may cause instrumentation failure or pathological fracture after surgery. In cases of instrumentation surgery for lumbar canal stenosis in RA, postoperative orthosis treatment is stricter and longer than usual due to osteopenia. All patients who took oral steroid at the time of surgery have received medical treatment according to the current guidelines.21) Preoperative treatment of osteoporosis is essential forsurgery in RA-related lumbar canal stenosis. The immunosuppressive condition caused by steroids or methotrexate as an antirheumatic drug may enhance the risk of delayed wound infection (Case 4). Two of the five patients with pulmonary fibrosis died of respiratory failure (42%) in the follow-up period, an outcome unrelated to the surgical management. Pulmonary fibrosis is a major life-limiting condition for a patient with RA-related lumbar canal stenosis and requires consideration-while deciding on treatment to be given.
Surgical treatment for lumbar canal stenosis in RA needs to be tailored individually for each patient on the basis of his or her preoperative general condition and the evaluation of risks and benefits associated with fixation surgery. Adequate preoperative assessment of respiratory function is important because pulmonary affections in RA, such as pulmonary fibrosis, were reported to have a marked adverse impact on the morbidity and premature mortality of RA patients.20,28) Amital et al. reported that pulmonary complications were directly responsible for 10% to 20% of overall mortality in RA patients.1) Deterioration of pulmonary fibrosis is life-threatening for a patient with RA. In this study, two patientswith pulmonary fibrosis died of respiratory failure, even though they didnot have perioperative pulmonary complications. Surgical treatment of lumbar canal stenosis is definitely not indicated for an RA patient with active pulmonary fibrosis and is avoided for advanced pulmonary fibrosis. A patient with osteoporosis as a consequence of RA and/or steroid treatment also has to be observed adequately to gauge the severity of condition. Decompression surgery for lumbar canal stenosis without instrumentation can be recommended for patients with pulmonary fibrosis requiring early ambulation and short anesthesia time. Lumbar fusion surgery for lumbar canal stenosis with RA is indicated only in cases with kyphosis or severe symptoms caused by intervertebral instability, and pedicle screw instrumentation system with hydroxyapatite granules or sublaminar tape is the preferred procedure in such cases. In cases of poor overall condition, decompression surgery without fixation and postoperative orthosis treatment are recommended. Closer follow-up is necessary after surgery for lumbar canal stenosis in RA compared to the other etiologies because delayed wound infection, instrumentation failure, pathological fracture, and respiratory deterioration are more frequent in RA than in non-RA patients.
References
- 1). Amital A, Shitrit D, Adir Y: The lung in rheumatoid arthritis. Presse Med 40: e31– 48, 2011. [DOI] [PubMed] [Google Scholar]
- 2). Ball J: Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis 30: 213– 223, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Crawford CH, Carreon LY, Djurasovic M, Glassman SD: Lumbar fusion outcomes in patients with rheumatoid arthritis. Eur Spine J 17: 822– 825, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Farragher TM, Lunt M, Plant D, Bunn DK, Barton A, Symmons DP: Benefit of early treatment in inflammatory polyarthritis patients with anti-cyclic citrullinated peptide antibodies versus those without antibodies. Arthritis Care Res 62: 664– 675, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Harzy T, Allali F, Bennani-Othmani M, Hajjaj-Hassouni N: Radiological characteristics of the lumbar spine in patients with rheumatoid arthritis. Presse Med 36: 1385– 1389, 2007. [DOI] [PubMed] [Google Scholar]
- 6). Helliwell PS, Zebouni LN, Porter G, Wright V: A clinical and radiological study of back pain in rheumatoid arthritis. Br J Rheumato 32: 216– 221, 1993. [DOI] [PubMed] [Google Scholar]
- 7). Heywood AW, Meyers OL: Rheumatoid arthritis of the thoracic and lumbar spine. J Bone Joint Surg Br 68: 362– 368, 1986. [DOI] [PubMed] [Google Scholar]
- 8). Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K: Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine 6: 354– 364, 1981. [DOI] [PubMed] [Google Scholar]
- 9). Hukins DWL: Disc structure and function, in Ghosh P. (ed): Biology of the Intervertebral Disc (vol. 1). Boca Raton, Florida, CRC Press, 1988, pp 1– 37 [Google Scholar]
- 10). Iizuka T, Yamada S: Possibility of enhancement for the pedicle screw fixations with HA sticks (hydroxyapatite sticks) augmentation: a preliminary report of clinical results in lumbar reconstruction surgery. The Internet J Spine Surg 3: 1, 2007. [Google Scholar]
- 11). Inaoka M, Tada K, Yonenobu K: Problems of posterior lumbar interbody fusion (PLIF) for the rheumatoid spondylitis of the lumbar spine. Arch Orthop Trauma Surg 122: 73– 79, 2002. [DOI] [PubMed] [Google Scholar]
- 12). Iwatsuki K, Yoshimine T, Yoshimura K, Ishihara M, Ohnishi Y, Goto Y: Intractable chronic low-back pain caused by ligamentopathia treated using a spinous process plate (S-plate). Clin Med Insights Arthritis Musculoskelet Disord 10: 1– 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Kawaguchi Y, Matsuno H, Kanamori M, Ishihara H, Ohmori K, Kimura T: Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech 16: 38– 43, 2003. [DOI] [PubMed] [Google Scholar]
- 14). Kawaji H, Miyamoto M, Gembun Y, Ito H: A case report of rapidly progressing cauda equina symptoms due to rheumatoid arthritis. J Nippon Med Sch 72: 290– 294, 2005. [DOI] [PubMed] [Google Scholar]
- 15). Kim DH, Hilibrand AS: Rheumatoid arthritis in the cervical spine. J Am Acad Orthop Surg 13: 463– 474, 2005. [DOI] [PubMed] [Google Scholar]
- 16). Koakutsu T, Morozumi N, Koizumi Y, Ishii Y: Lumbar radiculopathy caused by foraminal stenosis in rheumatoid arthritis. Ups J Med Sci 116: 133– 137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Lee SH, Kang YM, Park YM: Multiple vertebral involvement of rheumatoid arthritis in thoracolumbar spine: a case report. J Korean Med Sci 25: 472– 475, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Matsumoto M, Watanabe K, Ishii K, Tsuji T, Takaishi H, Nakamura M, Toyama Y, Chiba K: Posterior decompression surgery for extraforaminal entrapment of the fifth lumbar spinal nerve at the lumbosacral junction. J Neurosurg Spine 12: 72– 81, 2010. [DOI] [PubMed] [Google Scholar]
- 19). Nakase T, Fujiwara K, Kono J, Owaki H, Yonenobu K, Ochi T: [Characteristics and surgical treatment of lumbar spine in rheumatoid arthritis]. Orthopedic Surg 34: 219– 223, 1998. (Japanese) [Google Scholar]
- 20). Nannini C, Ryu JH, Matteson EL: Lung disease in rheumatoid arthritis. Curr Opin Rheumatol 20: 340– 346, 2008. [DOI] [PubMed] [Google Scholar]
- 21). Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y, Subcommittee to Study Diagnostic Criteria for Glucocorticoid-Induced Osteoporosis : Subcommittee to study diagnostic criteria for glucocorticoid-induced osteoporosis: guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23: 105– 109, 2005. [DOI] [PubMed] [Google Scholar]
- 22). Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL, Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O'Dell J, Turkiewicz AM, Furst DE, American College of Rheumatology : American College of Rheumatology: American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 59: 762– 784, 2008. [DOI] [PubMed] [Google Scholar]
- 23). Sakai T, Sairyo K, Hamada D, Higashino K, Katoh S, Takata Y, et al. : Radiological features of lumbar spinal lesions in patients with rheumatoid arthritis with special reference to the changes around intervertebral discs. Spine J 8: 605– 611, 2008. [DOI] [PubMed] [Google Scholar]
- 24). Scott DL, Wolfe F, Huizinga TW: Rheumatoid arthritis. Lancet 376: 1094– 1108, 2010. [DOI] [PubMed] [Google Scholar]
- 25). Shichikawa K, Matsui K, Oze K, Ota H: Rheumatoid spondylitis. Int Orthop 2: 53– 60, 1978. [Google Scholar]
- 26). Steinbrocker O, Traeger CH, Batterman RC: Therapeutic criteria in rheumatoid arthritis. JAMA 140: 659– 662, 1949. [DOI] [PubMed] [Google Scholar]
- 27). Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Ohshima S, et al. : Comparison of novel ultra-high molecular weight polyethylene tape versus conventional metal wire for sublaminar segmental fixation in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech 20: 449– 455, 2007. [DOI] [PubMed] [Google Scholar]
- 28). Tokuda H: [Lung involvements of RA, diagnosis and treatment]. Nippon Rinsho 60: 2423– 2428, 2002. (Japanese) [PubMed] [Google Scholar]
- 29). Yamashita T, Minaki Y, Ozaktay AC, Cavanaugh JM, King AI: A morphological study of the fibrous capsule of the human lumbar facet joint. Spine 21: 538– 543, 1996. [DOI] [PubMed] [Google Scholar]