Abstract
High-risk disease accounts for approximately 15% of prostate cancer diagnoses, but the current definitions include a heterogeneous group of patients with a range of prognoses. High-risk prostate cancers have the potential to progress to a lethal phenotype that can be fatal, in marked contrast to low-risk tumours deemed suitable for active surveillance,. The optimal management of this patient sub-group is evolving. A refined classification scheme is needed to enable the early and accurate identification of high-risk disease so that more effective treatment paradigms can be developed. Several principles have been established from clinical trials and are discussed in this review, while other questions remain unanswered. This review critically evaluates the existing literature focused on defining the high-risk population, the management therein, and future directions to optimize care.
Introduction
The diagnosis and treatment of prostate cancer can be placed in the context of a series of clinical states1. These states begin with localized disease, followed by the non-castrate rising prostate-specific antigen (PSA) state and the non-castrate metastatic state. Finally, there are the castration-resistant states which for most men are lethal within a few years (Figure 1). For all states, clinical management decisions and the design of clinical research are often based on a determination of risk. Tumours that are seemingly localized can range from those with a low malignant potential (which left untreated are unlikely to result in morbidity or reduce life expectancy), to those curable with a single modality directed exclusively to the gland itself, to those destined to recur locally or systemically despite optimal local therapy. It is the last category that encompasses tumours that are broadly classified as “high-risk” or alternatively, “locally advanced”.
Figure 1.
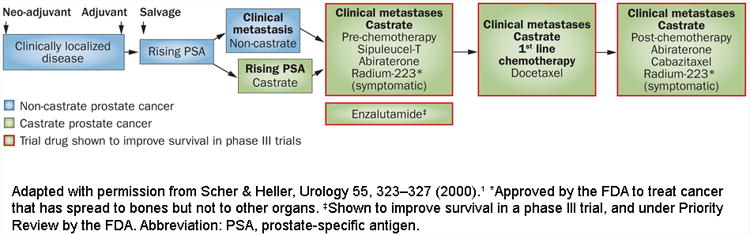
Clinical states of prostate cancer. Modified from Scher and Heller, Urology 55:323-7, 2000.1
Abbreviation: PSA, prostate-specific antigen.
The published literature on “high-risk” prostate cancer is extensive and increasing year by year. A search using “prostate cancer” with each of the three terms “high-risk”, “high-risk diagnosis”, and “high-risk treatment” yielded, respectively, 7189 publications, 4935 publications, and 4921 publications. Despite this, there remains no classification scheme that enables outcomes for high-risk prostate cancer to be determined reliably so that patient management is optimally informed. The situation is clouded further by the wide range of diagnostic methods used in reported series to classify patients, and by variations in the treatment itself even when looking only at studies based primarily on a surgical approach or a radiotherapy approach. The specific outcomes used also vary between studies, and few of these outcomes adequately represent how a patient functions, feels or how long he survives—which is arguably what matters most to patients. The fact that most reports are retrospective is an additional factor that limits the ability to formulate meaningful standards, and thus ultimately compromises counseling. Here we present a critical review of the published literature, and highlight key areas upon which to focus, to enable more-reliable treatment recommendations by physicians and better-informed decisions by patients.
Towards a Meaningful Definition of “High-Risk”
In the United States, approximately 238,590 men were expected to be diagnosed with prostate cancer in 2013, and 29,720 prostate cancer patients were anticipated to die of their disease in 2013.2 Many of the patients who die of prostate cancer present initially with tumours seemingly confined to the gland; this arguably represents true “high-risk” disease and new approaches are needed for these patients. By current estimates, “high-risk” disease accounts for 15% of all prostate cancer diagnoses3. The limitations of determining risk based on the T, N, M classification, which does not include Gleason score or PSA, have long been recognized. An important first step toward a more reliable schema was first proposed by D'Amico et al.,4 using an endpoint of PSA failure and defining “high-risk” as a clinical T stage ≥cT2c, a Gleason score ≥8, or a PSA >20 ng/mL; this definition has been adopted by the American Urological Association (AUA).5 The Radiation Therapy Oncology Group (RTOG) developed the first classification which associated specific baseline factors with overall survival and cause-specific survival, arguably more relevant measures. High risk in the RTOG classification includes 1) Gleason ≥8, or 2) Gleason =7 plus either ≥cT3 or node-positive; PSA adds little to this model for the prediction of cause-specific survival or overall survival.6 When combining the RTOG model with the Kattan nomogram, the ability to predict prostate cancer–specific survival is improved.7
More recent risk classifications for localized prostate cancer include a measure of the overall extent of tumour in biopsy specimens: this is based on the demonstration that the percentage of core involvement is associated with prostate cancer–specific mortality.8 One would intuit, for example, that the prognosis of a patient with cT3b, Gleason 9 (4+5) tumour in 16 of 16 pretreatment biopsy cores, and a baseline PSA of 65 ng/mL would have a worse outcome than a patient with cT3a, Gleason 8 (4+4) disease in 1 of 16 biopsy cores and the same PSA. The Cancer of the Prostate Risk Assessment (CAPRA) score was developed to better classify risk by accounting for disease extent within the gland. CAPRA considers the percentage of positive biopsy cores (>33% of positive cores) and age9 in addition to stage, PSA, and Gleason score, and was shown to predict prostate cancer–specific mortality, independent of treatment, in a validation set of 10,627 men.10 The National Comprehensive Cancer Network (NCCN) defines “high-risk” as T3a, Gleason ≥8, or PSA ≥20, and “very high risk” as T3b or T4 disease. Considering and recording the proportion of biopsies with ≤50% vs >50% tumor involvement also improved the NCCN risk stratification.8 Recognizing the difficulty in reliably and reproducibly determining the percentage of cores involved by tumour, many physicians continue to use more traditional nomograms based on T stage, PSA, and Gleason score, as originally developed by Kattan and coworkers to categorize their patients.11
Significant heterogeneity in prognosis has been observed using the available risk stratification schemes mentioned above. The range of outcomes using different schemas was highlighted in an analysis showing 5-year relapse-free survival probabilities ranging from 49–80% for the same patient population using different published schemas.12 Some, such as the Kattan nomograms, consider Gleason score and PSA as continuous as opposed to dichotomous variables and report the probability of a disease-specific outcome in a continuous fashion; consequently, what actually constitutes “high-risk” can indeed vary significantly.
A problem with all of these schemes is the inherent inaccuracy in determining T stage. Assessing disease by digital rectal exam has significant inter-observer variability and while understaging is more common, there is also a problem with overstaging. In one series, 23.5% of clinical T3a tumours defined by digital rectal exam were found to be pathologic T2 disease at radical prostatectomy.13 The American Joint Committee on Cancer staging system allows the use of imaging to refine the determination of T stage.14 Here again, the use of imaging is highly variable. In one report, the addition of multi-parametric magnetic resonance imaging (MRI) was shown to improve staging accuracy and to provide a quantitative volumetric assessment of the extent of tumour in the gland that could be compared to digital rectal exam assessment.15 The authors used diffusion-weighted imaging and dynamic contrast enhancement to perform MRI with an endorectal coil; this detected extracapsular extension with an accuracy of 91% and specificity of 99%, using pathologic staging at prostatectomy as the gold standard. This single-center study did not report clinical stage for comparison.16 Quantitative MRI has also been shown to improve performance in detecting clinically significant prostate cancer.17, 18 In a series of patients who underwent both extended systematic biopsy and MR-targeted biopsy, 12 of 51 patients (24%) had Gleason score upgraded after MR-targeted biopsy.18 MR spectroscopy is another potential biomarker that provides metabolic information and which is being studied to determine whether it can more reliably assess pathologic tumour grade and overall disease aggressiveness.19
To further refine risk, a number of groups are studying specific biologic determinants measured in tumour from the diagnostic biopsy samples, in addition to Gleason grading alone or in combination with the CAPRA score.20 These include markers of tumour proliferation such as Ki-67, alterations in specific pathways such as the PI3K/PTEN signaling axis, and copy number alterations at the DNA level. A four-gene signature assessing PTEN, SMAD4, cyclin D1, and secreted phosphoprotein 1 that was derived from a mouse model was prognostic of biochemical recurrence and metastasis in human samples.21 P16, Ki-67, MDM2, Cox-2, and PKA were identified by RTOG studies to be promising biomarkers that may be used to determine which high-risk patients may benefit from long-term androgen deprivation therapy (ADT).22 PCA3 is a prostate-specific noncoding mRNA that can be measured in the urine and is overexpressed in prostate cancer relative to benign prostatic tissue; it has been largely studied in the active surveillance setting to aid in management. Urinary PCA3 level has been correlated with Gleason score and tumor volume in radical prostatectomy specimens, though its use in identifying high-risk disease that would otherwise be undetected is uncertain.23, 24 Another genetic biomarker, the Prolaris® test, is a 46-gene panel associated with tumour proliferation d25 that is being marketed by Myriad Genetics (Salt Lake City, Utah). This 46-gene panel biomarker is designed to determine which tumors that are categorized as “low-risk” may be more aggressive and thus not appropriate for an active surveillance approach. The role of the test in management of high-risk tumours is unclear. A second test, marketed by Genomic Health (Redwood City, California) as the Oncotype DX® test, reports the expression of 17 genes to determine probability of adverse pathology at surgery and overall disease aggressiveness.26 It too is designed to better inform whether an active surveillance approach is appropriate and is not targeted to tumours determined to be high-risk by NCCN standard clinicopathologic criteria. The principle remains, however, of improving standard biomarkers by accounting for tumour genetics and biology. These tests are among a myriad of biomarkers in various stages of analytic and clinical validation.27
Treatment of “High-Risk” Prostate Cancer
Treatment for high-risk localized prostate cancer has evolved, based on evidence from clinical trials that have established important principles of management. The first principle is that treatment of the primary tumour is paramount: not only for local control but also to prevent further seeding of distant metastatic sites. ADT alone and external beam radiotherapy (EBRT) alone are each inferior to EBRT combined with ADT.28, 29 The optimal duration of long-term ADT is an open question. A number of trials have solidified the importance of the dose of radiation administered in achieving better biochemical control.30-31 Based on these trials, there has been a presumption that as a downstream consequence, trials conducted prior to the establishment of high-dose radiotherapy (75 Gy or greater) should be interpreted cautiously due to the acknowledgement that the doses used were sub-optimal by contemporary standards. However, given the fact that none of these studies to date have demonstrated an improvement in overall survival, one might argue that the evidence for the appropriate use of ADT may be more important than dose escalation; this is most likely related to the effect of ADT on micrometastatic disease.34
Radiation therapy and androgen deprivation therapy
A recent publication demonstrates an increasing trend of under treatment of high-risk prostate cancer, with many high-risk patients receiving ADT alone rather than curative treatment consisting of radiotherapy or radical prostatectomy.3 A Scandinavian randomised phase III trial, SPCG-7/SFUO-3 (ISRCTN01534787), showed that the addition of radiotherapy to total androgen blockade improved rates of survival and disease-free survival for high-risk patients (Table 1).35 The National Cancer Institute of Canada and the United Kingdom Medical Research Council together randomized 1,057 patients with high-risk prostate cancer receiving lifelong ADT to pelvic radiotherapy or no further treatment (ISRCTN24991896; NCT00002633). Similar to the results of the SPCG-7/SFUO-3 study, the addition of radiotherapy improved overall survival (74% vs. 66%, p=0.033) and prostate cancer–specific survival (65% vs. 49%, p=0.0001).36 This corresponds to very favorable number-needed-to-treat to prevent one prostate cancer–specific death (approximately 6.25-8.0).
Table 1. Improved outcomes in SPCG-7/SFUO-3 trial with addition of radiotherapy35.
| Study | Eligibility | Outcomes | ADT alone (n=439) | ADT (same regimen) plus radiotherapy (n=436) | P value |
|---|---|---|---|---|---|
| SPCG-7/SFUO-3 | cT1b-T2 and Gr 2-3, or cT3
|
||||
| 10y BFS | 25.3% | 74.1% | <0.001 | ||
| 10y PCSS | 76.1% | 88.1% | <0.001 | ||
| 10y OS | 60.0% | 70.4% | 0.004 | ||
| NCIC CTG PR.3/MRC UK PR07 | cT3-T4, cT2 with PSA >40, cT2 with PSA >30 and GS ≥8 | ||||
| 7y PCSS | 79% | 90% | 0.0001 | ||
| 7y OS | 66% | 74% | 0.03 |
Abbreviations: ADT, androgen deprivation therapy;BFS, biochemical free survival; OS, overall survival; Gr, grade; PCSS, prostate cancer specific survival; SFUO, Swedish Association for Urological Oncology; SPCG, Scandinavian Prostate Cancer Group;
No age threshold applies to the first principle which states that treating the primary tumour is essential. A recent study demonstrated that elderly men are more likely to have high-risk disease and are less likely to receive definitive therapy. However, when controlled for co-morbidities, even older men with high-grade disease face a substantial risk of dying from prostate cancer and will benefit from potentially curative therapy.37, 38 Although ADT alone is inferior to radiotherapy in high-risk disease, the combination is firmly established in high-risk disease.
A second principle is that long-term ADT in addition to radiotherapy is associated with superior outcomes, including survival, compared to radiotherapy alone (Table 2). RTOG 8531, conducted between 1987–1992, was the first trial to randomize locally advanced patients (n=977) with cT3 disease or lymph node–positive disease to radiation alone (60–70 Gy) versus radiation combined with life-long ADT. Approximately 15% of these patients had undergone radical prostatectomy but had evidence of positive margins and/or seminal vesicle invasion. Patients who received ADT with radiation experienced outcomes superior to those who received radiotherapy without ADT. At 10 years, the rates of local failure (23% vs 38%, p<0.0001), distant metastasis (24% vs 39%, p<0.0001), disease-specific mortality (16% vs 22%, p=0.0052), and overall survival (49% vs 39%, p=0.002) favored the combination arm.29 The European Organization for Research and Treatment of Cancer (EORTC) 22863 trial randomized 415 men to radiotherapy alone (70 Gy) vs radiotherapy plus concurrent and adjuvant goserelin for a total of 3 years. Approximately 90% of the patients had T3/4 disease. At 5-year follow-up, benefits were observed for the patients receiving both radiotherapy and 3 years of hormone therapy, in rates of local failure (1.7% vs 16.4%, p<0.0001), distant metastases (9.8% vs 29.2%, p<0.0001), disease-specific survival (74% vs 40%, p<0.0001), and overall survival (78% vs 62%, p=0.0002).28
Table 2. Improved outcomes with addition of long-term androgen deprivation therapy to radiation treatment.
| Study | Eligibility | Duration of ADT | Outcome | P value |
|---|---|---|---|---|
| RTOG 853129 | cT3 or regional lymphatic involvement (present in 30%) | Indefinite LHRH vs. no ADT | 49 vs. 39% 10y OS 24 vs. 39% DM |
0.002 <0.001 |
| RTOG 8610107 | Bulky T2-T4
|
4 mos CAB vs. no ADT | 43 vs. 34% 10y OS 23 vs. 36% PCSM 35 vs. 37% DM |
0.12 0.01 0.006 |
| RTOG 920243 | cT2c-T4
|
28mos CAB vs 4 mos CAB | 54 vs. 52% 10 y OS 88.7 vs. 83.9% 10 y DSS 14.8 vs. 22.8% DM |
0.36 0.0042 <0.0001 |
| EORTC 2286328 | High-grade T1-2 or any grade T3-T4 | 3y LHRH vs. no ADT | 78 vs. 62% 5y OS 94 vs. 79% DSS |
0.0002 0.0001 |
| EORTC 2296142 | cT1c-T2b with lymph node involvement, any cT2c-T4, or baseline PSA >40× upper limit of normal. | 3y CAB vs. 6 mos CAB | 85 vs. 81% 5y OS | 0.65 (non-inferiority) |
Abbreviations: ADT, androgen deprivation therapy; CAB, combined androgen blockade; DM, distant metastasis; DSS, disease-specific survival; LHRH, luteinizing-hormone–releasing hormone; OS, overall survival; PCSM, prostate cancer–specific mortality; RTOG, Radiation Therapy Oncology Group.
Together, these two trials support a significant benefit in clinical outcome with the addition of long-term ADT to EBRT doses up to 70 Gy. The challenge becomes our ability to extrapolate use of long-term ADT with dose-escalated EBRT in high-risk patients in the absence of level I evidence. RTOG 0815 (NCT00936390) will address the role of ADT in dose-escalated disease in intermediate-risk populations, while RTOG 0924 (NCT01368588) may clarify issue of long versus short term ADT (a stratification variable) in patients with high-risk disease receiving dose-escalated radiation.34, 39
Intermittent versus continuous hormone therapy
Intermittent androgen depletion is a treatment paradigm in which patients with metastastic or micrometastatic disease (rising PSA population) are formally treated in an on-and-off fashion. The central hypothesis is that by stopping ADT when patients are still responding, and allowing tumor cells that survive to regrow under androgen stimulation as testosterone levels rise, patients will retain sensitivity to androgen depletion. It was postulated further that ADT-related toxicities would be reduced during the “off” periods, resulting in a net gain in overall quality of life. A recently reported Southwest Oncology Group (SWOG) trial compared patients receiving intermittent ADT to patients receiving continuous ADT, and showed that among deaths where cause could be attributed, prostate cancer–specific death rates favored continuous therapy, and non–cancer-related deaths favored the intermittent arm; however, the authors concluded that equivalence was not confirmed in this study.40 In contrast, in another trial composed largely of patients selected for a rising PSA after EBRT, the results of intermittent ADT appeared to be comparable to continuously administered ADT.41 This study, unfortunately, had fairly short follow-up and it remains unclear how many of these patients might have been better served with local salvage treatment instead of ADT. As a result it remains unclear whether the remaining patients from this study with more extensive disease might have had a better outcome with continuous ADT.
Long-term versus short-term hormone therapy
Recognizing the long-term toxicities associated with continuous ADT, several trials have studied variable durations of hormone exposure in combination with radiotherapy in patients with high-risk disease (Table 2). EORTC 22961 (NCT00003026) randomized 970 men with locally advanced prostate cancer (defined as T1c-T2ab, N1-N2, or T2c-T4, N0-N2) to 6 months vs. 3 years of complete androgen blockade using a non-inferiority trial design. The short- and long-term ADT arms received the same treatment for the first 6 months, triptorelin plus flutamide/bicalutamide, with the long-term arm then continuing triptorelin alone for another 2.5 years. Short-term androgen suppression provided inferior overall survival (81% vs 85%, HR 1.42, p=0.65 for inferiority) and higher prostate cancer– specific mortality relative to 3 years of ADT (4.7% vs 3.2%, corresponding to HR 1.71, p=0.002).42 RTOG 9202 randomized 1554 patients receiving radiotherapy to 4 months versus 28 months of ADT. Ten-year outcomes showed improvements for the 28-month ADT group in disease-free survival (22.5% vs 13.2%, p< 0.0001), disease-specific survival (88.7% vs 83.9%, p=0.0042), and local recurrence (12.3% vs 22.2%, p< 0.0001). Across the study, no difference in overall survival was observed, but for the subset of patients with Gleason 8-10 disease, rates of overall survival at 5 years were 45% vs. 32% (p=0.0061), for the 28-month relative to 4-month exposure groups.43
A more recent phase 3 trial of 630 men with node-negative high-risk prostate cancer receiving pelvic radiotherapy (70 Gy) randomised them to 18 vs. 36 months of androgen blockade (NCT00223171).44 The median follow-up was 78 months, and 5-year overall survival was 86.1% vs. 91.1% (p=0.06) in the 18- and 36-month groups, with hazard ratio 1.15 (95% confidence interval 0.85–1.56). Non-inferiority of the shorter-duration ADT cannot be assumed with this design. In addition, cT1c-T2a/b represented 75% of patients, while cT3/4 represented only 25% of the cohort.
The evidence to date (overall trends and subset analysis) favors long-term use of ADT over short-term ADT in high-risk disease, while acknowledging that this is not definitive due to the previously described limitations of that evidence and the generalizability of study populations/definitions of high-risk disease. Some physicians and investigators interpret the lack of significance between short- and long-term ADT regimens as showing equivalence, implying that the observed 4% or 6% differences are not meaningful and that ADT beyond 6 months provides no additional benefit. Others feel 3 years of ADT should remain the standard and may cite EORTC 22961 which compared short- versus long-term ADT with a non-inferiority design, as this trial was stopped for futility at interim analysis.42 As this question is unlikely to be resolved any time soon, it is essential that patients be counseled on the trade-offs for long-term ADT so that they are in a position to make an informed decision on how they wish to be managed.
Among the risk:benefit calculus of short- versus long-term ADT are of course the toxicities associated with ADT. Principal differences in patient-reported outcomes (PRO) assessed after completing a year of ADT between long-term and short-term ADT exposure in the EORTC 22961 trial included increased hot flashes and insomnia and decreased sexual interest and sexual activity. No difference in overall quality of life was found.42 RTOG 9202 found grade ≥3 late radiotherapy toxicity (e.g., bowel, bladder) in 10% of patients with long-term ADT as compared with 7% in those who received short-term ADT. Rate of grade ≥3 hormone toxicities (hot flashes, etc.) was comparable between arms (approximately 5%), although it should be noted that this is a clinician-rating scale, rather than a PRO outcome, which is less ideal for assessing the patient's perspective.43 Metabolic and endocrine effects with ADT, such as bone loss, require management to mitigate. These side effects are not insignificant and management such as exercise, while important, is beyond the scope of this review and has been reviewed elsewhere (Grossman Clinical Endocrinology 2011).45-48
Improving radiotherapy outcomes
A third principle of prostate cancer management emphasizes the importance of assessing the appropriateness of the radiation delivery (dose and accuracy) using intermediate endpoints to assess treatment effects. Intermediate endpoints are crucial because trials focused on long-term endpoints such as time to metastatic disease or prolongation of life cannot yield results quickly enough to steer management of an individual patient or guide the choice of radiation dose to be used in phase 3 trials. Also, survival results can be confounded by use of new post-relapse/progression systemic therapies that are now approved by the United States Food and Drug Administration, as well as other regulatory bodies.49-55 These therapies have proven survival benefits in phase 3 trials of advanced prostate cancer, thus their late-stage use can be expected to skew the long-term follow-up survival data collected for trials of earlier-stage prostate cancer.
As there are several ways to improve radiation dose delivery to the tumour, it becomes essential to ensure that only those that are most likely to succeed in definitive phase 3 trials are brought forward. One early endpoint being used in this context is a post-treatment biopsy of the prostate, typically performed at 2 years.56 This was the endpoint used to show the impact of dose in high-risk disease relative to low-risk tumors.57
The observation that dose escalation in patients with high-risk disease conveyed significant benefit in terms of biochemical recurrence–free survival has been confirmed across 5 randomised trials (Table 3).32, 33, 58-60 One such trial reported by Kuban et al. randomised patients to radiotherapy doses of 70 vs. 78 Gy. In the high-risk cohort of patients (approximately 32–35% of the overall population), patients receiving the higher dose had improved biochemical recurrence–free survival: 79% for 78 Gy vs 57% for 70 Gy (p=0.018).32 Similarly, distant failure rates were 4% for 78 Gy vs 19% for 70 Gy (p<0.05), with all distant failures in the high-risk group occurring before 2.5 years. With 10-year follow-up, death from prostate cancer was 4% for the 78 Gy arm vs 16% for 70 Gy (p=0.05). Additional studies have suggested that improvements in local control with higher doses of radiation (>75.6 Gy) are associated with improvement in distant metastases and disease-specific survival.61
Table 3. Improved outcomes with dose-escalated radiotherapy in patients with high-risk prostate cancer.
| Publication | Design | Conclusions | Comments |
|---|---|---|---|
| Sathya, 200559 | 66 Gy EBRT vs. 40 Gy + 35 Gy Ir-192 implant | Improved PSA control with additional higher doses with implant | 60% high-risk; Improved 5y PSA control 63.3% vs. 34.4% in high-risk patients with dose escalation |
| Peeters, 200633 | 68 vs. 78 Gy EBRT | PSA control better in 78 Gy arm | 64% high-risk; Improved 5y PSA control 56% vs. 48% with dose escalation |
| Dearnaley, 200758 | 64 vs. 74 Gy EBRT (ADT on each arm) | 74 Gy improved PSA control | 45% high-risk; Improved 5y PSA control 57% vs. 43% (HR = 0.60) with dose escalation |
| Zietman, 201060 | 70.2 EBRT vs. 79.2 Gy with protons | Better 5y PSA control improved with 79.2 Gy | 5% high-risk; inadequate sample size of high-risk patients for subset analysis |
| Kuban, 201132 | 70 vs. 78 Gy EBRT | Patients with PSA >10 ng/mL or high-risk benefit from 78 Gy | 35% high-risk; 78 Gy improved PSA control (79% vs. 57%) and DMFS (96% vs. 81%) |
Abbreviations: ADT, androgen deprivation therapy; EBRT, external beam radiotherapy; DMFS, distant metastasis free survival; Ir-192, Iridium-192; PSA, prostate-specific antigen.
Improving local control rates and shortening treatment burden by adding brachytherapy
Another method to improve the outcome of high-risk patients receiving EBRT and androgen blockade is the addition of a brachytherapy boost. Brachytherapy allows for high-precision delivery of extreme radiation doses to the prostate. The tight conformality minimizes dose to the bladder, rectum, and urethra, resulting in an increase of the therapeutic ratio. Brachytherapy may be more convenient for the patient as the treatment course can be truncated from 9 weeks to 5 weeks.
Multiple institutional series have demonstrated a PSA relapse–free survival ranging from 70–98% with follow-up to 7–8 years[need refs here – are they just Vargas, D'Amico, and Fang (currently 62-64)?]. A large reported experience from Vargas and colleagues involved 560 patients, each with 1-3 risk factors, treated with high-dose rate brachytherapy and EBRT with or without neoadjuvant ADT (≤ 6 months vs no ADT). This study demonstrated a biochemical relapse–free survival rate ranging from 81–85%. Interestingly, the development of metastasis at 5 years was actually higher in those receiving neoadjuvant ADT (10% vs 5%, p=0.04), and this trend was consistent across risk groups.62 The fact that the addition of androgen blockade did not improve outcomes, in contrast to other studies,63 led to questioning the benefit of neoadjuvant ADT in the setting of high-dose radiation brachytherapy.62
Another retrospective study of 174 patients with Gleason 8–10 prostate cancer and PSA <15 ng/mL who underwent permanent interstitial brachytherapy, 91% of whom also received EBRT, demonstrated biochemical recurrence–free survival rates of 92.6% and 86.5% in patients treated with and without androgen blockade, respectively.64 This difference was not statistically significant and suggested that the use of ADT may not affect brachytherapy outcome. However, all of these types of post hoc analysis must be viewed with great caution, because potential selection biases could explain the findings. In addition, biochemical outcomes are not established as surrogates for more meaningful endpoints such as cause-specific survival and overall survival in patients with high-risk disease. Again, given the fact that none of the dose-escalation studies to date have demonstrated an improvement in overall survival, one might argue that the evidence for the appropriate use of ADT appears to trump the evidence for dose escalation.34
Radical prostatectomy
Radical prostatectomy is a second option for patients with high-risk prostate cancer, as noted in the NCCN, AUA, and European Association of Urology (EAU) guidelines. Studies reported from a number of institutions show 5-year PSA relapse–free survival rates ranging from 55%–71% and 10-year prostate cancer–specific survival rates from 72%–92%.65-68 The rates vary as a function of disease extent, and as previously noted, by the criteria used to define “high-risk”. As is the case with the various radiation therapy techniques, optimal surgical management also requires adherence to several principles. The radical prostatectomy procedure requires a) complete removal of the gland itself, b) confirming intra-operatively that the surgical margins are negative on frozen section, and for this high-risk population in particular, c) the performance of an extended pelvic lymph node dissection (ePLND). The procedure is best performed by an experienced high-volume surgeon, who have been shown as a group to have better outcomes.69, 70. Prior to surgery, patients should also be informed about the possible need for post-operative radiation therapy, as noted in the AUA and American Society for Radiation Oncology (ASTRO) consensus statements, which are considered in more detail below.71, 72
The first principle acknowledges that the survival benefit of radical prostatectomy will vary by patient, as a function of the patient's risk of death from prostate cancer and risk of death from other causes. Several large published retrospective series with a focus on high-risk disease have demonstrated favorable long-term prostate cancer–specific survival rates with radical prostatectomy and node dissection (Table 4).65-68, 73-76 In these large series, 10-year prostate cancer–specific survival rates were generally over 90%, and were remarkably consistent across the series reported. Such consistency and high survival rates may in part reflect definitions of high-risk disease, which commonly used the D'Amico classification and capture of patients with more favorable “high-risk disease”. As aptly pointed out by Spahn and colleagues in their series of 712 men with PSA >20 ng/ml at diagnosis, patients with one high-risk feature (e.g., PSA > 20 ng/ml) were more likely to have favorable histopathology (pT3a or lower, pGleason < 7, R0, pN0) than patients with more than one pre-operative high-risk factor, such as Gleason ≥ 8 and/or cT3 disease. In this series, 27% of patients with only PSA >20ng/ml had favorable histology at radical prostatectomy in comparison to 0% of patients with three risk factors (PSA > 20 ng/ml, cT3-4, and Gleason score 8). Similarly, prostate cancer–specific survival varied by the number of high-risk features: at 10years, it was 91% in those with only PSA >20 mg/ml and 65% in those with PSA >20 ng/ml and Gleason ≥8.67 This raises the importance of descriptive statistics to better define populations, including details of high-risk factors when reporting retrospective series. This also has significant implications for clinical trial design targeting therapeutic interventions in high-risk cohorts.
Table 4. Large retrospective series of radical prostatectomy in high-risk patients demonstrates favorable 10-year prostate cancer–specific survival.
| Author | Number high-risk patients | Definition of high-risk | Prostate cancer–specific survival (PCS) | Comments on population | Adjuvant therapy (adj) |
|---|---|---|---|---|---|
| Briganti, Joniau, et al., 201274 | 1366 | PSA >20 or cT3 or bx Gl > 8 | 10 yr PCS 91% 10 yr 98% vs 88% in organ confined vs non-organ confines PCS |
57% with cT3 75% with pT3-4 -adjuvant rx in 66.4% vs 16.8% in non-organ confined vs confined disease |
48% received adj therapy (ADT +/- RT). -8.2% adj RT -29.7% adj ADT -10.2% adj RT+ADT |
| Boorjian, 201173 | 1238 | PSA >20 or cT3 or bx Gl > 8 | 10 yrs PCS 92% | -33% cT3-4 | -40% received adj therapy - 6.9% RT -29.5% ADT - 4.1% both RT and ADT) |
| Stephenson, 200965 | 1962 | PSA >20 or cT3 or bx Gl > 8 | 10yr PCS 92% 15 yr PCS 81% |
-large study, high risk represented 17% of overall population | -Adj therapy not reported |
| Ward, 200575 | 841 | cT3 | 10yr PCS 90% 15yr PCS 79% |
18% Gleason > 8 Mean PSA=10.2 (4.7-23.7) |
-51% received adj RT -16% received adj ADT |
| Yossepowitch, 200876 | 1359 per D'Amico def'n 938 per NCCN def'n |
8 high risk definitions compared | 10yr PCS 93% per D'Amico 10yr PCM 92% per NCCN |
-10yr cum incidence varied by definition (3-11%) | 30-39% received ADT and/or RT within 5 yrs, but almost all at BCR/or salvage; no adj ADT and only 31cases of adj RT |
| Eggener, 201166 | 631 modeling/726 validation cohort | High risk per se not defined, but subset Gleason > 8subset reported | 10yr PCS 82-87% for Gleason > 8 | PCS by age: 70-79yo: 82% 60-69: 87% <60: 85% | -Adj therapy not reported |
| Spahn, 201067 | 712 | PSA > 20ng/ml | 10yr PCS estimated 89.8% | 44% cT3-4 20% Gleason > 8 |
Adj RT varied by number of additional risk factors (11.9-21.6%) Adjuvant ADT similarly varied based on additional risk factors (35.4%-84.1%) |
| Zwergel, 200768 | 275 | PSA > 20ng/ml | 10 yr PCS 83% | 75% also pT3 49% Gl > 8 |
-129/275 (47%) immediate ADT (almost all before yr 2000 when practice patterns changed at this single institution study) -Survival did not differ between immediate vs deferred ADT 2/275 adj RT (<1%) |
Another theme that has emerged from retrospective surgical series that dovetails with the concept of more favorable versus less favorable “high-risk” disease, is the concept of which patients are most likely to benefit from adjuvant therapy. As seen in Table 4, use of adjuvant radiation therapy and/or ADT is variable across retrospective surgical outcomes series. Use of adjuvant radiation, for example, varied between 10.5%-51% depending on the series. Since these were retrospective series rather than controlled trials, the use of adjuvant therapy likely varied by physician, patient, and institutional practice. For instance, one series found patients with more than one adverse risk feature were more likely to receive adjuvant therapy than those with an isolated adverse or high-risk feature.67 Data on randomized controlled trials for adjuvant radiation are reviewed separately below (see third principle, post-operative radiation therapy).
There are no large phase 3 trials assessing the value of radical prostatectomy exclusively in men with high-risk disease, unlike the data from the definitive radiation therapy trials. However, two recently reported prospective randomized phase 3 trials (which included a few high-risk patients) concluded that 1) radical prostatectomy did not confer a survival benefit to men with localized disease, or 2) that the benefit was modest at best.77 The implication was that a significant proportion of men were undergoing an operation from which they would not benefit, but which could cause harm through the loss of erectile function and urinary control. However sobering the reality of “overtreatment” for some cancers may be, these trials also provide insight into outcomes for high-risk disease. The Prostate Cancer Intervention Versus Observation Trial (PIVOT) study (NCT00007644) randomized 731 men with newly diagnosed prostate cancer to watchful waiting or radical prostatectomy. The study did not reach the originally planned accrual target (>2000), and no overall survival benefit was seen, although bone metastasis–free survival rates favored radical prostatectomy (10.6% vs 4.7% for watchful waiting; p<0.001).78 Radical prostatectomy did however prolong prostate cancer–specific survival in the 251 patients with PSA >10 ng/mL (HR 0.36, 95% CI 15–89%), with a trend for the 157 patients with high-risk disease (defined as stage T2c or PSA level >20 ng/mL or Gleason score ≥8) (HR 0.40, 95% CI 0.16–1.00).
The Scandinavian SPCG-4 study also randomized patients to radical prostatectomy or watchful waiting.77 This trial showed that at 15 years, radical prostatectomy decreased prostate cancer–specific mortality (14.6% vs 20.7%, p=0.01) and the cumulative incidence of bone metastases (21.7% vs 33.4%, p<0.001) in comparison to watchful waiting. Some may argue this is not a sufficient benefit for a group as a whole, which further emphasizes the importance of identifying those who need surgery because of the risk of their cancer to cause symptoms, to metastasize, or to shorten survival. The decision is further complicated by the need to determine for which patients radical prostatectomy will not be sufficient. Or stated differently, to determine at what point it is necessary to plan a combined modality approach that includes additional local therapy (radiation to primary site and/or pelvic lymph nodes), and systemic therapies to address micrometastatic disease that was undetected at the time of diagnosis or at prostatectomy.
Extended pelvic lymph node dissection
The second principle emphasizes the importance of an ePLND. The morbidity of extended nodal procedures which removed nodal chains that extend to the pelvic side wall and/or the pelvic brim (sacral promontory) as well as perhaps stage migration of cT1c disease of low risk led to modifications in the procedure such that fewer lymph nodes were removed. Even so, some surgeons still hesitate to perform an ePLND because of potential complications. Reported rates of complications vary widely in frequency, from 2–51%.79 In two studies, complications were more frequent for an ePLND than for a limited PLND: 35.9% vs. 2% (p<0.001)80 and 19.8% vs. 8.2%, p<0.001).81 Complication rates below 5% have also been reported for ePLND.82-84 Lymphocele formation is perhaps among the most common complication with node dissection, which, in a large series reported by Briganti and colleagues, significantly differed between limited PLND and ePLND (4.6% vs 10.3%, p<0.01). No increased deep vein thrombosis, blood loss, or pelvic hematomas were found.81 Although lymphocele is among the more commonly reported complication in the literature, this has also been reported as low as <5% with ePLND,85 perhaps owing to different definitions of lymphocele and post-prostatectomy imaging practices across series, as more routine post-operative imaging will detect more sub-clinical lymphoceles. A prospective phase 3 German multi-institutional study comparing ePLND with limited PLND will attempt to address the role of ePLND [do you have a study registration number?]. The increasing proportion of men with low-risk disease, in whom nodal spread is rare, led many surgeons to abandon PLND entirely or to remove at most 1 or 2 nodes from each side. Such is the case, unfortunately, reflected by trends reporting less extensive PLND with robotic prostatectomy than with open prostatectomy, despite the evidence that nodal dissection can be competently performed with either procedure.86-88
A variety of pre-operative nomograms and risk assessment algorithms have been reported to estimate the frequency of nodal spread at prostatectomy. At Memorial Sloan Kettering Cancer Center (MSKCC), the policy is to perform a lymph node dissection when the predicted probability of spread is 2% or more, with a plan to remove 20 or more nodes.89 Some may argue that this probability is too low, but for patients with high-risk disease, where the frequency of nodal spread can vary from 30-40%, the dissection is essential for three reasons: the therapeutic benefit, the more accurate staging to estimate prognosis and as therapy, and to inform the need for subsequent therapy.90 Two systematic reviews suggested that ePLND increased the detection of positive nodes, with an associated improvement in survival. The observed survival benefit was attributed to the elimination of micrometastatic disease.79, 91 A retrospective single-center analysis of men with lymph node–positive disease who were treated with radical prostatectomy and lymph node dissection found that 28% of these men remained free from biochemical recurrence at 10 years.92 This, along with other retrospective series, favors completion of radical prostatectomy (rather than aborting the procedure) when intra-operative node dissection finds node-positive disease on frozen section.93 Additionally, a prospective randomized trial of extended versus standard lymph node dissection in open prostatectomy cases demonstrated improved 10-year biochemical relapse–free survival rates among those with high-risk disease (51.1% vs 71.4%; p = 0.036).94
Post-operative radiation therapy (adjuvant and salvage)
Adjuvant radiation therapy
The third principle is to assess the need for post-operative radiation therapy in patients with clinically localized prostate cancer treated with radical prostatectomy. Adjuvant radiotherapy is administered to men with a high risk of local recurrence when the PSA is undetectable (<0.2 ng/mL) and based on pathologic features from prostatectomy. Adjuvant radiotherapy is typically administered 3-6 months postoperatively when incontinence has stabilized or resolved. Support for its use is based on three prospective randomized trials showing a clinical benefit or survival benefit for adjuvant radiotherapy in patients with pT3 disease or positive margins, with the highest level of evidence for the latter.95-98
SWOG 8794 randomized 425 men with pT3 disease or positive margins to observation or adjuvant radiation therapy of 60-64 Gy. In an updated publication with long-term follow-up (median approximately 12.5 years), both overall survival (HR 0.72; 95% CI 0.55-0.96, p=0.023) and metastasis-free survival (HR 0.71; 95% CI 0.54-0.94, p=0.016) favored adjuvant radiotherapy over observation.97 The initial trial publication (median 10.6-year follow-up) did not reach statistical significance for these endpoints, though it did find differences in PSA relapse–free survival favoring adjuvant radiotherapy (10.3 years for radiotherapyvs 3.1 years for observation; HR 0.43; 95% CI 0.31-0.58, p<.001).99
EORTC 22911 enrolled 1005 patients randomized to post-prostatectomy radiotherapy or no postsurgical treatment, with a median follow-up of 10.6 years. This study demonstrated an improvement in biochemical relapse–free survival (74.0% vs 52.6%, p<0.0001) and local failure (7.0% vs 16.5%, p<0.0001) in patients receiving adjuvant radiotherapy, but failed to demonstrate an improvement in overall survival (80.7% vs 76.9%; p=0.20) or metastasis-free survival (11.3% vs 11.0%; p=0.94). Salvage radiotherapy was administered in 56% of patients with biochemical recurrence on the observation arm, although the trigger to treat in the salvage setting was variable. Examining the observation arm retrospectively, using an intent to treat approach, demonstrated that 83.7% of patients did not fail locally.96 The question then is whether it is worth treating 100% of patients to reduce the relapse rate by 9.7% (83.7% to 74.0%). It is for this reason that many groups favor a salvage radiation therapy approach. The German Cancer Society enrolled 388 patients onto trial ARO 96-02, comparing post-prostatectomy radiotherapy to no postsurgical treatment, which demonstrated an improvement in 5-year biochemical progression–free survival.98 Unlike SWOG 8794, no differences in rates of distant metastases or overall survival were seen in EORTC 22911 or ARO 96-02.95, 96, 98
Adjuvant radiotherapy holds potential for toxicities. In SWOG 8794, men received adjuvant radiotherapy versus observation post-prostatectomy, and this trial reported rectal complications (proctitis or rectal bleeding) in 3.3% vs 0% (p=0.02), total urinary incontinence in 6.5% vs 2.8% (p=0.11), and urethral stricture in 17.8% vs 9.5% (p=0.02); although, the time points of these occurrences were not reported.100 Toxicities may impair quality of life; hence, the patient's tolerance for such risk is weighed in decision making. Interestingly, impairment in quality of life may be “front loaded”, with improvements over time; hence, this should be part of the discussion with patients. In SWOG 8794, initially acute gastrointestinal and genitourinary effects were worse with adjuvant radiotherapy when compared with observation, but at 2 years, no differences were observed.97 The EORTC 22911 trial demonstrated only a 3% rate of grade 3 late toxicity, with no grade 4 late effects seen.95 Moreover, patient reported outcomes on 5-year quality of life measures were improved with adjuvant radiotherapy when compared to observation.100
Salvage radiation therapy
In patients with a rising or detectable PSA after radical prostatectomy, salvage EBRT is often considered. Rising PSA indicates continued cancer growth but does not necessarily mean that left untreated, a patient will develop symptoms or metastasis or succumb to his disease. In these cases, the question is whether the source of the PSA is only the prostate bed itself — which could potentially be treated with salvage EBRT; nodal disease — which could be treated by surgery, radiation or a systemic approach; or a systemic failure (distant metastasis) which would require systemic therapy. The PSA may also come from a combination of these locations.
Radiation therapy that is given when a previously undetectable PSA becomes detectable is considered “salvage radiation therapy”. Proponents of salvage radiotherapy over adjuvant radiotherapy argue that the use of PSA testing to detect recurrent disease may still allow effective radiation to be delivered when disease is localized to the prostatic bed. Salvage radiation selects for men who have evidence of biochemical recurrence, avoiding overtreatment of men who are not destined to recur and restricting to a smaller cohort the potential complications of radiotherapy. An important consideration, however, is that even when a recurrence is documented, the risk of death from competing comorbidities may exceed the risk of developing prostate cancer–related symptoms or dying from prostate cancer.
Although, no randomized trials have been completed to demonstrate a survival benefit for salvage radiotherapy, evidence from retrospective and observational studies suggest it may be effective for controlling local recurrence, decreasing distant metastasis, and lowering prostate cancer mortality. For such reasons, the AUA/ASTRO guidelines72 consider the strength of evidence for recommending salvage radiation a grade C. That said, if a patient is to consider salvage radiotherapy, the effectiveness is maximized if radiation is administered when PSA levels are low (<0.5 ng/mL), which requires close monitoring of patients when a recurrence is documented. In one study, progression-free survival varied from approximately 20% to 50% depending on the PSA level at which salvage radiotherapy was offered. The 6-year progression-free survival was 48% in patients with a PSA <0.5 ng/mL and 18% if the PSA level was >1.5 ng/mL.101
An important question to be addressed in trials is to formally compare the strategy of adjuvant radiotherapy relative to early salvage radiotherapy using a low PSA as the trigger to administer treatment. Some have argued that “in most trials reporting a ‘benefit’ for the adjuvant approach, salvage therapy was not administered until it was less likely to be effective, with the idea being that, by identifying patients who are clearly destined to fail locally based on the pathologic findings, would better enable adjuvant treatment to be given early to those who need it. However, since many of the patients included in the major trials described above actually had “detectable” PSAs, and their outcomes were better if irradiated early, and since so-called “salvage” EBRT is only modestly effective, caution is recommended in advising patients with adverse pathological features to delay adjuvant EBRT. In randomized adjuvant radiation therapy trials, adverse pathology at prostatectomy was associated with biochemical recurrence rates around 60%. 72, 96, 97 Two phase 3 randomized trials are being conducted by the Medical Research Council (RADICALS, Radiotherapy and Combined Androgen Deprivation after Local Surgery) and the Trans-Tasmanian Radiation oncology Group (RAVES, Radiotherapy Adjuvant vs. Early Salvage following Radical Prostatectomy) that will address whether adjuvant versus salvage radiation therapy is associated with improvements in prostate cancer–specific mortality or biochemical progression–free survival, respectively. The AUA/ASTRO guidelines provide the best summary and recommendations for patients in the postoperative setting.72
Radiation therapy vs. radical prostatectomy
A frequently asked question is whether surgically based approaches are superior to radiation therapy–based approaches or vice versa. Although no prospective randomized trials have ever been completed, several studies have retrospectively compared radical prostatectomy with radiotherapy. A combined analysis involving patients from the Mayo Clinic and Fox Chase Cancer Center included 1,238 patients undergoing radical prostatectomy and 609 patients undergoing EBRT.73 Three hundred forty-four of the patients receiving radiotherapy also received ADT. All patients had high-risk prostate cancer classified as PSA ≥20 ng/mL, Gleason score 8-10, or ≥T3 disease. The 10-year cancer-specific survival rates were 92%, 92%, and 88% for patients receiving radical prostatectomy, EBRT and ADT, and EBRT alone, respectively (p=0.06). After multivariable risk adjustment, there was no significant difference in distant metastasis or cause-specific survival. However, the risk of all-cause mortality was greater after EBRT with ADT when compared to radical prostatectomy. Several reasons could explain this finding of increased all cause mortality: the first being selection bias for patients with greater medical comorbidities to be treated with radiation rather than surgery. All retrospective series suffer this inherent bias, and in the absence of comorbidity scores for all patients, it is difficult to either prove or disprove. This study did however attempt to control for comorbidities (when available) using the Charlson comorbidity index and did not find this to influence outcomes. Alternatively, some argue that the potential adverse cardiovascular effects of androgen deprivation therapy may have contributed to greater cardiovascular deaths and all cause mortality, a controversial topic in the literature.43, 73, 102-104
Several additional limitations should be mentioned about the Mayo/Fox Chase series. The median radiation dose of 72 Gy used in this study is suboptimal as suggested by the multiple randomized trials demonstrating a clinical benefit with higher doses of radiation. In addition, the radiation target volume included the prostate and seminal vesicles with omission of the pelvic lymph nodes.73 The pelvic lymph nodes are often included in the radiation treatment volume for patients with high-risk disease. With escalated doses of radiation and incorporation of pelvic lymph nodes in the radiotherapy field, further improvements in clinical outcome might have been observed for the radiation treatment arms.
A smaller retrospective study from Italy reported on 288 patients with high-risk prostate cancer. One hundred sixty-two patients underwent EBRT in combination with 9 months of ADT; 122 patients underwent radical prostatectomy with pelvic lymph node sampling. Also, patients treated with radiotherapy either received a total dose of 80 Gy in standard fractions of 2 Gy each or a hypofractionated course to 62 Gy in 20 fractions of 3.1 Gy each. The target was the prostate and seminal vesicles, with no pelvic lymph node treatment. The median follow-up was approximately 3 years for each group. This study demonstrated an improvement in 3-year biochemical failure–free survival favoring radiation (86.8% vs. 69.8%; p=0.001).105 A significant limitation of this study was the use of biochemical failure as a primary endpoint. Different definitions of biochemical failure were used for radiation therapy and surgery, causing difficulty in comparing outcome across modalities. Additionally, the 9-month duration of ADT may not have been optimal, as multiple trials have demonstrated a survival benefit for longer-term hormone therapy up to 2-3 years. Lastly, because biochemical failures often occur after 4 years, especially in patients receiving ADT, many failures may have been missed with the relatively short follow-up.
The largest retrospective study evaluated patients with cT1c-T3b prostate cancer who underwent either radical prostatectomy with pelvic lymphadenectomy or radiotherapy at MSKCC. Radiation was delivered to the prostate with omission of the pelvic lymph nodes to a total dose of 81-86.4 Gy. Three to six months of ADT was given in combination with radiation treatment. The 8-year distant metastasis–free survival was similar (97% vs. 93%) for radical prostatectomy and radiotherapy, respectively, with overlapping confidence intervals. In the high-risk cohort of patients, an absolute benefit of 7.8% in distant metastasis–free survival was suggested favoring radical prostatectomy.106
Several limitations of the MSKCC study should be mentioned. Long-term ADT was not routinely used in conjunction with radiation in many of the studies. As mentioned above, the addition of long-term ADT to radiation therapy decreases metastases by 40-50% when compared to short-term ADT, and improves survival. Second, the pelvic lymph node irradiation was not routinely performed. Patients with high-risk prostate cancer are at increased risk for harboring microscopic lymph node involvement and pelvic lymph node irradiation is part of treatment for all prospective randomized trials establishing the role of hormone therapy and radiation in this patient population.28, 29, 43, 107 Third, image guidance delivery of radiation was not routinely incorporated. Image guidance radiotherapy improves biochemical tumor control in patients with high-risk prostate cancer and is associated with lower rates of urinary toxicity.108 Fourth, there was an imbalance with higher pretreatment risk factors in the radiation group compared to the surgery group including higher PSA, clinical stage, and Gleason score. Also, patients receiving radiation were less likely to remain disease-free based on the preoperative Kattan nomogram, which predicts for disease recurrence after radical prostatectomy. Of note, the pretreatment nomogram predicting for 5-year probability of distant metastasis109 rather than disease recurrence may have been more appropriate for this study when the primary endpoint was to compare the rate of distant metastasis. Last, patients undergoing radical prostatectomy were more likely to undergo early salvage treatment after treatment failure than the radiation cohort. Salvage radiation was offered after failure at 13 months in patients undergoing surgery and after 69 months in patients who underwent radiotherapy.106 Data demonstrate that the earlier salvage treatment is offered, the greater chance of success. Earlier salvage treatment is associated with improved progression-free survival when compared to delayed treatment.101 Furthermore, recent studies have demonstrated the efficacy of brachytherapy as a salvage procedure in patients with biochemical failures after EBRT.110, 111
Retrospective comparisons of survival after radical prostatectomy and radiation therapy are inherently difficult because of selection biases in the general health of men with high-risk disease selected for surgery when compared with those selected for radiation treatment. For example, Giordano et al. in a comparison study showed that men treated by radical prostatectomy had better survival than men in the general population who did not have prostate cancer.112
More recently, Eifler et al. showed that death from non–prostate cancer causes was lower in 11,000 men who underwent a radical prostatectomy compared to men in the general population.113 Second, patients receiving radiation therapy are more likely to have more advanced T stage disease, Gleason score, and higher PSA.73, 106, 112 Also, differences in definitions between radiation and radical prostatectomy make it difficult for biochemical failure to be used as a primary endpoint. Rather, studies need long-term follow-up with endpoints of distant metastasis–free survival and cause-specific survival. Many of the above-mentioned studies attempt to account for this selection bias with multivariate regression models and competing risk-regression analysis. However, with the retrospective nature of the studies, the ability to completely account for differences in baseline characteristics between the patient cohorts is practically impossible.
Randomized trials focusing not only on survival but also quality of life are essential. Currently, the Prostate Testing for Cancer and Treatment (ProtecT) trial is ongoing and hopes to address both survival and quality of life questions.114 This trial includes patients diagnosed with prostate cancer of all risk categories. However, it is unclear if the trial will successfully address the relative effectiveness of each modality in the high-risk cohort after accrual.
Ultimately, prostate cancer treatment will be individualized for each patient. Current risk stratifications tools are inadequate for tailoring therapy. These tools only address tumor characteristics and not patient characteristics, such as their comorbidities. Biomarker-driven decision analyses are being developed to predict disease aggressiveness, response to treatment, and toxicity from treatment. Biomarkers may allow us to better assess the benefit versus the toxicity of each treatment, alone or in combination, to guide decision makers. Rather than choosing only surgery or radiation treatment, patients with highly aggressive tumors may benefit from combined modality treatment of surgery, radiation, and ADT, similar to that in other disease sites such as rectal or breast cancer. Furthermore, by accounting for patient comorbidities in addition to tumor characteristics, biomarkers may improve our treatment decisions by accounting for the relative risk of death from cancer versus other causes. Altogether, a multidisciplinary effort between urologists, radiation oncologists, and medical oncologists is critical to improve treatment outcomes for patients. In practice the modality used to treat localized prostate cancer appears to be heavily influenced by the type of physician seen and referral patterns, with <5% seeing all three specialties.115, 119 For the high-risk patient gauging among various treatment options, however, multidisciplinary consultations and collaborations may not only improve satisfaction, but potentially outcomes (.117-119
Future Directions
Technical advances and refinements in surgery and radiation therapy have enabled the outcomes for patients with “high-risk” prostate cancer to be improved both from the point of view of cancer control and reducing the morbidity associated with treatment.32, 107, 120-122 The better outcomes have been shown through well-designed and executed clinical trials. Now, with further advances in surgical technique, our understanding of radiation biology and technical advances in radiation delivery systems and methodologies, and advances in the systemic therapy of prostate cancer which have led to the approval of 5 new agents with diverse mechanisms of action based on a survival benefit, the field is uniquely poised to shift the paradigm even further and to begin to extend the limits of curability beyond what can be achieved with any single or dual modality approach.
Unfortunately, as the various definitions of “high-risk” include patients with a wide range of prognoses, so too do the published reports reflect a wide range of quality, limiting our ability to establish practice standards based on hard evidence. Clinical trials are experiments, with an objective, defined eligibility, an intervention, outcomes, statistical methods, and conclusions designed to determine whether the objective was met and how to move forward. Too many reports are retrospective analyses of a particular modality or treatment approach. Furthermore, these reports differ in the cases treated, how disease extent was determined, the intervention (e.g., radiation portal, dose and dose rate, and for both radiation and surgery, whether nodal radiation or node dissection was performed and if so, how complete), the specifics and the timing of the assessments used to monitor disease following the intervention, the specific outcomes reported, the follow-up period, and last but not least, the interpretation of the data. Few reports include a statistical design, and for those that do, the conclusions are misinterpreted. For example, demonstrating benefit in an analysis of a subset of patients enrolled on a trial is not level 1 evidence, it is a “hypothesis-generating conclusion” to be studied in future trials.
Moving forward: optimizing future studies
(Re)-defining high-risk
High-risk disease must ultimately be defined and placed in a clinically meaningful context. For most men, this is represented by control of the primary tumor and treatment of the metastatic disease that is ultimately the major cause of morbidity and mortality from prostate cancer. These outcomes and the need for treatment must be balanced against the competing causes of death in this generally elderly population. Arguably, preventing morbidity by controlling the disease to the point where a patient dies “with it” rather than “of it” can be considered a therapeutic success. This review highlights the range of endpoints (i.e., clinical outcome) used to evaluate an intervention in variably defined “high-risk” populations. The endpoints include biochemical recurrence, local recurrence, metastasis-free survival, overall survival, and prostate cancer–specific survival. There are others. Patient-reported outcomes, which are essential for a patient to consider the risk/reward ratio of an intervention, are rarely included, yet this ratio is of particular importance when considering whether to continue a treatment that might have a modest gain in overall survival at the expense of a reduced quality of life. This heterogeneity in both definitions and endpoints limits the ability to place outcomes in context, determine utility, or to provide clinicians, patients, regulators, and payers with meaningful data.
The large retrospective series by Boorijian and colleagues comparing radical prostatectomy and EBRT highlights these difficulties. The investigators report a 10-year prostate cancer–free survival rate of 92% both for patients who underwent radical prostatectomy or EBRT with ADT.73 While it may be argued that the high survival rate underscores the effectiveness of the therapy, it also may be attributed to our failure to predict truly high-risk disease. The historic clinicopathologic variables such as T stage are challenged by both intra- and inter-clinician judgment, limiting it's validity and utility as a biomarker; Gleason score is subject to interpretation and inter-observer variability, sampling error, while PSA lacks sensitivity and specificity; these risk factors may simply not be adequate, even in combination. Assessing tumor burden by quantifying the percentage of core involvement, such as with the CAPRA score, has shown enhanced performance for the determination of risk, but is not a paradigm shift. As mentioned previously, analytically valid biomarkers that have been clinically validated are sorely needed to more reliably assess risk, such as those that assess biologic determinants in the tumor itself and associate with an aggressive disease phenotype. While much of the development in this arena is being applied to the decision of active surveillance versus treatment in classically defined low-risk disease, the same sensitivity is required to distinguish those with high-risk disease so that clinical trials can address this population of patients as well.
While few practitioners would disagree that a man with Gleason 10 T3b disease has a significant risk of mortality from his prostate cancer, there are few measures to help identify a patient with, for example, Gleason 7, T1-T2 disease that may also actually have a similar risk of death from prostate cancer. The pathway to biomarker development is treacherous as the limitations that plague trial design apply to biomarker research; moreover, the majority of tumor biomarkers are troubled by a lack of analytic validation (e.g., reliability, reproducibility of an assay) and clinical validation (determines that a test is associated with a clinical outcome), and have not yet demonstrated clinical utility.
More consistent use of meaningful clinical endpoints for validation
Comparing biochemical recurrence rates or PSA relapse–free survival times between surgery and radiation is inherently flawed, given that the PSA nadirs are achieved at different time points and patterns of biochemical relapse cannot be reliably compared. PSA relapse–free survival can be considered as a screening endpoint for a phase 2 intervention but does not in itself represent a clinical benefit. For one, the rise in PSA may represent recurrent local disease, locoregional disease, systemic disease or all three. Furthermore, it is well recognized that of men who relapse after primary treatment and enter the rising PSA state, only a small proportion require immediate intervention based on their probability of developing metastasis, symptoms or dying of their cancers.
More important is the risk of developing metastatic disease, at which point the risk of dying of prostate cancer exceeds that from other causes. Thus it is important that new biomarkers be clinically validated using more downstream measures such as metastasis-free, overall, or prostate cancer–specific survival which more clearly reflect patient benefit. It is also important to clearly show that the “new” marker adds predictive accuracy to the currently available risk classification criteria currently in use.4, 6, 7
Therapeutic strategies
The boundaries of what is considered high-risk, and markers to define such populations, need to be standardized and validated to advance the field. From this, the impact of different therapeutic interventions can be assessed more reliably and promising approaches considered for more definitive testing in the phase 3 setting. Narrowing the definition of high-risk disease by increasing specificity may lead to a smaller proportion or subset of patients, but is the only way to generate the evidence needed to establish standards and change practice.
Intervention strategies to improve outcomes for high-risk populations can take several approaches, either alone or in combination: improving existing technologies, using multi-modality approaches, and in particular moving the armamentarium for metastatic disease earlier in the disease course. Improving upon existing modalities is a common pattern of clinical trial development used in high-risk disease. For example, investigating the benefit of extended lymph node dissection with prostatectomy, whole pelvic radiotherapy, use of hypofractionated versus conventional radiation therapy, or lengthening the duration of ADT with radiation have all been evaluated and reviewed previously. Use of multi-modality therapy is another common approach, such as radical prostatectomy with adjuvant radiation or ADT with EBRT; if the boundary of high-risk disease is pushed to micrometastatic disease, then systemic therapies will likely be a critical aspect to multi-modality therapy as well.
Lastly, another common strategy for high-risk localized disease that is integrally linked to a multi-modality approach is to apply therapies proven to be effective in metastatic populations and evaluate the same intervention in earlier disease states. The phase II trial (NCT00924469) utilizing abiraterone with ADT in a neoadjuvant setting prior to radical prostatectomy is one such example; this trial demonstrated a 30% pathologic complete response rate and holds potential to have eliminated early castration-resistant clones.123 A phase II trial of ADT with ipilimumab (CTLA-4 blockade) in patients with locally advanced disease found that a higher proportion achieved an undetectable PSA (54% vs 38%) with the addition of ipilimumab.124 CALGB 90203 randomized high-risk patients to neoadjuvant ADT with docetaxel or immediate radical prostatectomy and has completed accrual and will inform of this potential neoadjuvant strategy. Similarly, building upon standard ADT with radiation is a series of trials adding other systemic therapies such as enzalutamide (NCT02023463), TAK700 (NCT01546987), or taxane chemotherapy (NCT00651326; NCT00116142). With all of these trials, it will be years before the data have matured to determine if a survival advantage or metastasis-free survival is demonstrated. That said, if the population selected is a more accurately defined, more homogenous high-risk population, then these endpoints may be reached sooner. These endpoints are arguably the most clinically relevant and the best comparisons for surgery versus radiation therapy, which serve as the backbone for localized disease therapy.
Conclusions
The future of clinical trials in the high-risk population awaits a paradigm shift, away from the standard risk definitions and design challenges that have limited the existing literature. As highlighted in this review, there are multiple open questions for the field, some of which await follow-up from existing trials and others that will require new trials with new designs. Importantly, however, there is consensus across disciplines that risk stratification is in need of refinement and standardization, and that innovative strategies to optimize care are needed for the truly high-risk patient.
Box 1.
Common definitions of high-risk prostate cancer.
| Organization | Definition |
|---|---|
| American Urologic Association | Pre-op PSA >20 ng/ml, and/or pre-op Gleason score of 8-10, and/or clinical stage >= T2c |
| European Association of Urology | Pre-op PSA >20 ng/ml, and/or pre-op Gleason score of 8-10, and/or clinical stage >= T3a |
| Radiation Therapy Oncology Group | High: T1-2 and Gleason 8-10, T3 or N1 with GS7 Very High: T3 or N1 with Gleason 8-10 |
| National Comprehensive Cancer Network | High: Pre-op PSA >20 ng/ml, pre-op Gleason score of 8-10, or clinical stage T3a Very High: T3b-T4 |
| Cancer of the Prostate Risk Assessment (CAPRA) | Includes age, PSA, clinical stage, Gleason score, percentage of positive cores |
Box 2.
Multiple trials have been conducted that have established the key principles of management for radiotherapy of clinically localized prostate cancer.
Principle 1: Definitive treatment of the primary tumour is essential: ADT alone is inadequate.
Principle 2: ADT in addition to radiation therapy is associated with superior outcomes compared to radiation therapy alone.
Principle 3: The importance of radiation dose using intermediate endpoints (PSA) to assess treatment effects.
Abbreviations: ADT, androgen deprivation therapy; RTOG, Radiation Therapy Oncology Group.
Box 3.
Key principles for surgical management of localized prostate cancer:
Principle 1: The survival benefit of radical prostatectomy is a function of the patient's risk.
Principle 2: The importance of an extended pelvic lymph node dissection.
Principle 3: Assessing the need for post-operative radiation therapy.
Key Points.
High-risk disease represents a subset of prostate cancer patients who have a significant chance of developing systemic or local recurrence; thus, such patients are at higher risk for symptoms and/or death from prostate cancer.
Definitions vary for what constitutes high-risk disease in localized prostate cancer, but are historically based on clinicopathologic findings including clinical stage, Gleason score, and PSA.
Much of the literature on this subject has been limited by variations in definition, lack of prospective randomized trials, limitations in statistical plan (e.g., underpowered studies), need for long term follow-up, and selection of sub-optimal endpoints.
Several key principles for radiotherapy have been established, including the importance of dose, and the addition of androgen deprivation therapy.
Treatment of potential lymph node involvement, either surgically or with extended pelvic radiation, is favored in high-risk disease, but lacks level I evidence.
Needed: 1) Re-defining high-risk populations and 2) clinical trials implementing novel multi-modality therapy.
Review Criteria.
A formal literature search was performed using the PubMed database and the following terms: “prostate cancer” with “high-risk”, “high-risk diagnosis”, or “high-risk treatment”. Data from randomized clinical trials was preferentially included whenever possible. The authors used their own judgment about which papers to include from the literature search based on the relevance of the article to the clinical scenario. This review also includes a summary of the authors' work and knowledge based on reading the oncology literature as well as guidelines from the American Urological Association (AUA), European Association of Urology (EUA), American Society of Radiation Oncology (ASTRO), and National Comprehensive Cancer Network (NCCN).
References
- 1.Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000;55:323–7. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Amico AV, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 5.Thompson I, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Roach M, et al. Four prognostic groups predict long-term survival from prostate cancer following radiotherapy alone on Radiation Therapy Oncology Group clinical trials. Int J Radiat Oncol Biol Phys. 2000;47:609–15. doi: 10.1016/s0360-3016(00)00578-2. [DOI] [PubMed] [Google Scholar]
- 7.Roach M, 3rd, et al. Defining high risk prostate cancer with risk groups and nomograms: implications for designing clinical trials. J Urol. 2006;176:S16–20. doi: 10.1016/j.juro.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, et al. Percentage of positive biopsy cores: a better risk stratification model for prostate cancer? Int J Radiat Oncol Biol Phys. 2012;83:1141–8. doi: 10.1016/j.ijrobp.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101:878–87. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 12.Yossepowitch O, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493–9. doi: 10.1016/j.juro.2007.03.105. discussion 499. [DOI] [PubMed] [Google Scholar]
- 13.Gosselaar C, Kranse R, Roobol MJ, Roemeling S, Schroder FH. The interobserver variability of digital rectal examination in a large randomized trial for the screening of prostate cancer. Prostate. 2008;68:985–93. doi: 10.1002/pros.20759. [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th. Springer; New York: 2011. [Google Scholar]
- 15.Yakar D, et al. Predictive value of MRI in the localization, staging, volume estimation, assessment of aggressiveness, and guidance of radiotherapy and biopsies in prostate cancer. J Magn Reson Imaging. 2012;35:20–31. doi: 10.1002/jmri.22790. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JQ, Loughlin KR, Zou KH, Haker S, Tempany CM. Role of endorectal coil magnetic resonance imaging in treatment of patients with prostate cancer and in determining radical prostatectomy surgical margin status: report of a single surgeon's practice. Urology. 2007;69:1134–7. doi: 10.1016/j.urology.2007.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Y, et al. Quantitative analysis of multiparametric prostate MR images: differentiation between prostate cancer and normal tissue and correlation with Gleason score--a computer-aided diagnosis development study. Radiology. 2013;267:787–96. doi: 10.1148/radiol.13121454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puech P, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268:461–9. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari P, Kurhanewicz J, Madabhushi A. Multi-kernel graph embedding for detection, Gleason grading of prostate cancer via MRI/MRS. Med Image Anal. 2013;17:219–35. doi: 10.1016/j.media.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooperberg MR, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31:1428–34. doi: 10.1200/JCO.2012.46.4396. [DOI] [PubMed] [Google Scholar]
- 21.Ding Z, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–73. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roach M, 3rd, Waldman F, Pollack A. Predictive models in external beam radiotherapy for clinically localized prostate cancer. Cancer. 2009;115:3112–20. doi: 10.1002/cncr.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durand X, et al. The value of urinary prostate cancer gene 3 (PCA3) scores in predicting pathological features at radical prostatectomy. BJU Int. 2012;110:43–9. doi: 10.1111/j.1464-410X.2011.10682.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin DW, et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res. 2013;19:2442–50. doi: 10.1158/1078-0432.CCR-12-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shore N, et al. Clinical utility of a biopsy-based cell cycle gene expression assay in localized prostate cancer. Curr Med Res Opin. 2013 doi: 10.1185/03007995.2013.873398. [DOI] [PubMed] [Google Scholar]
- 26.Knezevic D, et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics. 2013;14:690. doi: 10.1186/1471-2164-14-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan MJ, Cordon-Cardo C. Predicting high-risk disease using tissue biomarkers. Curr Opin Urol. 2013;23:245–51. doi: 10.1097/MOU.0b013e32835f89cc. [DOI] [PubMed] [Google Scholar]
- 28.Bolla M, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 29.Pilepich MV, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 30.Beckendorf V, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–63. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Creak A, et al. Randomised pilot study of dose escalation using conformal radiotherapy in prostate cancer: long-term follow-up. Br J Cancer. 2013;109:651–7. doi: 10.1038/bjc.2013.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuban DA, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79:1310–7. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Peeters ST, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–6. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 34.Roach M., 3rd Dose escalated external beam radiotherapy versus neoadjuvant androgen deprivation therapy and conventional dose external beam radiotherapy for clinically localized prostate cancer:do we need both? Strahlenther Onkol. 2007;183 Spec No 2:26–8. doi: 10.1007/s00066-007-2011-8. [DOI] [PubMed] [Google Scholar]
- 35.Widmark A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–8. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 36.Warde P, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–11. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–41. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu-Yao GL, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–9. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichol AM, Warde P, Bristow RG. Optimal treatment of intermediate-risk prostate carcinoma with radiotherapy: clinical and translational issues. Cancer. 2005;104:891–905. doi: 10.1002/cncr.21257. [DOI] [PubMed] [Google Scholar]
- 40.Hussain M, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–25. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crook JM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolla M, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz EM, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 44.Nabid A, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized trial. J Clin Oncol; ASCO Annual Meeting; May 31 - June 4, 2013; Chicago, Illinois. 2013. suppl; abstr LBA4510. [Google Scholar]
- 45.Ahmadi H, Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int. 2013;111:543–8. doi: 10.1111/j.1464-410X.2012.11774.x. [DOI] [PubMed] [Google Scholar]
- 46.Cormie P, et al. Can Supervised Exercise Prevent Treatment Toxicity in Prostate Cancer Patients Initiating Androgen Deprivation Therapy: A Randomised Controlled Trial. BJU Int. 2014 doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 47.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32:335–46. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 48.Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med. 2009;24 Suppl 2:S389–94. doi: 10.1007/s11606-009-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Bono JS, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Bono JS, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 51.Fizazi K, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 52.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 53.Parker C, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 54.Ryan CJ, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 56.Zelefsky MJ, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 57.Levegrun S, et al. Risk group dependence of dose-response for biopsy outcome after three-dimensional conformal radiation therapy of prostate cancer. Radiother Oncol. 2002;63:11–26. doi: 10.1016/s0167-8140(02)00062-2. [DOI] [PubMed] [Google Scholar]
- 58.Dearnaley DP, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 59.Sathya JR, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192–9. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 60.Zietman AL, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–73. doi: 10.1016/j.juro.2007.11.063. discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vargas C, et al. High-dose radiation employing external beam radiotherapy and high-dose rate brachytherapy with and without neoadjuvant androgen deprivation for prostate cancer patients with intermediate- and high-risk features. Prostate Cancer Prostatic Dis. 2006;9:245–53. doi: 10.1038/sj.pcan.4500882. [DOI] [PubMed] [Google Scholar]
- 63.D'Amico AV, et al. Risk of death from prostate cancer after brachytherapy alone or with radiation, androgen suppression therapy, or both in men with high-risk disease. J Clin Oncol. 2009;27:3923–8. doi: 10.1200/JCO.2008.20.3992. [DOI] [PubMed] [Google Scholar]
- 64.Fang LC, et al. High-risk prostate cancer with Gleason score 8-10 and PSA level </=15 ng/mL treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2011;81:992–6. doi: 10.1016/j.ijrobp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Stephenson AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eggener SE, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–75. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spahn M, et al. Outcome predictors of radical prostatectomy in patients with prostate-specific antigen greater than 20 ng/ml: a European multi-institutional study of 712 patients. Eur Urol. 2010;58:1–7. doi: 10.1016/j.eururo.2010.03.001. discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 68.Zwergel U, et al. Outcome of prostate cancer patients with initial PSA> or =20 ng/ml undergoing radical prostatectomy. Eur Urol. 2007;52:1058–65. doi: 10.1016/j.eururo.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 69.Klein EA, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179:2212–6. doi: 10.1016/j.juro.2008.01.107. discussion 2216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Begg CB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 71.Valicenti RK, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822–8. doi: 10.1016/j.ijrobp.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 72.Thompson IM, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–9. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 73.Boorjian SA, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–91. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Briganti A, et al. Identifying the best candidate for radical prostatectomy among patients with high-risk prostate cancer. Eur Urol. 2012;61:584–92. doi: 10.1016/j.eururo.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 75.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005;95:751–6. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 76.Yossepowitch O, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. Eur Urol. 2008;53:950–9. doi: 10.1016/j.eururo.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bill-Axelson A, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 78.Wilt TJ, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Briganti A, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–65. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 80.Stone NN, Stock RG, Unger P. Laparoscopic pelvic lymph node dissection for prostate cancer: comparison of the extended and modified techniques. J Urol. 1997;158:1891–4. doi: 10.1016/s0022-5347(01)64161-2. [DOI] [PubMed] [Google Scholar]
- 81.Briganti A, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol. 2006;50:1006–13. doi: 10.1016/j.eururo.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 82.Bader P, Burkhard FC, Markwalder R, Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. 2002;168:514–8. doi: 10.1016/s0022-5347(05)64670-8. discussion 518. [DOI] [PubMed] [Google Scholar]
- 83.Keller H, Lehmann J, Beier J. Radical perineal prostatectomy and simultaneous extended pelvic lymph node dissection via the same incision. Eur Urol. 2007;52:384–8. doi: 10.1016/j.eururo.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 84.Wyler SF, et al. Laparoscopic extended pelvic lymph node dissection for high-risk prostate cancer. Urology. 2006;68:883–7. doi: 10.1016/j.urology.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 85.Liss MA, et al. Outcomes and complications of pelvic lymph node dissection during robotic-assisted radical prostatectomy. World J Urol. 2013;31:481–8. doi: 10.1007/s00345-013-1056-9. [DOI] [PubMed] [Google Scholar]
- 86.Feifer AH, et al. Temporal trends and predictors of pelvic lymph node dissection in open or minimally invasive radical prostatectomy. Cancer. 2011;117:3933–42. doi: 10.1002/cncr.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gandaglia G, et al. The impact of robot-assisted radical prostatectomy on the use and extent of pelvic lymph node dissection in the “post-dissemination” period. Eur J Surg Oncol. 2014 doi: 10.1016/j.ejso.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 88.Yuh B, et al. Eur Urol. 2013 [Google Scholar]
- 89.Weingartner K, et al. Anatomical basis for pelvic lymphadenectomy in prostate cancer: results of an autopsy study and implications for the clinic. J Urol. 1996;156:1969–71. doi: 10.1016/s0022-5347(01)65406-5. [DOI] [PubMed] [Google Scholar]
- 90.Heidenreich A, Ohlmann CH, Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol. 2007;52:29–37. doi: 10.1016/j.eururo.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 91.Wagner M, Sokoloff M, Daneshmand S. The role of pelvic lymphadenectomy for prostate cancer--therapeutic? J Urol. 2008;179:408–13. doi: 10.1016/j.juro.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 92.Touijer KA, Mazzola CR, Sjoberg DD, Scardino PT, Eastham JA. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur Urol. 2014;65:20–5. doi: 10.1016/j.eururo.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 93.Engel J, et al. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol. 2010;57:754–61. doi: 10.1016/j.eururo.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 94.Ji J, Yuan H, Wang L, Hou J. Is the impact of the extent of lymphadenectomy in radical prostatectomy related to the disease risk? A single center prospective study. J Surg Res. 2012;178:779–84. doi: 10.1016/j.jss.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 95.Bolla M, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 96.Bolla M, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 97.Thompson IM, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiegel T, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 99.Thompson IM, Jr, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. Jama. 2006;296:2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 100.Moinpour CM, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26:112–20. doi: 10.1200/JCO.2006.10.4505. [DOI] [PubMed] [Google Scholar]
- 101.Stephenson AJ, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen PL, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. Jama. 2011;306:2359–66. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 104.Punnen S, Cooperberg MR, Sadetsky N, Carroll PR. Androgen deprivation therapy and cardiovascular risk. J Clin Oncol. 2011;29:3510–6. doi: 10.1200/JCO.2011.35.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arcangeli G, et al. Retrospective comparison of external beam radiotherapy and radical prostatectomy in high-risk, clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:975–82. doi: 10.1016/j.ijrobp.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 106.Zelefsky MJ, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–13. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roach M, 3rd, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 108.Zelefsky MJ, et al. Predicting biochemical tumor control after brachytherapy for clinically localized prostate cancer: The Memorial Sloan-Kettering Cancer Center experience. Brachytherapy. 2012;11:245–9. doi: 10.1016/j.brachy.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Kattan MW, et al. Pretreatment nomogram that predicts 5-year probability of metastasis following three-dimensional conformal radiation therapy for localized prostate cancer. J Clin Oncol. 2003;21:4568–71. doi: 10.1200/JCO.2003.05.046. [DOI] [PubMed] [Google Scholar]
- 110.Hsu CC, et al. Feasibility of MR imaging/MR spectroscopy-planned focal partial salvage permanent prostate implant (PPI) for localized recurrence after initial PPI for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:370–7. doi: 10.1016/j.ijrobp.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 111.Chen CP, et al. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Radiat Oncol Biol Phys. 2013;86:324–9. doi: 10.1016/j.ijrobp.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 112.Giordano SH, et al. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–66. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eifler JB, et al. Causes of death after radical prostatectomy at a large tertiary center. J Urol. 2012;188:798–801. doi: 10.1016/j.juro.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 114.Lane JA, et al. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer. 2010;46:3095–101. doi: 10.1016/j.ejca.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 115.Jang TL, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–50. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sommers BD, et al. Predictors of patient preferences and treatment choices for localized prostate cancer. Cancer. 2008;113:2058–67. doi: 10.1002/cncr.23807. [DOI] [PubMed] [Google Scholar]
- 117.Bastian PJ, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61:1096–106. doi: 10.1016/j.eururo.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 118.Bellmunt J, et al. Advances in the management of high-risk localised and metastatic prostate cancer. BJU Int. 2012;109 Suppl 2:8–13. doi: 10.1111/j.1464-410X.2011.10871.x. [DOI] [PubMed] [Google Scholar]
- 119.Gomella LG, et al. Enhancing prostate cancer care through the multidisciplinary clinic approach: a 15-year experience. J Oncol Pract. 2010;6:e5–e10. doi: 10.1200/JOP.2010.000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 121.Jacobs EF, Boris R, Masterson TA. Advances in Robotic-Assisted Radical Prostatectomy over Time. Prostate Cancer. 2013;2013:902686. doi: 10.1155/2013/902686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Turpen R, Atalah H, Su LM. Technical advances in robot-assisted laparoscopic radical prostatectomy. Ther Adv Urol. 2009;1:251–8. doi: 10.1177/1756287210364207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taplin ME, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. J Clin Oncol; ASCO Annual Meeting; June 1-5, 2012; Chicago, Illinois. 2012. suppl; abstr 4521. [Google Scholar]
- 124.Tollefson MK, et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. ASCO Genitourinary Cancers Symposium; March 5-7, 2010; San Francisco, CA. 2010. Abstract 168. [Google Scholar]


