Abstract
To evaluate the long-term outcome and functional recovery of intramedullary medullocervical ependymoma (IME), the clinical charts of 38 surgically treated consecutive cases of IME were reviewed. Follow-up was obtained prospectively. The mean age of the patients (19 male and 19 female) was 35.3 years (range: 11–60 years). Complete resection was achieved in 33 (86.8%) patients. Fourteen patients worsened postoperatively; five and seven of these improved to their baseline levels within 1 and 3 months, respectively. By 1 year postoperatively, 17 patients returned to work. After a mean follow-up duration of 81.5 months, 31 patients improved or stabilized, and 3 had recurrence. The means of the modified McCormick grade (mMG) scores before the operation, at discharge, 1 year after the operation, and at the most recent evaluation were 1.76, 2.13, 1.82, and 1.84, respectively. A favorable long-term outcome of the mMG was associated with a good preoperative status (mMG I) (odds ratio [OR] = 9.956, p = 0.008) and well-defined tumor boundary (OR = 7.829, p = 0.035). Improvements in the postoperative walking dysfunction and paresthesia over time were associated with the absence of preoperative walking dysfunction (p = 0.047) and paresthesia (p = 0.028), respectively. The 12-year progression/recurrence-free survival and overall survival rates were 92.0% and 93.7%, respectively. The study suggests that the goal of surgery is to stabilize the preoperative neurological function and that a favorable outcome may be achieved in patients with good preoperative statuses and well-defined tumor boundaries. Surgery should be performed as soon as possible after the diagnoses and before the neurological functions deteriorate.
Keywords: cervical cord, ependymoma, intramedullary, medulla oblongata, outcome
Introduction
Ependymomas are an uncommon group of central nervous system (CNS) neuroectodermal neoplasms constituting 2–8% of all primary CNS tumors, 30–60% of spinal cord gliomas, and up to 12% of pediatric brain tumors.13,15,29,30,33) A considerable number of retrospective studies on intramedullary spinal ependymomas have been reported in the last decade,1,5,8,12–14,16,20,22,23,26) confirming the existence of various predictors of the disease, including histology,22) preoperative neurological function,4) and surgical resection.5) Nevertheless, few of these were performed on patients harboring an intramedullary medullocervical ependymoma (IME). Additionally, many series had a long recruitment period with limited follow-up. Due to the location of the tumor, IME results in neurological dysfunction (e.g., quadriplegia, respiration disorders, and dysphagia) that impairs the patient's quality of life.36) Additionally, the high potential risk of operation and lack of accepted and recognized prognostic factors for IME have led to the absence of a standardized therapeutic strategy.12,19,35) Therefore, the following are of significant value to elucidate: the pertinent prognostic factors, neurological function recovery, and long-term outcome. Here, we review our 2000–2010 institutional experience with 38 cases of IME.
Materials and Methods
Patient population
A retrospective medical record review of 38 patients (19 male and 19 female; mean and median ages, 35.3 ± 11.5 and 35.5 years, respectively; range, 11–60 years) with IME who underwent surgery between January 2000 and January 2010 at the Beijing Tiantan Hospital, Capital Medical University, was conducted.
The clinical data included the patient gender, age at surgery, symptoms, duration of symptoms, modified McCormick grade (mMG) (measured at the initial diagnosis, in the preoperative time period, at discharge after the operation, 1 year after the operation, and most recent follow-up), tumor features (volume, length, boundary, and localization) (Figs. 1–4), radiotherapy if received, World Health Organization (WHO) classification of the ependymoma (Grade II and Grade III), postoperative complications, follow-up time, and treatment for recurrent patients. Two independent neuropathologists conducted the pathologic examinations. The follow-up was obtained prospectively in clinic or by questionnaire.
Fig. 1.
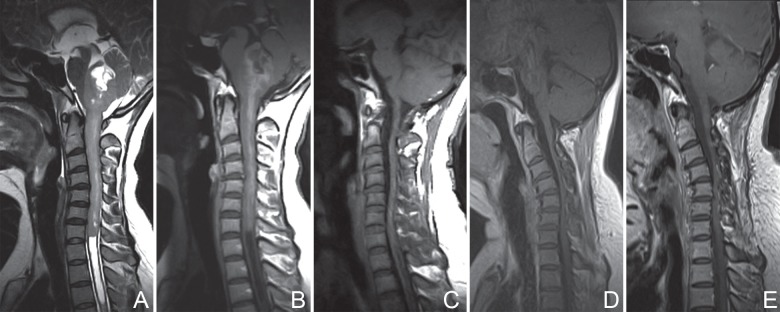
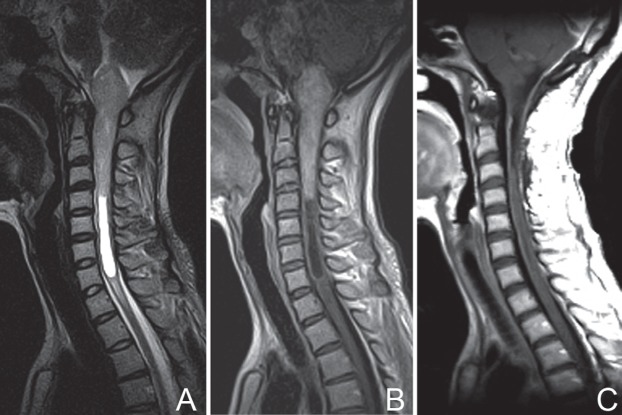
Patient No. 18. Preoperative T2-weighted MRI (A) and T1-weighted imaging with contrast enhancement (B) showing regions with tumor involvement (from medulla oblongata rostrally to seventh cervical vertebra caudally) and a well-defined tumor boundary. Postoperative MRI scans with contrast enhancement at 1 week (C), 3 years (D), and 5 years (E), illustrating total removal of the lesion (C) and no recurrence (D, E). After a period of 80 months of follow-up, the patient improved from a preoperative mMG III to mMG II. mMG: modified McCormick grade, MRI: magnetic resonance imaging.
Definition of IME
Intramedullary ependymoma, involving both the medulla oblongata rostrally and cervical spinal cord caudally with a varied number of spinal levels involved, was defined as IME, while exophytic, extramedullary, or posterior fossa/IVth ventricular ependymomas were not included. When an IME involved the medulla oblongata and only the first cervical spinal cord, the tumor location was labeled Medulla-C1.
Neurological evaluation
The patients' neurological statuses were evaluated using the mMG (Grade I, neurologically normal or with mild neurological deficits; Grade II, sensorimotor deficit affecting the function of the corresponding limb; Grade III, more severe neurological deficits, brace required; Grade IV, severe deficit, wheelchair required; Grade V, death from the disease).5,27)
Radiological evaluation
In all patients, magnetic resonance imaging (MRI) scans with contrast enhancement were evaluated preoperatively, at discharge, 3 and 6 months after operation, and every 1 or 2 years thereafter. Postoperative MRI with contrast enhancement was used to evaluate the extent of surgical resection.
The definition of the tumor boundary was based only on T2-weighted MRI scans (Fig. 5). A well-defined boundary either (A) had a clearly identifiable subarachnoid space between the tumor and healthy spinal cord or (B) lacked an identifiable subarachnoid space but the contour line between the tumor and healthy spine was smooth (Fig. 5C), which corresponded to intraoperative findings; there was also a clearly identifiable transition between the tumor and the normal parenchyma. An ill-defined boundary was a boundary that lacked an identifiable subarachnoid space and for which the contour line was obscure. When most of the boundary was ill defined, even if some parts were well defined, the tumor boundary was determined to be ill defined.
Fig. 5.
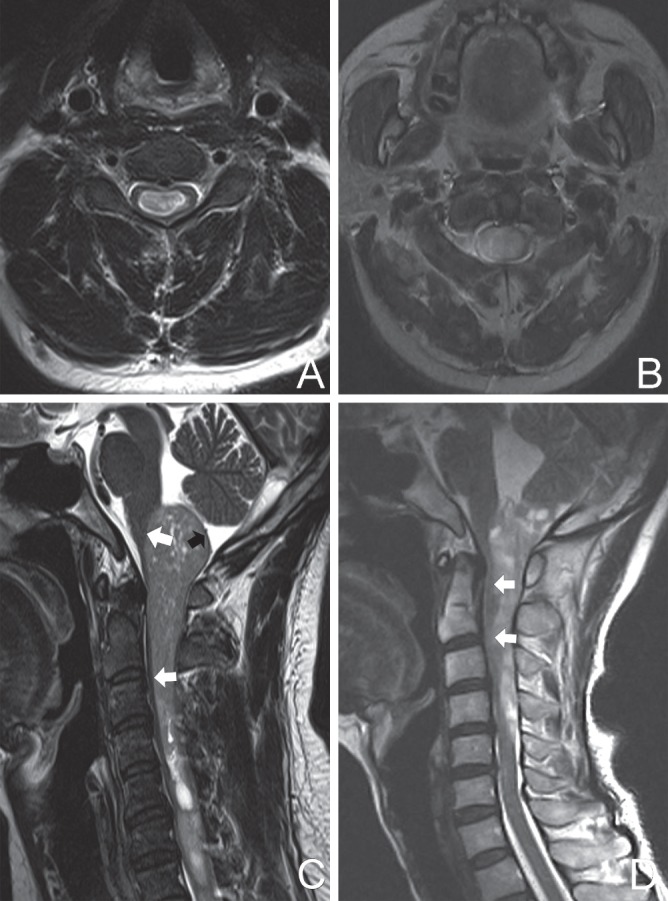
Preoperative MRI scans illustrating the existence (A) or absence (B) of a subarachnoid space between the tumor and parenchyma. An isointense ring (thin-slice parenchyma) surrounding dorsal surface of the tumor (black arrow) (C) indicated an intramedullary tumor and contour line of the ventral surface of the tumor (white arrow) (C) was smooth; this tumor had a well-defined boundary even though it lacked an identifiable subarachnoid apace. The contour line (white arrow) (D) was obscure and the boundary was determined to be ill defined. MRI: magnetic resonance imaging.
Tumors consisted of two independent parts, the posterior fossa part and spinal parts. The tumor volume was calculated as the sum of the two parts by the Coniglobus formula, which was described by the following: Vtumor = (D1 × D2 × D3 )/2, where D1, D2, and D3 were the maximal diameters in the axial, sagittal, and coronal T1-weighted MRI scans, respectively, with administration of gadolinium.
Surgical technique
For all patients, a standard midline incision was made, and suboccipital midline approach and laminoplasty were performed to avoid future spinal instability. Intraoperative monitoring of evoked potentials, including spinal cord somatosensory evoked potentials (SEPs), motor-evoked potentials, and brainstem auditory evoked potential (BAEP), was adopted to prevent neurosurgical functional impairment. During the operation, needle electrodes were used to record the BAEPs. If the monitor showed intraoperative abnormalities in Waves I, III, or V, such as the prolongation of the latency by 10%, an amplitude that decreased by 50%, or significant changes in the form of the Wave, the surgeon stopped, corrected the dangerous manoeuvres, identified the tumor boundary, and reduced the brainstem retraction. The spinal midline was identified as precisely as possible before the myelotomy was performed to reduce neurological deficits; in our series, this was performed with consideration of the anatomic landmarks of the posterior spinal arteries on the normal spinal cord rostral and caudal to the lesion. Additionally, intraoperative ultrasonography and dorsal cord mapping were used in 5 (13.2%) and 4 (10.5%) patients, respectively, because anatomic landmarks had shifted due to severe deformation of the spinal cord from a significant lesion space-occupying effect. The strip electrode for dorsal cord mapping was a multielectrode grid containing eight parallel Teflon-coated steel wires that were spaced 1 mm apart. Peripheral electrodes were placed at the bilateral tibial, ulnar, or median nerves. The grid was placed on the dorsal column surface. After stimulating one of the bilateral peripheral nerves, the multielectrode grid recorded the conducted SEPs from the dorsal surface overlying the lesion. The amplitude corresponded to the topographic anatomy of the dorsal cord. The maximum amplitude indicated a dense aggregation of the dorsal column fibres. The midline was located between the two maximum amplitudes.
Statistical analysis
We used the “mean ± standard deviation” to describe all measurement data. Logistic regression analysis (IBM SPSS Statistical Package v. 19.0) was performed to evaluate the effects of the age, gender, duration of the symptom, preoperative mMG (I or II–IV), tumor boundary (well or ill defined) (Fig. 5), and tumor location (number of spinal levels with tumor involvement) on the most recent mMG. The incidences of the postoperative neurological function improvement (walking function, motor weakness, and paresthesia), tumor progression/ recurrence (P/R), and overall survival as a function of time postoperatively were analyzed with Kaplan–Meier method.14) A p value < 0.05 was considered significant.
Results
Preoperative findings
The average duration of a symptom was 20.1 ± 32.3 months (range: 1–144 months). On admission, all patients presented with neurological deficits of variable severity. Pain was nonspecific in 22 (57.9%) patients and was primarily localized in the neck or head (90.9%) and shoulder (9.1%). Paresthesia, motor weakness, and walking deficits occurred in 21 (55.3%), 12 (31.6%), and 12 (31.6%) patients, respectively. Other symptoms included bladder dysfunction (n = 11, 28.9%), headache (n = 10, 26.3%), dizziness (n = 6, 15.8%), vomiting (n = 4, 10.5%), ataxia (n = 3, 7.9%), nausea (n = 3), involuntary neck movement (n = 2, 5.3%), and cranial nerve (CN) deficits (CN XII, n = 4; CNs IX–XI, n = 10; CN VIII, n = 3; CN VII, n = 2; and CN VI, n = 2). One patient (2.6%) was diagnosed as neurofibromatosis Type 2. The means of the tumor length (range: 1.5–20 cm) and tumor volume (range: 1.5–62.5 cm3) were 5.9 ± 3.6 cm and 19.4 ± 15.0 cm3, respectively. The tumor was involved in a mean of 3.5 ± 1.8 levels (range: 2–8 levels). A well-defined tumor boundary was observed in 24 (63.2%) patients and the remaining patients (14, 36.8%) had ill-defined tumor boundaries.
Four patients (10.5%) rejected operation after considering the surgical risks at the initial diagnosis when the mMG was recorded as an initial mMG (mMG I, n = 2; mMG II, n = 2). All four patients were followed up closely, and they unfortunately experienced neurological function deterioration; as a consequence, the mMG declined from I to II in two patients and from II to III in the other two patients during a mean observation period of 25 ± 7.4 months (range: 18–34 months) preoperatively. After their neurological statuses were sufficiently aggravated, the four patients were retransferred to our hospital and decided to accept surgical treatment. The remaining 34 (89.5%) patients accepted the operations that we recommended to them at the first clinical consultation in our hospital, as their initial mMGs were similar to their preoperative mMGs (Table 1). Two patients (5.3%) had a previous operation before being admitted to our hospital. The preoperative mMGs were as follows: Grade I, n = 18; Grade II, n = 11; and Grade III, n = 9; the mean and median of the grade were 1.8 ± 0.8 and 2, respectively.
Table 1.
Clinical data of the 38 cases of intramedullary medullocervical ependymoma
| No | Age (years) | Sex | Tumor location | Chief complaints at admission | Tumor border | DS (months) | mMG | Surgical removal | Main surgical complication | FUP (months) | P/R | Recent status | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Pre- | Post- | 1 year | Recent | ||||||||||||
| 1 | 24 | F | Medulla-C1 | Motor weakness, leg | WD | 3 | II | III | II | II | TR | 141 | No | AWD | ||
| 2 | 40 | F | Medulla-C1 | Paraparesis | WD | 6 | III | III | II | I | TR | Dysphagia | 139 | No | NED | |
| 3 | 36 | M | Medulla-C1 | Pain, neck | WD | 2 | I | I | I | I | TR | 138 | No | AWD | ||
| 4 | 36 | M | Medulla-C4 | Paresthesia, hands and legs | WD | 144 | II | III | IV | IV | IV | TR | Respiratory deficit | 133 | No | AWD |
| 5 | 40 | M | Medulla-C2 | Paresthesia, hands and legs | WD | 24 | I | I | I | I | TR | 131 | No | NED | ||
| 6 | 33 | F | Medulla-C4 | Paresthesia, hand | WD | 12 | II | III | III | II | TR | 130 | No | AWD | ||
| 7 | 32 | F | Medulla-C2 | Pain, neck | WD | 18 | I | I | I | I | TR | 122 | No | AWD | ||
| 8 | 36 | F | Medulla-C1 | Pain, neck | WD | 3 | I | I | I | I | TR | Intracranial infection | 116 | No | NED | |
| 9 | 24 | F | Medulla-C4 | Paresthesia, hand | WD | 18 | I | I | I | I | TR | 115 | No | AWD | ||
| 10 | 35 | M | Medulla-C6 | Paresthesia, hand and leg | ID | 36 | II | III | IV | III | III | TR | Respiratory deficit | 114 | No | AWD |
| 11 | 33 | M | Medulla-C3 | Paraparesis | ID | 1 | III | IV | III | III | STR | Intracranial infection | 114 | No | AWD | |
| 12 | 32 | M | Medulla-C3 | Paresthesia, hands and legs | WD | 2 | I | I | I | I | TR | 105 | No | AWD | ||
| 13 | 11 | M | Medulla-C5 | Motor weakness, hand and leg | WD | 18 | III | III | III | II | TR | Respiratory deficit | 97 | No | AWD | |
| 14 | 60 | M | Medulla-C1 | Pain, neck | ID | 6 | I | II | II | II | TR | CSF leak | 95 | No | AWD | |
| 15 | 20 | M | Medulla-C1 | Motor weakness, hand and leg | ID | 3 | II | I | I | I | TR | 95 | No | NED | ||
| 16 | 35 | F | Medulla-C2 | Pain, neck | WD | 1 | I | I | I | I | TR | 82 | No | AWD | ||
| 17 | 18 | F | Medulla-C1 | Pain, hand | WD | 132 | I | II | III | II | V | TR | Dysphagia | 51 | Yes | DOD |
| 18 | 36 | F | Medulla-C7 | Paresthesia, hand and leg | WD | 24 | III | IV | III | II | TR | Dysphagia | 80 | No | AWD | |
| 19 | 26 | M | Medulla-C1 | Paraparesis | WD | 12 | I | III | II | V | TR | Respiratory deficit | 45 | Yes | DOD | |
| 20 | 42 | M | Medulla-C1 | Pain, neck | ID | 6 | II | III | III | III | TR | Dysphagia | 78 | No | AWD | |
| 21 | 14 | M | Medulla-C1 | Motor weakness, leg | ID | 12 | II | I | I | I | TR | 75 | No | NED | ||
| 22 | 29 | F | Medulla-C2 | Paresthesia, hands | ID | 48 | III | III | II | II | STR | Respiratory deficit | 75 | No | AWD | |
| 23 | 39 | F | Medulla-C2 | Pain, neck | WD | 12 | I | I | I | I | TR | 71 | No | AWD | ||
| 24 | 38 | M | Medulla-C3 | Tetraparesis | ID | 7 | III | IV | IV | III | TR | CSF leak | 61 | No | AWD | |
| 25 | 25 | F | Medulla-C2 | Paresthesia, hand | WD | 1 | I | II | I | I | TR | 60 | No | AWD | ||
| 26 | 28 | F | Medulla-C4 | Paresthesia, hands and legs | ID | 3 | II | II | II | II | STR | 59 | No | AWD | ||
| 27 | 46 | F | Medulla-C2 | Tetraparesis | WD | 14 | I | III | II | I | TR | 56 | No | NED | ||
| 28 | 46 | M | Medulla-C4 | Motor weakness, hand and leg | WD | 84 | I | II | III | II | II | STR | 53 | No | AWD | |
| 29 | 58 | F | Medulla-C1 | Paraparesis | WD | 18 | I | II | II | I | TR | 53 | No | NED | ||
| 30 | 41 | F | Medulla-C6 | Tetraparesis | WD | 12 | II | III | I | I | TR | 50 | No | AWD | ||
| 31 | 29 | M | Medulla-C1 | Pain, neck | WD | 2 | I | I | I | I | TR | Respiratory deficit | 48 | No | NED | |
| 32 | 57 | M | Medulla-C1 | Paraparesis | ID | 3 | I | III | II | II | TR | Respiratory deficit | 47 | No | AWD | |
| 33 | 30 | F | Medulla-C1 | Pain, neck | WD | 6 | I | I | I | I | TR | 48 | No | NED | ||
| 34 | 43 | M | Medulla-C3 | Paresthesia, hands | ID | 9 | I | III | II | II | TR | 48 | Yes | AWD | ||
| 35 | 24 | M | Medulla-C6 | Tetraparesis | ID | 12 | III | IV | III | II | TR | Respiratory deficit | 46 | No | AWD | |
| 36 | 45 | F | Medulla-C1 | Paresthesia, legs | WD | 7 | I | II | I | I | TR | 45 | No | AWD | ||
| 37 | 44 | F | Medulla-C3 | Paresthesia, hands and legs | ID | 36 | II | II | II | II | TR | 42 | No | AWD | ||
| 38 | 55 | M | Medulla-C1 | Paresthesia, hand and leg | ID | 6 | II | II | II | II | STR | 42 | No | AWD | ||
AWD: alive with deficit, DOD: die of disease, DS: duration of symptoms, FUP: follow-up period, ID: ill defined, mMG: modified McCormick grade, NED: no evidence of deficit, P/R: progression/recurrence, Pre-: preoperative, Post-: postoperative at discharge, STR: subtotal resection, TR: total resection, WD: well defined.
Surgery and morbidity
Overall, gross total resection (GTR) was achieved in 33 (86.8%) patients, and there was subtotal resection (STR) in 5 (13.2%) patients without surgical mortality. The reasons for GTR failure were an ill-defined tumor boundary (n = 4) and the loss of SEPs in the right upper extremity (n = 1). Overall, histological examination revealed a benign ependymoma (WHO Grade II) in 35 (92.1%) patients and an anaplastic ependymoma (WHO Grade III) in 3 (7.9%) patients (Cases 7, 29, and 33). Surgical morbidity occurred in 16 patients (42.1%), including respiratory difficulty (n = 9, 23.7%), lower CN function impairment (new onset [n = 2, 5.3%]; worsening of preexisting impairment [n = 3, 7.9%]), cerebrospinal fluid leakage (n = 2, 5.3%), intracranial infection (n = 2, 5.3%), pneumonia (n = 1, 2.6%), development of a stress ulcer (n = 1, 2.6%), and hydrocephalus (n = 1, 2.6%). Tracheostomy was performed to prevent suffocation or respiratory failure in 11 (28.9%) patients due to respiratory difficulty (n = 8) and severe lower CN deficiencies (n = 3). Five of the eight patients required ventilator assistance for 16.4 ± 14.8 days (range: 5–41 days) due to severe respiratory dysfunction, and of all the patients improved and could breathe independently before discharge. The tracheostomies were closed at 42.4 ± 34.1 days (range: 18–138 days) after surgery. We recommended radiotherapy to patients with STR. Three of the five STR cases and one patient with anaplastic ependymoma underwent postoperative adjuvant radiotherapy (gamma knife, n = 1; linear accelerator, n = 3) immediately after discharge from the hospital.
Short-term outcomes
Compared to the preoperative mMG, the mMG at discharge improved in t patients (5.3%), stabilized in 22 (57.9%), and worsened in 14 (36.8%), including 6 of the 9 patients for whom the preoperative mMG was III and 8 of the 29 preoperative mMG I and II cases (Table 2). The worsening of the mMG at discharge was related to a poor preoperative mMG (odds ratio [OR], 5.250; 95% confidence interval [CI], 1.052–26.197; p = 0.044). The mean of the mMG was 2.1 ± 1.1, which was significantly worse than the preoperative mMG (t = −3.366, p = 0.002) (Fig. 6). Changes in paresthesia, motor weakness, and walking deficits are described in Table 3. New onset of parethesia, motor weakness, and walking deficits occurred in 11 (28.9%), 13 (34.2%), and 5 (13.2%) patients, respectively. In 14 patients with perioperative neurological decline, 5 (35.7%) and 7 (50%) patients improved to their baseline within 1 and 3 months, respectively.
Table 2.
Transition of the postoperative mMG categorized based on the preoperative mMG
| Preoperative mMG | At discharge | 1 year | Recent | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | I | II | III | IV | V | ||
| I | 13 | 3 | 2 | 14 | 3 | 1 | 14 | 3 | 1 | 18 | ||||
| II | 2 | 6 | 3 | 3 | 7 | 1 | 3 | 6 | 1 | 1 | 11 | |||
| III | 3 | 6 | 2 | 5 | 2 | 1 | 4 | 3 | 1 | 9 | ||||
| Total | 15 | 9 | 8 | 6 | 17 | 12 | 7 | 2 | 18 | 13 | 4 | 1 | 2 | 38 |
mMG: modified McCormick grade.
Fig. 6.
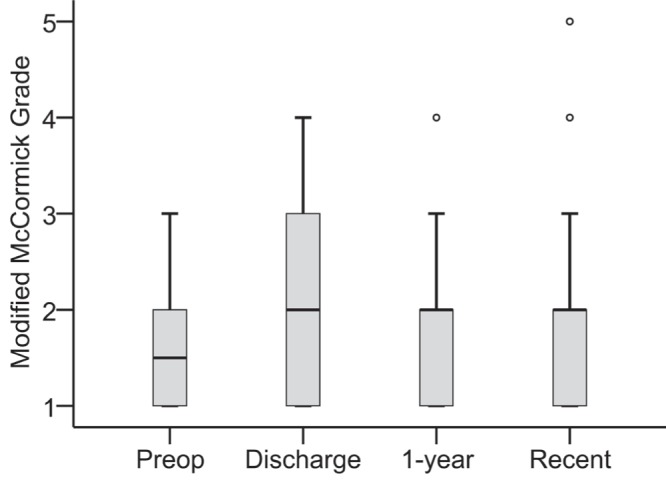
Average preoperative, postoperative, and follow-up mMGs showing an increase in the score immediately after surgery, a return to the preoperative levels by 1 year after surgery, and a stabilized score at the most recent follow-up evaluation compared to that of 1 year after surgery. Circles indicate outliers. mMG: modified McCormick grade.
Table 3.
Transition of the postoperative neurological symptoms categorized using the preoperative neurological symptoms
| Preoperative | At discharge | 1 year | Recent | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wor | Un | Im | Wor | Un | Im | Wor | Un | Im | ||
| Paresthesia | 11 | 8 | 2 | 5 | 4 | 12 | 2 | 5 | 14 | 21 |
| No paresthesia | 11 | 6 | 4 | 13 | 3 | 14 | 17 | |||
| Motor weakness | 2 | 7 | 3 | 2 | 2 | 8 | 2 | 10 | 12 | |
| Full strength | 13 | 13 | 8 | 18 | 6 | 20 | 26 | |||
| Walking deficit | 4 | 5 | 3 | 2 | 4 | 6 | 2 | 2 | 8 | 12 |
| Normal gait | 5 | 21 | 3 | 23 | 1 | 25 | 26 | |||
Im: improve, Un: unchange, Wor: worsen.
At 1 year postoperatively, the mMG scores (mean, 1.8 ± 0.9), which improved in 5 (13.2%) patients, stabilized in 26 (68.4%), and worsened in 7 (18.4%), were similar to the preoperative mMGs (t = −0.572, p = 0.571). Seven (53.1%) of the 32 patients with postoperative paresthesia, 10 (40%) of 25 with postoperative motor weakness, and 5 (35.7%) of 14 with postoperative walking deficits improved by various degrees (Table 3). By 1 year postoperatively, 17 (44.7%) patients had a full- or part-time job.
Long-term outcomes
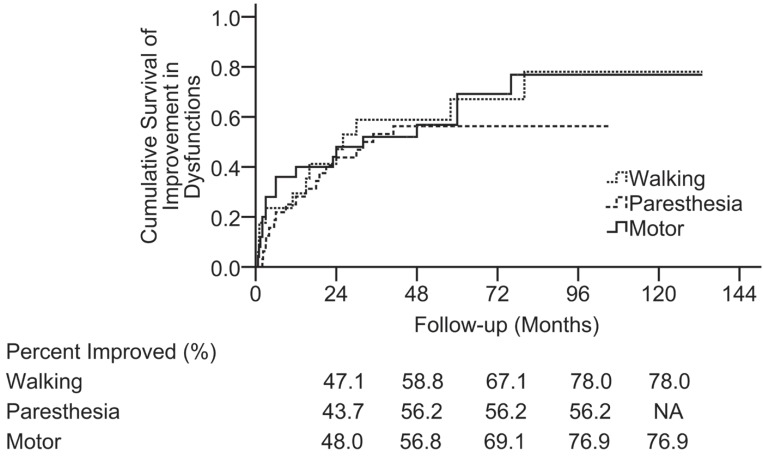
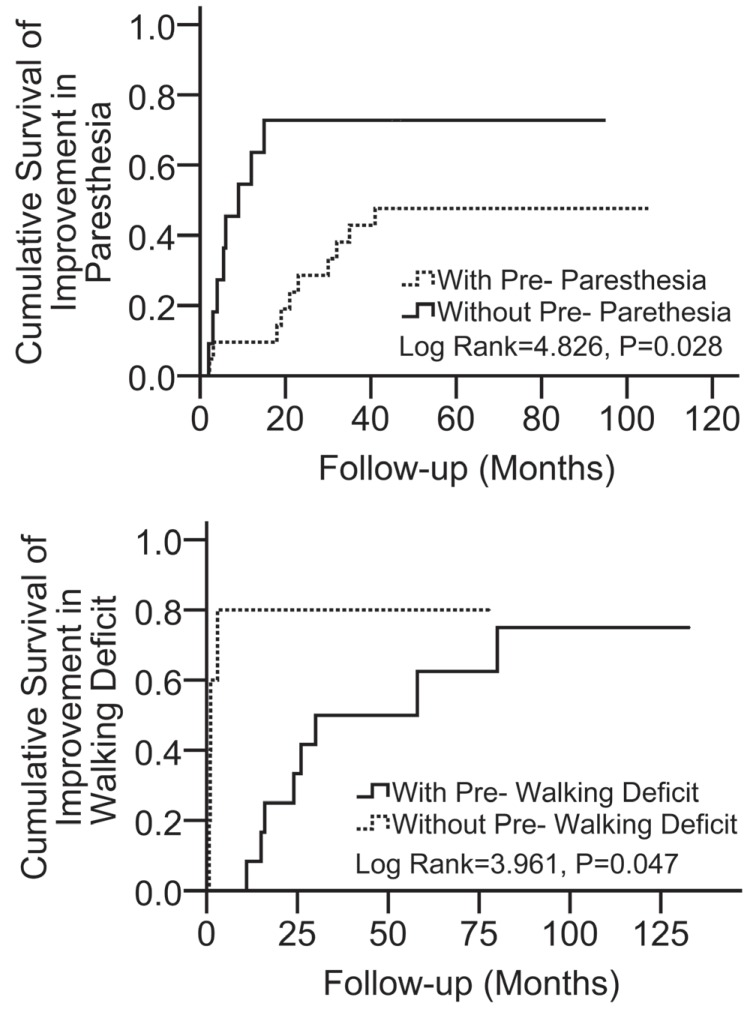
At the final assessment, 3 (7.9%) patients relapsed according to MRI scans after a mean recurrence-free period of 32.7 months, among whom 2 (5.3%) died of recurrence without treatment and 1 patient underwent total resection and obtained a good outcome. Thirty-six patients were still alive after a mean follow-up duration of 81.5 ± 33.7 months (range: 41.6–140.8 months). Compression of the spinal cord by misplaced or mobile lamina of the vertebral arch did not occur after laminoplasty in any of the patients. The improvements in the symptoms observed at the end of the follow-up are detailed in Table 3. By 96 months postoperatively, in patients with postoperative paresthesia, motor, or walking dysfunction, paresthesia symptoms improved to minimal or none in 56.2%, motor weakness improved to full or near-full strength in 76.9%, and walking deficits improved to normal gait in 78.0% (Fig. 7). Improvement in postoperative paresthesia (Fig. 8A) and walking dysfunction (Fig. 8B) over time were associated with the absence of preoperative paresthesia (log rank = 4.826, p = 0.028) and walking deficits (log rank = 3.961, p = 0.047), respectively.
Fig. 7.
Kaplan–Meier plots illustrating the incidence of the postoperative walking, sensory, and motor function improvement as a function of time. By 96 months postoperatively, 78.0% of patients with postoperative walking deficit symptoms had improvement in these symptoms (to normal gait), 56.2% with postoperative paresthesia had improved (to minimal or none), and 76.9% with postoperative motor weakness had improved to full or near-full strength. These dysfunctions improved at similar rates. The vertical axis is indicated as percentage.
Fig. 8.
Kaplan–Meier plots demonstrating the incidence of postoperative paresthesia (A) and walking deficit (B) improvement over time, based on the presence or absence of preoperative paresthesia and walking dysfunction, respectively. The absence of preoperative paresthesia and walking dysfunction was associated with favorable long-term improvement in paresthesia (to minimal or none) and walking dysfunction (to normal gait), respectively. The vertical axis is indicated as percentage.
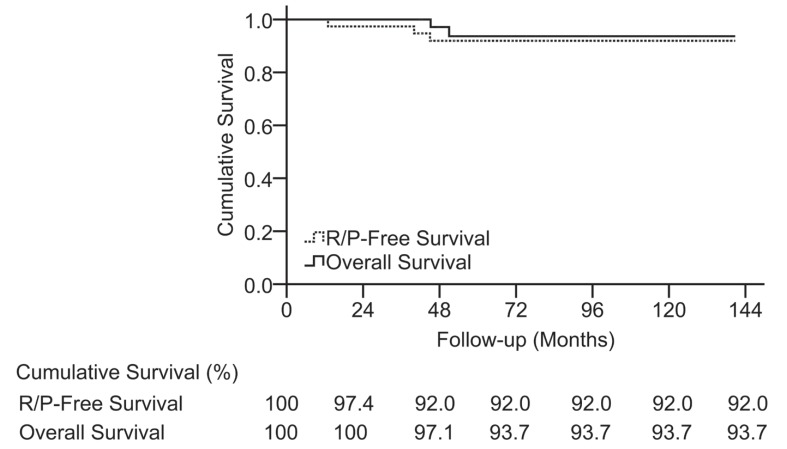
The most recent mMG scores (mean, 1.8 ± 1.1) were as follows: Grade I, n = 18; Grade II, n = 13; Grade III, n = 4; Grade IV, n = 1; and Grade V, n = 2, which were similar to the preoperative mMGs (t = −0.464, p = 0.646), indicating that the main effect of operation was to stabilize the preoperative neurological function. In a comparison between the preoperative and most recent mMGs, 8 (21.1%) patients improved, 23 (60.5%) were stable, and 7 (18.4%) worsened, including 2 (5.3%) deaths. On the whole, 32 (84.2%) patients lived independently, and among them, 14 (36.8%) patients were able to carry out normal activity with or without minor signs of disease, 12 (31.6%) patients could carry out normal activity with effort and some signs or symptoms of disease, and 6 (15.8%) patients could care for themselves without assistance (Table 2). A favorable long-term neurological function outcome was associated with the preoperative status (mMG I) (OR, 9.956; 95% CI, 1.846–53.690; p = 0.008) and a well-defined tumor boundary (OR, 7.829; 95% CI, 1.155–53.088; p = 0.035) (Table 4). The P/R-free survival rate was 97.4% at 3 years and 92.0% at 5, 10, and 12 years. The overall survival rate was 100% at 3 years and 93.7% at 5, 10, and 12 years (Fig. 9).
Table 4.
Favorable prognostic factors for intramedullary medullocervical ependymoma
| Variables | Recent mMG | Multivariate logistic regression | ||||
|---|---|---|---|---|---|---|
| I | II–V | OR | 95% CI | p | ||
| Gender | Male | 6 | 13 | 0.269 | ||
| Female | 12 | 7 | ||||
| Age, years | ≥ 35 | 10 | 11 | 0.858 | ||
| < 35 | 8 | 9 | ||||
| Duration of symptoms, months | ≥ 18 | 4 | 8 | 0.164 | ||
| < 18 | 14 | 12 | ||||
| Preop mMG | II–IV | 4 | 16 | Ref | ||
| I | 14 | 4 | 9.956 | 1.846–53.690 | 0.008 | |
| Tumor boundary | Ill defined | 2 | 12 | Ref | ||
| Well defined | 16 | 8 | 7.829 | 1.155–53.088 | 0.035 | |
| Tumour localization, levels | 4–8 | 3 | 12 | 0.097 | ||
| 2–3 | 15 | 8 | ||||
CI: confidence interval, mMG: modified McCormick grade, OR: odds ratio, Ref: reference.
Fig. 9.
Kaplan–Meier survival analysis illustrating the long-term recurrence-free survival rate and overall survival rate in the series. The vertical axis units are indicated as percentages.
Additional statistical analysis
Due to the small number of patients with STR, lesions of WHO grade III, and postoperative radio-therapy, analyses of the effect of histopathology, surgical resection, or radiotherapy on the long-term outcome were not performed. The association between the long-term neurological outcomes and improvement of the postoperative symptoms (paresthesia, motor weakness, and walking deficits) with the following variables was not independently significant (p > 0.05): age, gender, duration of symptoms, and tumor location (number of involved spinal levels).
Discussion
The main focus of the present study was the neurological status outcome and incidence of functional improvement over time in 38 consecutive cases of IME resection performed over a 10-year period. The mMG27) includes four different levels to describe a patient's independence status. Although detailed neurological evaluation shows a slight improvement or deterioration in status during follow-up, the mMG on postoperative examination does not change accordingly,12) especially in distinguishing “slight deficit” from “no evidence of disease.” Thus, it is evident that the mMG does not sufficiently characterize the patient status. Therefore, in many studies, the mMG was modified according to the research design, and the grades were redefined12,23,26) or a new grade was added to the evaluation system (e.g., mMG I was redefined as “no deficit”12) or “neurologically normal”1)). In our series, the final status was additionally evaluated as “alive with deficit” or “no evidence of deficit” to identify the status of patients with or without deficit who had a recent follow-up status of mMG I; the status of patients who died from the disease was defined as “Grade V, die from the disease” (Table 1).
Surgery
Most reports acknowledge that the extent of tumor removal was the most significant prognostic factor influencing the postoperative outcome, with minimal postoperative neurological deficits.2,5,8,13,14,18,22–27,29,30,33,35,37) GTR was found to be superior to partial resection with radiation.14,30) GTR at the time of initial surgery should be the primary goal in the removal of these lesions.23)
One study found that the primary goal of the operation was to stabilize the state of illness,1) as reported by previous authors,12,14,26,32) which did not significantly differ from our goal. Many surgeons suggest that surgery must be performed as soon as possible in patients with evolving neurological deficits or before the onset of deterioration.1,4,9–12,26,29,31) The indications for surgery should be carefully discussed with patients who did not have any preoperative neurological deficits but who instead only have pain (mMG score I).1) In the earlier stages of disease, the tumor is deep within the spinal cord and cannot be identified from the appearance of the spinal cord. For this reason, early operation cannot avoid the risk of cord traction and manipulation, leading to the inevitable impairment of the normal spinal cord. When the tumor has reached a medium volume and is closer to the posterior surface of spinal cord, the contour of the spinal cord may still be recognized; therefore, the risk of surgical damage to the surrounding functional cord (dorsal column function) is reduced.35) Further study of the natural history of intramedullary ependymoma is required to demonstrate the relationship between the tumor growth curve and the increasing severity of symptoms and to identify a critical point of accelerated deterioration of neurological function, which will provide valuable information for the surgical decision-making process.
In our series, four patients rejected operation primarily due to the surgical risk; unfortunately, all four patients showed neurological function deterioration, and after finally undergoing surgery, their neurological statuses were unable to return to their initial status. Postponing surgery may play a role in transforming asymptomatic patients into symptomatic ones due to the continuous tumor growth, which results in the worsening of symptoms. We suggest that surgery be performed as early as possible after diagnosis to help the patient before symptoms are aggravated, but careful consideration of surgery should be taken for patients who lack neurological deficits.12,34)
Clinical outcomes
In the early postoperative course, 9–67% of patients experienced neurological status aggravation.12,14,16,26) Evaluating the mMG at discharge, the improvement, stabilization, and deterioration rates were 5.3%, 57.9%, and 36.8% (14 patients), respectively. Five (35.7%) of the 14 patients improved to their baseline within 1 month, which was comparable to another series, and the recovery rate might benefit from aggressive postoperative rehabilitation and steroid treatment for postoperative edema.7,14,35) Postoperative neurological decline was associated with a poor preoperative status, which indicates that reduced preoperative neurological function does not respond well to the operation perioperatively.
Postoperative symptoms (e.g., paresthesia, motor, or walking dysfunction) began to improve within 1 month with improvement continuing during the postoperative follow-up, which in part parallels the experience of other research groups.14,17,23) At the most recent evaluation, the majority of the postoperative symptoms improved to a normal or near-normal level. The improvements in the postoperative paresthesia and walking deficits over time were associated with their absence as preoperative symptoms.26,34) To minimize these dysfunctions, it is imperative to perform the myelotomy along the midline, which we would identify from anatomic landmarks, ultrasonography, or dorsal cord mapping.
The clinical improvement was significant within the first 1 year and maintained during follow-up and at the final evaluation; compared to the preoperative status, 60.5%, 21.1%, and 18.4% of patients presented with stable, improved, and worsened mMGs, respectively, which was in accordance with previously published studies.12,14,23) Karikari et al.22) reported that 20% of patients improved, 69% remained the same, and 10.9% worsened. During the observation period, 7.9% of the tumors recurred and the P/R-free survival rate at 10 years was 92.0%. Others reported 48–75% P/R-free survival rate at 10 years, which was quite different from our results; this might be due to small number (three cases) of patients with WHO Grade III tumors in our series and the regrowth inhibition after devascularization of the residual tumors.3,16,28) Two of the three patients rejected surgery after P/R and died within 1 year, which decreased the overall survival rate from 100% at 3 years to 93.7% at 10 years.
Prognostic variables
Poor preoperative status is a significant predictor of poor outcomes.6,8,12,15,16,21,22,29) Patients with worse preoperative functional statuses had fewer opportunities for neurological improvement.8) Our study also indicated that a good preoperative status (mMG I) is a favorable predictor, as has been previously reported in other studies.16,26) Bansal et al.4) concluded that the preoperative neurological condition was the strongest predicting factor of the functional outcome beyond the histological differentiation of the intramedullary tumors. Furthermore, early diagnosis and operation have been suggested to be vital for positive outcomes.4,8) It has been postulated that once the normal plasticity of the spinal cord is impaired, surgery offers few advantages with regard to functional improvement. Additionally, operations and radiotherapy can increase impairment to the medulla and spine.
Tumor boundary has been considered a prognostic factor in previously published studies. The existence of a subarachnoid space guarantees safety in aggressive resection, en bloc resection, and the avoidance of a residual tumor.5,14) A multivariate regression analysis supported the significant effect of a well-defined boundary on favorable postoperative neurological function, and we found that an ill-defined boundary with tight adhesion between the lesion and normal tissue increases the difficulty of surgery, whereas a distinct boundary contributes to complete resection. An attempt at complete removal without a well-defined plane would inevitably lead to relatively poor prognosis.
Limitations of the present study
The limitations of the present study are as follows: (1) this was a single-center study with a relatively small number of patients; (2) this was a nonrandomized trial lacking a control group and was unable to compare the outcomes between patients who did or did not undergo operation; and (3) the study included 38 cases that were limited to the histology of ependymoma and with the lesion location in the medullocervical regions alone. Because of the small number of patients, we did not analyze the effect of complete resection, histology, and postoperative radiotherapy on the outcomes.
Conclusions
The primary predictors of a good outcome after surgery are a good preoperative status and a well-defined tumor boundary. Patients lacking preoperative paresthesia or walking deficits have favorable functional improvement in these two symptoms postoperatively. Half of the patients with perioperative neurological decline improve to baseline within 3 months, and nearly half of all patients have a full- or part-time job within 1 year. Surgical risks depend on the tumor size and disease stage, and patients should be informed of these risks. The goal of surgery is to stabilize the preoperative neurological function. Operation is recommended for all patients with IME, including patients with minimal neurological signs, and should be performed as soon as possible once the diagnosis has been made and before neurological function further deteriorates.
Fig. 2.
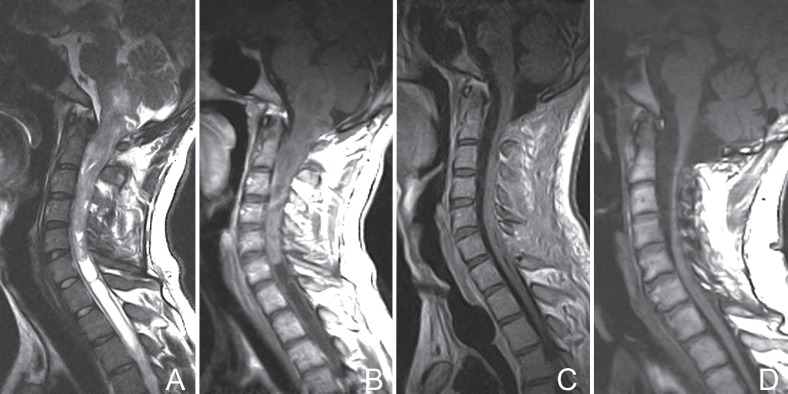
Patient No. 35. Preoperative T2-weighted MRI (A) and T1-weighted imaging with contrast enhancement (B), showing significant deformation of the medulla oblongata and fourth ventricle, and an ill-defined tumor boundary. Postoperative MRI scans with contrast enhancement at 1 week (C) and 2 years (D), demonstrating complete resection of the lesion (C) and mild myelatrophy without recurrence (D). At 46 months postoperatively, the patient improved in neurological function with an mMG of II. mMG: modified McCormick grade, MRI: magnetic resonance imaging.
Fig. 3.
Patient No. 9. Preoperative T2-weighted MRI (A) with a well-defined tumor boundary and T1-weighted imaging with contrast enhancement (B). Postoperative MRI scans with contrast enhancement at 1 week (C) showing total removal of the tumor. MRI: magnetic resonance imaging.
Fig. 4.
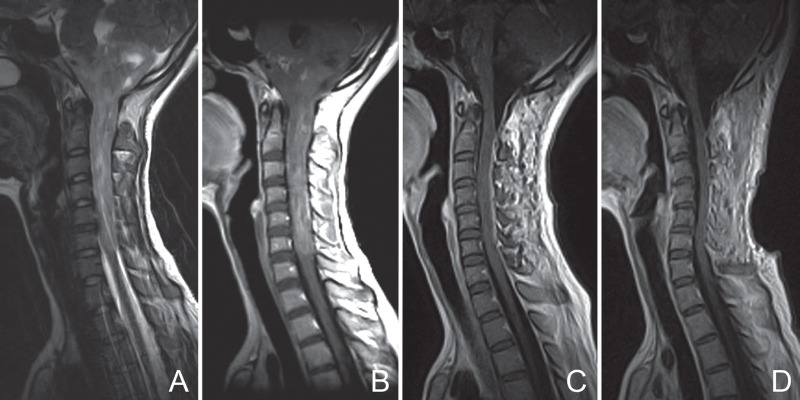
Patient No. 10. Preoperative T2-weighted MRI (A) with an ill-defined tumor boundary and T1-weighted imaging with contrast enhancement (B) illustrating mild to moderate inhomogeneous enhancement. Postoperative MRI scans with contrast enhancement at 1 week (C) and 6 months (D) showing total resection. MRI: magnetic resonance imaging.
Acknowledgments
We would like to acknowledge the contributions of Drs. Liang Wang, Xin-Ru Xiao, and Jie Tang from the Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, who were responsible for data collection and technical help, as well as that of Dr. Ming-Ran Wang from the Department of Electrophysiology, Beijing Neurosurgical Institute, who provided technical help for the intraoperative monitoring of the evoked potentials.
References
- 1). Aghakhani N, David P, Parker F, Lacroix C, Benoudiba F, Tadie M: Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery 62: 1279– 1285; discussion 1285–1286, 2008. [DOI] [PubMed] [Google Scholar]
- 2). Ahyai A, Woerner U, Markakis E: Surgical treatment of intramedullary tumors (spinal cord and medulla oblongata). Analysis of 16 cases. Neurosurg Rev 13: 45– 52, 1990. [DOI] [PubMed] [Google Scholar]
- 3). Akyurek S, Chang EL, Yu TK, Little D, Allen PK, McCutcheon I, Mahajan A, Maor MH, Woo SY: Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol 80: 177– 183, 2006. [DOI] [PubMed] [Google Scholar]
- 4). Bansal S, Suri A, Borkar SA, Kale SS, Singh M, Mahapatra AK: Management of intramedullary tumors in children: analysis of 82 operated cases. Childs Nerv Syst 28: 2063– 2069, 2012. [DOI] [PubMed] [Google Scholar]
- 5). Bostrom A, von Lehe M, Hartmann W, Pietsch T, Feuss M, Bostrom JP, Schramm J, Simon M: Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery 68: 302– 308; discussion 309, 2011. [DOI] [PubMed] [Google Scholar]
- 6). Brotchi J, Bruneau M, Lefranc F, Balériaux D: Surgery of intraspinal cord tumors. Clin Neurosurg 53: 209– 216, 2006. [PubMed] [Google Scholar]
- 7). Brotchi J, Dewitte O, Levivier M, Balériaux D, Vandesteene A, Raftopoulos C, Flament-Durand J, Noterman J: A survey of 65 tumors within the spinal cord: surgical results and the importance of preoperative magnetic resonance imaging. Neurosurgery 29: 651– 656; discussion 656–657, 1991. [PubMed] [Google Scholar]
- 8). Chang UK, Choe WJ, Chung SK, Chung CK, Kim HJ: Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol 57: 133– 139, 2002. [DOI] [PubMed] [Google Scholar]
- 9). Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ: Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg 93: 183– 193, 2000. [DOI] [PubMed] [Google Scholar]
- 10). Cooper PR, Epstein F: Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg 63: 492– 499, 1985. [DOI] [PubMed] [Google Scholar]
- 11). Cristante L, Herrmann HD: Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery 35: 69– 74; discussion 74–76, 1994. [DOI] [PubMed] [Google Scholar]
- 12). Eroes CA, Zausinger S, Kreth FW, Goldbrunner R, Tonn JC: Intramedullary low grade astrocytoma and ependymoma. Surgical results and predicting factors for clinical outcome. Acta Neurochir (Wien) 152: 611– 618, 2010. [DOI] [PubMed] [Google Scholar]
- 13). Ferrante L, Mastronardi L, Celli P, Lunardi P, Acqui M, Fortuna A: Intramedullary spinal cord ependymomas—a study of 45 cases with long-term follow-up. Acta Neurochir (Wien) 119: 74– 79, 1992. [DOI] [PubMed] [Google Scholar]
- 14). Garcés-Ambrossi GL, McGirt MJ, Mehta VA, Sciubba DM, Witham TF, Bydon A, Wolinksy JP, Jallo GI, Gokaslan ZL: Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine 11: 591– 599, 2009. [DOI] [PubMed] [Google Scholar]
- 15). Guyotat J, Metellus P, Giorgi R, Barrie M, Jouvet A, Fevre-Montange M, Chinot O, Durand A, Figarella-Branger D: Infratentorial ependymomas: prognostic factors and outcome analysis in a multi-center retrospective series of 106 adult patients. Acta Neurochir (Wien) 151: 947– 960, 2009. [DOI] [PubMed] [Google Scholar]
- 16). Halvorsen CM, Kolstad F, Hald J, Johannesen TB, Krossnes BK, Langmoen IA, Lied B, Rønning P, Skaar S, Spetalen S, Helseth E: Long-term outcome after resection of intraspinal ependymomas: report of 86 consecutive cases. Neurosurgery 67: 1622– 1631; discussion 1631, 2010. [DOI] [PubMed] [Google Scholar]
- 17). Hanbali F, Fourney DR, Marmor E, Suki D, Rhines LD, Weinberg JS, McCutcheon IE, Suk I, Gokaslan ZL: Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery 51: 1162– 1172; discussion 1172–1174, 2002. [DOI] [PubMed] [Google Scholar]
- 18). Hejazi N, Hassler W: Microsurgical treatment of intramedullary spinal cord tumors. Neurol Med Chir (Tokyo) 38: 266– 271; discussion 271–273, 1998. [DOI] [PubMed] [Google Scholar]
- 19). Hoshimaru M, Koyama T, Hashimoto N: [Micro-surgery of cervical intramedullary ependymomas extending into the medulla oblongata]. No Shinkei Geka 28: 517– 522, 2000. (Japanese) [PubMed] [Google Scholar]
- 20). Hsu W, Pradilla G, Constantini S, Jallo GI: Surgical considerations of spinal ependymomas in the pediatric population. Childs Nerv Syst 25: 1253– 1259, 2009. [DOI] [PubMed] [Google Scholar]
- 21). Jenkinson MD, Simpson C, Nicholas RS, Miles J, Findlay GF, Pigott TJ: Outcome predictors and complications in the management of intradural spinal tumours. Eur Spine J 15: 203– 210, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Karikari IO, Nimjee SM, Hodges TR, Cutrell E, Hughes BD, Powers CJ, Mehta AI, Hardin C, Bagley CA, Isaacs RE, Haglund MM, Friedman AH: Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery 68: 188– 197; discussion 197, 2011. [DOI] [PubMed] [Google Scholar]
- 23). Kucia EJ, Bambakidis NC, Chang SW, Spetzler RF: Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Neurosurgery 68: 57– 63; discussion 63, 2011. [DOI] [PubMed] [Google Scholar]
- 24). Kyoshima K, Akaishi K, Tokushige K, Muraoka H, Oikawa S, Watanabe A, Koyama J, Kobayashi S, Unoki T, Goto T, Wada N, Uehara T: Surgical experience with resection en bloc of intramedullary astrocytomas and ependymomas in the cervical and cervicothoracic region. J Clin Neurosci 11: 623– 628, 2004. [DOI] [PubMed] [Google Scholar]
- 25). Little AS, Sheean T, Manoharan R, Darbar A, Teo C: The management of completely resected childhood intracranial ependymoma: the argument for observation only. Childs Nerv Syst 25: 281– 284, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Matsuyama Y, Sakai Y, Katayama Y, Imagama S, Ito Z, Wakao N, Sato K, Kamiya M, Yukawa Y, Kanemura T, Yanase M, Ishiguro N: Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine 10: 404– 413, 2009. [DOI] [PubMed] [Google Scholar]
- 27). McCormick PC, Torres R, Post KD, Stein BM: Intramedullary ependymoma of the spinal cord. J Neurosurg 72: 523– 532, 1990. [DOI] [PubMed] [Google Scholar]
- 28). Pica A, Miller R, Villà S, Kadish SP, Anacak Y, Abusaris H, Ozyigit G, Baumert BG, Zaucha R, Haller G, Weber DC: The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: a retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys 74: 1114– 1120, 2009. [DOI] [PubMed] [Google Scholar]
- 29). Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G: Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 56: 972– 981; discussion 972–981, 2005. [PubMed] [Google Scholar]
- 30). Rodríguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG: Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005). J Surg Res 156: 340– 351, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, Stolke D, Wiedemayer H: Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord 43: 34– 41, 2005. [DOI] [PubMed] [Google Scholar]
- 32). Schwartz TH, McCormick PC: Intramedullary ependymomas: clinical presentation, surgical treatment strategies and prognosis. J Neurooncol 47: 211– 218, 2000. [DOI] [PubMed] [Google Scholar]
- 33). Vitanovics D, Bálint K, Hanzély Z, Banczerowski P, Afra D: Ependymoma in adults: surgery, reoperation and radiotherapy for survival. Pathol Oncol Res 16: 93– 99, 2010. [DOI] [PubMed] [Google Scholar]
- 34). Woodworth GF, Chaichana KL, McGirt MJ, Sciubba DM, Jallo GI, Gokaslan Z, Wolinsky JP, Witham TF: Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery 61: 99– 105; discussion 105–106, 2007. [DOI] [PubMed] [Google Scholar]
- 35). Xu QW, Bao WM, Mao RL, Yang GY: Aggressive surgery for intramedullary tumor of cervical spinal cord. Surg Neurol 46: 322– 328, 1996. [DOI] [PubMed] [Google Scholar]
- 36). Yokota H, Yokoyama K, Noguchi H, Uchiyama Y: Two-stage operation for high cervical intramedullary ependymoma in young adult. Br J Neurosurg 26: 540– 541, 2012. [DOI] [PubMed] [Google Scholar]
- 37). Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D: Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst 26: 905– 911, 2010. [DOI] [PubMed] [Google Scholar]