Abstract
In the research for the treatment of spinal cord injury (SCI), the evaluation of motor function in model rats must be as objective, noninvasive, and ethical as possible. The maximum speed and acceleration of a mouse measured using a SCANET system were previously reported to vary significantly according to severity of SCI. In the present study, the motor performance of SCI model rats was examined with SCANET and assessed for Basso-Beattie-Bresnahan (BBB) score to determine the usefulness of the SCANET system in evaluating functional recovery after SCI. Maximum speed and acceleration within the measurement period correlated significantly with BBB scores. Furthermore, among several phased kinematic factors used in BBB scores, the capability of “plantar stepping” was associated with a drastic increase in maximum speed and acceleration after SCI. Therefore, evaluation of maximum speed and acceleration using a SCANET system is a useful method for rat models of SCI and can complement open field scoring scales.
Keywords: acceleration, locomotor ability, hindlimb function, speed, spinal cord injury
Introduction
Open field scores such as the Basso-Beattie-Bresnahan (BBB) score for rats1) and Basso Mouse Scale (BMS) for mice2) are widely used as simple and reliable methods for evaluating motor performance after spinal cord injury (SCI) in rodents. However, eliminating subjectivity from these methods requires assessment by multiple skilled examiners in a blinded manner. In addition, since these ratings are not linear, the potential for quantitative comparison or analysis of scores is limited.7) A complementary objective method is thus indispensable.
We previously reported the usefulness of the SCANET system, with which an animal can be evaluated easily, noninvasively, and reproducibly.4,5) Maximum speed and acceleration calculated from raw data obtained by SCANET effectively distinguished between a thoracic transection model, a contusion model, and uninjured mice.8) Nevertheless, the previous reports leave several concerns. First, the effectiveness of the SCANET on rats has not been demonstrated. The length of each side of the box in the system is 45 cm, which limits the running space for a rat compared to that of a mouse under the same condition. Whether a rat is capable of reaching top speed and demonstrating its best performance is unclear. Next, in the contusion model, although a correlation between open field score and maximum speed was suggested in a prior study,8) the data may have reflected only the natural recovery process, because longitudinal data were included for the calculation of the correlation coefficient. To describe the correlation more precisely, data should be examined at each time point. Third, the superiority of the SCANET to other methods has not been determined. The key advantage of the SCANET is its ability to evaluate an animal at the time of its best performance, whereas other examinations are performed at an arbitrary time. Therefore, the invalidity of evaluating an animal at an arbitrary time should also be demonstrated.
To address these issues, we used contusion-model rats to evaluate the correlation between open field score and maximum speed/acceleration at each time point until 6 weeks after injury. Average speed was calculated as the performance at an arbitrary time and also compared to open field score. We further examined the locomotive factors used to determine open field scores for an in-depth investigation of their relationships with maximum speed/acceleration.
Materials and Methods
I. SCI
The present study used 35 6-week-old adult female Sprague-Dawley rats (body weight, 152–174 g). Animals were anesthetized by intraperitoneal injection of ketamine and xylazine, and the dorsal surface of the dura mater at the T10 level was exposed by laminectomy. Severe contusion SCI was induced in each rat using an IH impactor (Precision Systems and Instrumentation; impact force 250 kdyn), as reported previously.3,9) The muscles and skin were closed in layers, and the animals were placed in a temperature-controlled chamber until thermoregulation was reestablished. Manual voiding of the bladder was performed twice daily until reflex bladder emptying was reestablished. All experiments and procedures in our study were approved by the Keio University Animal Research Committee in accordance with the Laboratory Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Maryland, USA), and the Guidelines and Policies for Animal Surgery provided by the Animal Study Committees of the Central Institute for Experimental Animals and of Keio University.
II. Behavioral analyses
The SCANET MV-40 (MELQUEST, Toyama) is a device that allows behavioral analysis of an animal using high-density-arranged infrared sensors. The infrared sensors are distributed in all directions at 6-mm intervals parallel to the floor of a transparent 45 × 45-cm box. The animal can move freely on the floor. These movements are detected by the infrared sensors, and the coordinates of the center of the animal are recorded on a computer every 0.1s. The height of the sensor from the floor was set at 4 cm. One animal at a time was placed in the box, and after waiting for 10s until the animal settled down, its coordinates were recorded for 5 minutes, as reported previously.8) Measurement was performed in the same environment before SCI, 1 day after SCI, and weekly until 6 weeks postoperatively.
Motor function of the hindlimbs in each rat was also evaluated using BBB score. Individual animals were allowed to move freely for 5 minutes, and hindlimb movements were observed by two examiners. The average score was used as the recorded value.
III. Data analysis
The distance moved every 0.1 s was calculated as speed (m/s) for each interval of time. Next, the rate of change in speed per 0.1 s was calculated as acceleration (m/s2) for each interval of time. Example data are shown in Table 1. Since the coordinate per unit in raw data was equivalent to 6 mm, the formulae were as follows:
where Xn, Yn, Dn, Sn, and An are the x and y coordinates, moving distance, speed, and acceleration, respectively, at a time point n. Maximum speed and maximum acceleration over 5 minutes were extracted as the real performance of each animal. Maximum deceleration was also calculated as an indicator of braking performance of the animal. Moreover, the average speed during movement was computed as the total distance moved by each animal divided by its total time spent moving.
Table 1.
Example of data for coordinates, speed, and acceleration
| Time | X | Y | Distance (m) | Speed (m/s) | Acceleration (m/s2) |
|---|---|---|---|---|---|
| · | · | · | · | · | · |
| · | Xn | Yn | · | · | · |
| · | Xn + 1 | Yn + 1 | Dn | Sn | · |
| · | · | · | · | Sn + 1 | An |
| 34.31.0 | 60 | 38 | · | · | · |
| 34.31.1 | 60 | 38 | 0 | 0 | · |
| 34.31.2 | 60 | 39 | 0.006 | 0.06 | 0.6 |
| 34.31.3 | 59.5 | 44.5 | 0.0331361 | 0.331361 | 2.71361 |
| 34.31.4 | 59 | 50 | 0.0331361 | 0.331361 | 0 |
| 34.31.5 | 58.5 | 50 | 0.0067082 | 0.067082 | –2.6428 |
| 34.31.6 | 58.5 | 50 | 0 | 0 | –0.67082 |
| · | · | · | · | · | · |
Raw serial data of time and X–Y coordinates for a 0.6-s epoch are shown. Data are recorded every 0.1 s, so 3000 rows are recorded in 5 minutes in practice. Speed and acceleration were calculated using our custom program (see text for formulae). For this 0.6-s epoch, maximum speed is 0.33 m/s, and maximum acceleration is 2.71 m/s2.
IV. Statistical analysis
All values are reported as means ± standard error of the mean. The strength of correlation between each parameter and BBB score was determined using the Pearson correlation coefficient.
Results
I. Speed and acceleration correlate significantly with open field scores
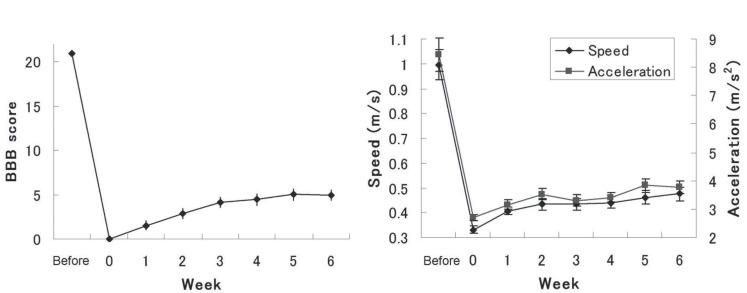
The time courses of BBB scores are shown in Fig. 1. BBB scores were 0 at Day 1 after SCI and gradually recovered to plateau around five points, as reported previously.6)
Fig. 1.
Time course of Basso-Beattie-Bresnahan (BBB) scores, maximum speed, and maximum acceleration for rats. (Left) Rats scored near zero at Day 1 after spinal cord injury (SCI), with subsequent gradual recovery: most reached a plateau after 3 weeks. (Right) Both maximum speed and maximum acceleration gradually recovered and reached a plateau within 3 weeks after SCI.
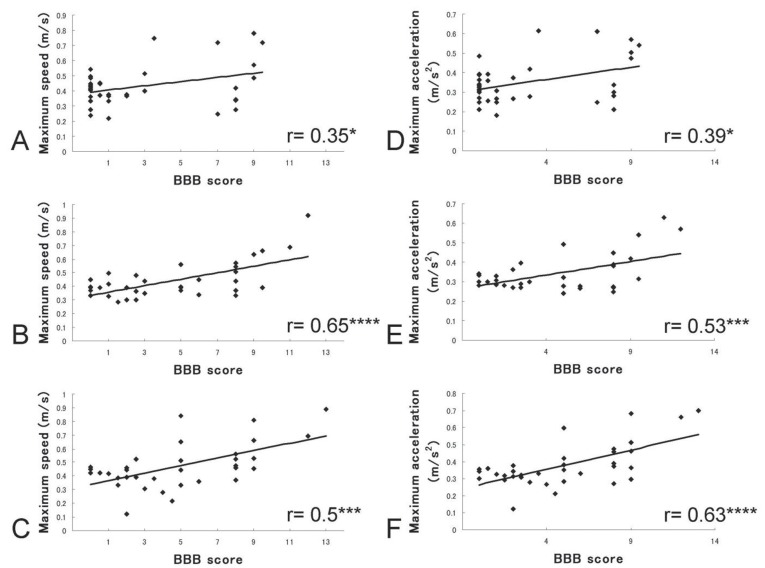
Maximum speed gradually recovered from 0.33 m/s at 1 day to 0.48 m/s at 42 days after SCI. Likewise, maximum acceleration recovered from 2.7 m/s2 to 3.76 m/s2 (Fig. 1). Correlation coefficients between BBB scores and maximum speed were 0.22 (p = 0.21) at 1 week, 0.35 (p < 0.05) at 2 weeks, 0.54 (p < 0.001) at 3 weeks, 0.65 (p < 0.0001) at 4 weeks, 0.43 (p < 0.01) at 5 weeks, and 0.58 (p < 0.001) at 6 weeks after SCI. Significant relationships were found from 2 to 6 weeks. Similar to maximum speed, significant correlations between BBB scores and maximum acceleration were observed from 1 week to 6 weeks. Correlation coefficients were 0.35 (p < 0.05) at 1 week, 0.39 (p < 0.05) at 2 weeks, 0.50 (p < 0.01) at 3 weeks, 0.53 (p < 0.001) at 4 weeks, 0.50 at 5 weeks (p < 0.01), and 0.63 (p < 0.0001) at 6 weeks after SCI. Representative diagrams are shown in Fig. 2. The maximum speed and maximum acceleration using data from each time point correlated significantly with hindlimb function in SCI model rats.
Fig. 2.
Correlation between Basso-Beattie-Bresnahan (BBB) scores and maximum speed and acceleration. A–C: Scatter diagrams of BBB scores and maximum speed at 2, 4, and 6 weeks after spinal cord injury (SCI). The distribution of BBB scores shifted as time advanced, but a significant correlation was preserved. D–F: Scatter diagrams of BBB scores and maximum acceleration at 2, 4, and 6 weeks after SCI, respectively. A significant correlation was again apparent. *p < 0.05, ***p < 0.001, ****p < 0.0001.
II. In severe SCI models, maximum speed and acceleration are higher in animals capable of plantar stepping
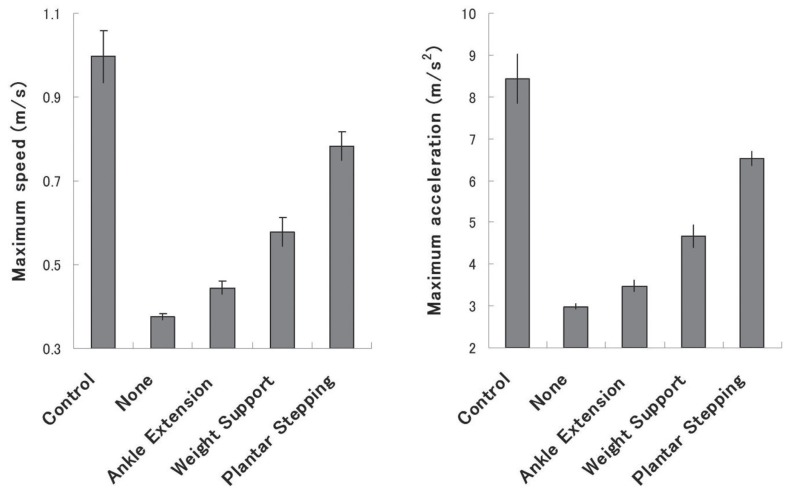
BBB scoring is based on several kinematic factors. However, few previous reports have clarified the relative importance of each factor to evaluate hindlimb functions. We therefore examined the relationships between maximum speed and acceleration and the factors “ankle extension,” “weight support,” and “plantar stepping” from the longitudinal data of all rats. Relationships between each category and hindlimb status were defined as follows: none, ankle extension impossible on either or both hindlimbs (n = 148); ankle extension, ankle extension possible on both hindlimbs, but not weight support (n = 66); weight support, weight support possible, but frequent to consistent plantar stepping impossible on either or both hindlimbs (n = 14); and plantar stepping, frequent to consistent plantar stepping possible on both hindlimbs (n = 7). Maximum speed and acceleration were dramatically increased in animals capable of plantar stepping (Fig. 3), suggesting plantar stepping as the most important locomotive factor in severe SCI model rats.
Fig. 3.
Relationship between kinematic factors and maximum speed or acceleration. (Left) The relationship between maximum speed and kinematic factors of the Basso-Beattie-Bresnahan (BBB) score. Maximum speed demonstrated a stepwise increase with improved factors. “Plantar stepping” was associated with a dramatic increase in maximum speed. (Right) The same tendency was observed for maximum acceleration. Statistical analysis was not performed, because these data are longitudinal.
III. Braking performance depends on hindlimb ability in SCI model rats
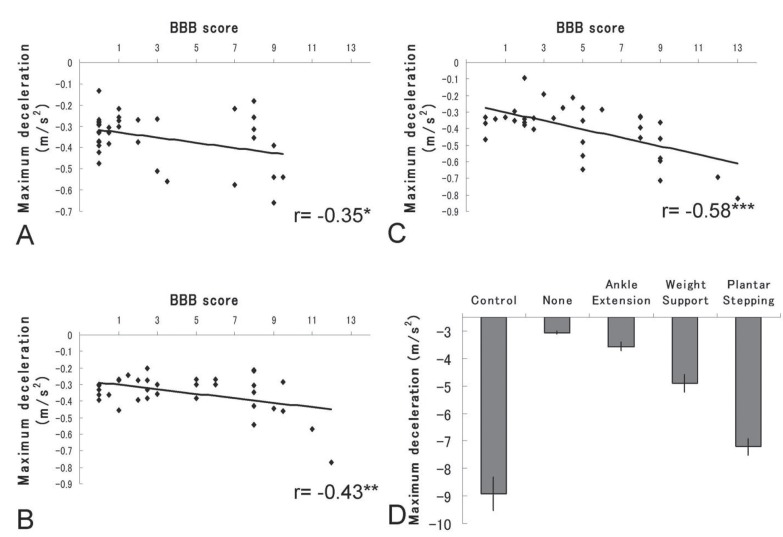
To evaluate locomotor ability, braking performance should also be addressed. However, no previous reports have examined braking performance in rodents after SCI. Braking performance is expressed as a decrease in speed, that is, deceleration. We extracted maximum deceleration from the acquired data. Representative scatter diagrams of maximum deceleration and BBB scores are shown in Fig. 4. A significant correlation was observed between braking performance and BBB scores, with correlation coefficients of −0.35 (p < 0.05) at 1 week, −0.35 (p < 0.05) at 2 weeks, −0.48 (p < 0.01) at 3 weeks, −0.43 (p < 0.01) at 4 weeks, −0.47 (p < 0.01) at 5 weeks, and −0.58 (p < 0.001) at 6 weeks. Moreover, braking performance improved in animals with plantar stepping compared with other kinematic factors, suggesting that braking performance also depends on hindlimb function in SCI model rats.
Fig. 4.
Characteristics of braking ability. A–C: Representative scatter diagrams of Basso-Beattie-Bresnahan (BBB) scores and maximum deceleration at 2, 4, and 6 weeks after spinal cord injury (SCI). Significant correlations were observed for all time periods. D: Relationship between braking ability and kinematic factors. Similar to maximum speed and acceleration, braking ability increased markedly with the presence of “Plantar stepping.” *p < 0.05, **p < 0.01, ***p < 0.001.
IV. Performance at an arbitrary time point does not reflect motor function
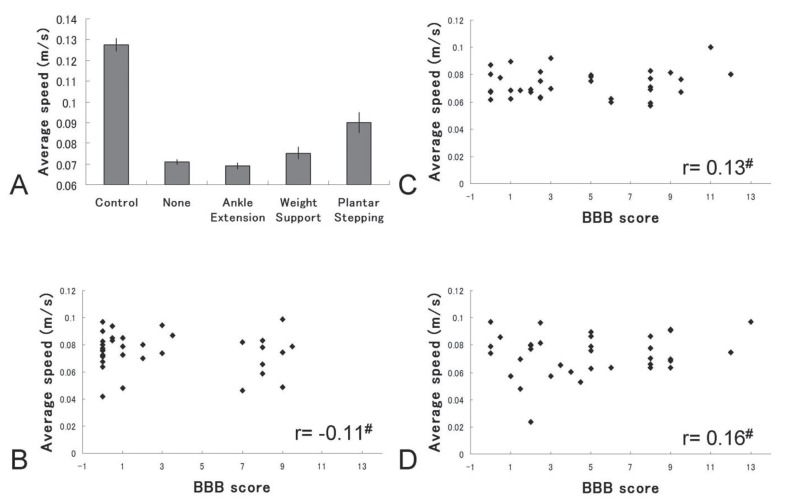
As an animal may move vigorously at one time and barely move at another time, speed and acceleration are always variable. Therefore, when an examiner evaluates movement performance using arbitrary timing, the results may not reflect true motor function. To investigate this possibility, we calculated average speed (total distance moved divided by total time spent moving) and compared it with BBB score, under the assumption that loco-motor ability reflected hindlimb function. Indeed, average speed was reduced from 0.13 m/s to 0.06 m/s with loss of hindlimb function, and showed a stepwise increase with improved factors (Fig. 5). However, no significant correlation was found between average speed and BBB score. Accurate hindlimb performance thus could not be determined when measurement was executed using arbitrary timing, even if the animal was voluntarily moving as the examiner intended.
Fig. 5.
Average speed. A: Average speed demonstrated a stepwise increase with improved factors. B–D: Representative scatter diagram of Basso-Beattie-Bresnahan (BBB) scores and average speed, calculated from the total distance moved and total time spent moving at 2, 4, and 6 weeks after spinal cord injury (SCI). No significant correlation was apparent. #p > 0.05.
Discussion
Locomotion of rats is performed using both fore-limbs and hindlimbs. If the hindlimbs are impaired, locomotor ability declines, but a long distance can still be moved using only the forelimbs, given sufficient time. Hence, to assess locomotor ability, the examiner should consider the distance moved per unit time, that is, speed. Furthermore, since speed determined over an arbitrary time period does not always reflect hindlimb function, the best performance should be extracted. From another perspective, locomotor ability is dependent on the force to move, and force can be determined by acceleration, assuming a constant mass of the animal. The present study revealed that maximum speed and maximum acceleration in severe SCI model rats bear significant relationships to BBB scores, which essentially focus on hindlimb functions rather than locomotor ability.
The most serious problem with existing behavioral evaluations for rats is noncooperation, and the performance of an animal does not always reflect maximal ability. Most examinations including the inclined test, grid walk, beam walk, and manual footprint analysis require the cooperation of the animal to perform the tested motion. However, since we found that performance at an arbitrary time point bears no relationship to hindlimb function, those motions are not guaranteed to reflect the best performance. To use these examinations, several sessions must be carried out, and the best result should be used.
Simplicity is also required for repeated examinations over time. Automatic footprint or kinematic analysis using a high-speed camera can provide reliable results, but analysis and extraction of certain results from the enormous amount of data they generate are labor intensive. In the present system, the examiner needs only to place the animal in the box for 5 minutes, so stress for both the examiner and animal is substantially reduced. We built our own program to compute parameters, but the original SCANET manufacturer is constructing an automatic program to make processing extremely easy.
The most important issue for using the SCANET system is to regulate the environment, including time of testing, brightness, odor, and surrounding sound. In particular, loud noises or sudden movements may surprise the animal and lead to short-term increases in locomotor ability, and the present system may record these accidental situations. Another limitation of the present method is the impossibility of detecting slight hindlimb-specific differences among rats in severe condition. Assessing maximum speed and acceleration relies on movement of the whole body; hence, subtle differences in hindlimb state are not detectable.
In the evaluation of locomotor function for SCI model rats, multiple perspectives are desirable. Evaluation of the maximum speed and acceleration of rats using the SCANET system is objective, ethical, and simple, and SCANET values can complement open field scores. For mild SCI animals or other disease model animals, further examination will be required to determine the efficacy of the SCANET system.
Acknowledgments
The authors are grateful to Ms Harada for the special care of the rats. This work was supported by the Project for Realization of Regenerative Medicine and Support for the core institutes for iPS cell research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
References
- 1). Basso DM, Beattie MS, Bresnahan JC: A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1– 21, 1995. [DOI] [PubMed] [Google Scholar]
- 2). Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG: Basso Mouse Scale for locomotion detects differences in recovery after SCI in five common mouse strains. J Neurotrauma 23: 635– 659, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW: Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci 24: 4432– 4443, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Mikami Y, Okano H, Sakaguchi M, Nakamura M, Shimazaki T, Okano HJ, Kawakami Y, Toyama Y, Toda M: Implantation of dendritic cells in injured adult spinal cord results in activation of endogenous neural stem/progenitor cells leading to de novo neurogenesis and functional recovery. J Neurosci Res 76: 453– 465, 2004. [DOI] [PubMed] [Google Scholar]
- 5). Mikami Y, Toda M, Watanabe M, Nakamura M, Toyama Y, Kawakami Y: A simple and reliable behavioral analysis of locomotor function after spinal cord injury in mice. Technical note. J Neurosurg 97: 142– 147, 2002. [DOI] [PubMed] [Google Scholar]
- 6). Nessler JA, De Leon RD, Sharp K, Kwak E, Minakata K, Reinkensmeyer DJ: Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: characterization of intrinsic recovery and comparison with BBB. J Neurotrauma 23: 882– 896, 2006. [DOI] [PubMed] [Google Scholar]
- 7). Semler J, Wellmann K, Wirth F, Stein G, Angelova S, Ashrafi M, Schempf G, Ankerne J, Ozsoy O, Ozsoy U, Schoenau E, Angelov DN, Irintchev A: Objective measures of motor dysfunction after compression spinal cord injury in adult rats: correlations with locomotor rating scores. J Neurotrauma 28: 1247– 1258, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Shinozaki M, Takahashi Y, Mukaino M, Saito N, Toyama Y, Okano H, Nakamura M: Novel concept of motor functional analysis for spinal cord injury in adult mice. J Biomed Biotechnol 2011: Article ID 157458, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, Nagai T, Katoh H, Kohda K, Matsuzaki Y, Yuzaki M, Ikeda E, Toyama Y, Nakamura M, Yamanaka S, Okano H: Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA 107: 12704– 12709, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]