The retromer-associated DNAJ protein Rme-8 is necessary for normal Notch recycling, and reductions in Rme-8 sensitize cells so that additional loss-of-sorting retromer or ESCRT-0 components have catastrophic effects.
Abstract
Notch signaling is a major regulator of cell fate, proliferation, and differentiation. Like other signaling pathways, its activity is strongly influenced by intracellular trafficking. Besides contributing to signal activation and down-regulation, differential fluxes between trafficking routes can cause aberrant Notch pathway activation. Investigating the function of the retromer-associated DNAJ protein Rme-8 in vivo, we demonstrate a critical role in regulating Notch receptor recycling. In the absence of Rme-8, Notch accumulated in enlarged tubulated Rab4-positive endosomes, and as a consequence, signaling was compromised. Strikingly, when the retromer component Vps26 was depleted at the same time, Notch no longer accumulated and instead was ectopically activated. Likewise, depletion of ESCRT-0 components Hrs or Stam in combination with Rme-8 also led to high levels of ectopic Notch activity. Together, these results highlight the importance of Rme-8 in coordinating normal endocytic recycling route and reveal that its absence predisposes toward conditions in which pathological Notch signaling can occur.
Introduction
The highly conserved Notch signaling pathway is a key regulator of many developmental decisions, controlling processes such as proliferation and differentiation. Inappropriate pathway activation is associated with several human diseases, including hematopoietic and solid tumors (Lobry et al., 2011). As a consequence, tight spatiotemporal regulation of Notch activity is essential. Intracellular trafficking is an important element in such regulation, influencing steps involved in signal activation as well as in preventing inappropriate signaling (Brou, 2009).
In canonical signaling, Notch is activated by ligands of the DSL (Delta [Dl], Serrate, Lag-2) family presented by neighboring cells. Upon ligand binding, Notch undergoes two consecutive proteolytic cleavages, catalyzed by an ADAM (A Disintegrin And Metalloproteinase) metalloprotease (S2 cleavage) and the γ-secretase complex (S3 cleavage). The latter releases the Notch intracellular domain (NICD) from the membrane, which translocates to the nucleus, where it regulates transcription of a variety of target genes (Bray, 2006). Signaling is highly sensitive to levels, localization, and modifications of the membrane proteins, which in turn are dependent on vesicular trafficking routes. For example, regulated trafficking is important for active ligand function and for turnover of inactive Notch receptors (Brou, 2009). In addition, ligand-independent trafficking of the full-length receptor to the late endosome and lysosome can result in activation (Fortini and Bilder, 2009). In Drosophila melanogaster, the ring finger protein Deltex (Dx) is one factor that promotes signaling via this alternate late-endosomal mechanism, which, like ligand induced activation, relies on γ-secretase–mediated cleavage (Baron, 2012).
For endocytosed cargoes to reach the correct final destination, they have to be sorted into distinct endosomal compartments (e.g., Fig. S3 K). A significant fraction of endocytosed Notch is transferred into intraluminal vesicles (ILVs) of so-called multivesicular bodies (MVBs), destined for degradation once MVBs fuse with lysosomes (Henne et al., 2011). The sorting and internalization of cargoes into ILVs is performed by endosomal sorting required for transport (ESCRT) complexes. Mutations affecting this process result in Notch being retained in the limiting membrane of MVBs where conditions facilitate proteolytic release of NICD, resulting in ectopic signaling (Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Vaccari et al., 2008; Hori et al., 2011)
Besides being routed to lysosomes for degradation, internalized proteins can be recycled back to the plasma membrane or transferred to the Golgi. Early endosomes, marked by Rab5 GTPase, are the hub where internalized cargoes are sorted into different domains depending on their destination. For example, Rab4 marks a tubular subdomain of early endosomes that is involved in rapid recycling of cargoes to the plasma membrane. Cargoes destined for recycling are concentrated in tubular extensions through the actions of complexes, including retromer (Jovic et al., 2010). First identified as a result of its role in retrograde transport from early endosomes to trans-Golgi, retromer is also involved in direct endosome-to-plasma membrane transport of proteins such as the β2-adrenergic receptor (Seaman, 2012; Seaman et al., 2013). It consists of two subcomplexes, a cargo-sorting subcomplex, composed of Vps35/Vps26/Vps29, and a sorting nexin subcomplex, which promotes tubulation (McGough and Cullen, 2011). The associated proteins include the Rme-8 (receptor-mediated endocytosis-8), a member of the DNAJ (J-domain containing) protein family of co-chaperones, whose absence leads to highly branched endosomal tubules and missorting of cargoes (Popoff et al., 2009; Shi et al., 2009; Freeman et al., 2014). Indeed, pathogenic mutations in Rme-8 and in Vps35 have been found in some instances of familial Parkinson’s disease, underscoring the importance of endosomal recycling pathways (Vilariño-Güell et al., 2011, 2014).
First identified as a protein required for endocytosis in Caenorhabditis elegans (Girard et al., 2005), evidence now indicates that Rme-8 is primarily involved in postendocytic transport steps. Although the Hsc40-binding DNAJ domain is the only obvious feature of its sequence, biochemical studies have shown that the mammalian protein can bind to retromer components (SNX1) and WASH complex (the major endosomal actin polymerization-promoting complex) through other parts of the protein (Popoff et al., 2009; Shi et al., 2009; Freeman et al., 2014). Rme-8 may therefore link together requisite elements of the transport machinery in endocytic tubules (Freeman et al., 2014), but it remains to be established what role the DNAJ domain or Hsc40 play in this process.
Although previous studies have not detected a requirement for retromer in normal Notch function (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pocha et al., 2011), our small scale screen for factors influencing Notch, along with larger screens by others (Le Bras et al., 2012), identified Rme-8 as a potential regulator. Therefore, we have investigated here the role of Rme-8 in Notch trafficking and in the organization of the endosomal network in vivo. Our results indicate that Rme-8 is necessary for normal Notch recycling. Furthermore, they reveal that reductions in Rme-8 sensitize the cells so that additional loss of sorting retromer or ESCRT-0 components have catastrophic effects. The fact that such synthetic combinations result in ectopic Notch activity and tissue overgrowth has major implications in the context of human disease mutations affecting Rme-8 and related genes.
Results
Rme-8 is a positive regulator of the Notch pathway
In a small-scale genetic screen, using an RNAi (Ri) collection against known trafficking proteins, we identified the DNAJ protein Rme-8 as a positive modulator of the Notch pathway. Rme-8 knockdown at late stages of wing development (using salEPv-Gal4 to express Rme-8 Ri; Fig. 1 A; Cruz et al., 2009) gave rise to wings with thickened veins (L2 and L3) and, occasionally, small deltas (Fig. 1 C, arrows). This phenotype is reminiscent of reduced Notch function as illustrated by phenotypes produced when the Notch pathway transcription factor, Su(H), was perturbed using the same conditions (Fig. 1 D). Additional defects, such as posterior vein loss and more severe changes in wing morphology were also observed, indicating that Rme-8 has Notch-independent roles (Fig. 1 C, arrowhead). However, the penetrance of the vein thickening phenotypes suggests that the Notch pathway may be particularly sensitive to Rme-8 depletion.
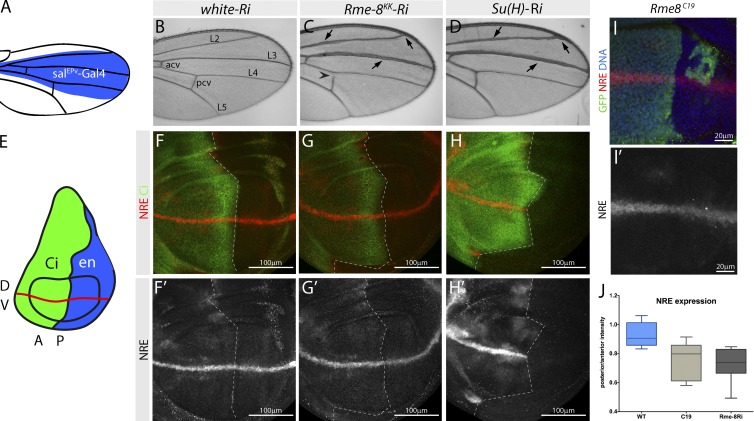
Figure 1.
Rme8 regulates Notch pathway function. (A) salPEv-Gal4 expression domain in an adult wing. (B–D) Adult wing from flies expressing w (B), Rme-8 (C), or Su(H) (D) Ri with salPEv-Gal4. Rme-8 knockdown results in vein thickening and small Dl’s, similar to Su(H)-Ri treatment (arrows). Also note loss of posterior cross-vein (arrowhead; Pcv). L2–5 veins; Acv, anterior cross-vein. (E) en-Gal4 expression domain in the posterior (P, blue) wing disc, as used in this and all subsequent knockdown experiments (F–H′) NRE-mCherry expression in wing discs from flies expressing w (F), Rme-8 (G), or Su(H) (H) Ri (right of dashed line). Rme-8-Ri slightly reduced NRE expression, Su(H)-Ri causes loss of NRE expression. Cubitus interruptus (Ci) marks the unaffected anterior (A) compartment in this and all subsequent experiments. (I and I′) NRE-mCherry expression is decreased in Rme-8 (Rme-8c19) mutant cells (GFP negative). Mutant clones were generated using FRT42D PCNA, Ubi-GFP; hh-flp. (J) Box plot of anterior/posterior pixel intensity ratio, measured in comparable posterior and anterior regions of control, RmeC19 mutant (C19; n = 7), and Rme-8 knockdown (Rme-8Ri, n = 12) discs. Boxes indicate 25th to 75th percentiles, whiskers represent maximum and minimum values in the dataset, and lines show the median values.
To verify that Rme-8 depletion affected Notch activity, we assessed the consequences on a Notch responsive element (NRE) reporter, NRE-mCherry, when Rme-8-Ri was expressed in the posterior domain of wing imaginal discs (using engrailed [en]-Gal4; Fig. 1 E). Normally, NRE-mCherry is strongly expressed in a stripe along the dorsoventral (D/V) boundary with lower levels in flanking inter-vein regions (Fig. 1 F; Housden et al., 2012). Knockdown of Rme-8 (Fig. S1 A) led to reduced NRE-mCherry expression, both at the D/V boundary and in surrounding regions (Fig. 1, G and J), although to a substantially lesser extent than depletion of Su(H) in the same way (Fig. 1 H). In these (and subsequent experiments), similar phenotypes were obtained using two independent Ri lines that target different regions in Rme-8 transcripts (Rme-8KK Ri and Rme-8GD Ri, the former gave slightly stronger phenotypes). Likewise, NRE-mCherry expression was reduced in clones of Rme-8 mutant cells, generated with a previously described x ray–induced loss-of-function allele (Rme-8C19; Fig. 1, I and J; Chang et al., 2004). Together, these data indicate that Rme-8 is required for full Notch pathway activity and that it normally acts as a positive regulator of Notch function.
Aberrant Notch and Wingless (Wg) localization in the absence of Rme-8
Because Rme-8 is an evolutionary conserved endosomal-associated protein (Zhang et al., 2001; Chang et al., 2004; Girard et al., 2005; Fujibayashi et al., 2008; Girard and McPherson, 2008; Popoff et al., 2009; Shi et al., 2009), it likely affects Notch signaling by altering trafficking of key pathway components. We therefore investigated the consequences of Rme-8 knockdown on the distribution of Notch and its ligand, Dl. Both proteins accumulated together in enlarged intracellular puncta in Rme-8–depleted territories (Fig. 2 A, arrowheads), demonstrating that Rme-8 is required for proper Notch and Dl trafficking. Concomitant with the increase in intracellular accumulation, their levels at the plasma membrane were decreased (Fig. 2, A–D; and Fig. S1 B). Furthermore, the intracellular puncta contained both intracellular (NICD) and extracellular domains of Notch (Notch extracellular domain [NECD]), suggesting that the uncleaved form of the receptor accumulates upon Rme-8 depletion (Fig. 2, B–B″, arrowheads). Similar intracellular Notch puncta were also present in Rme-8 mutant cells (Fig. 2 E, Rme-8C19; and Fig. S1 C, Rme-8A9). Although both Notch and Dl were affected, the reduced signaling appeared autonomous to Rme-8–depleted cells, suggesting that the receptor is particularly compromised (rather than the ligand). Phenotypes in the ovary also indicated a requirement for Rme-8 in signal receiving follicle cells rather than the signal-sending oocyte (unpublished data) arguing that the primary defect arises from changes to Notch function.
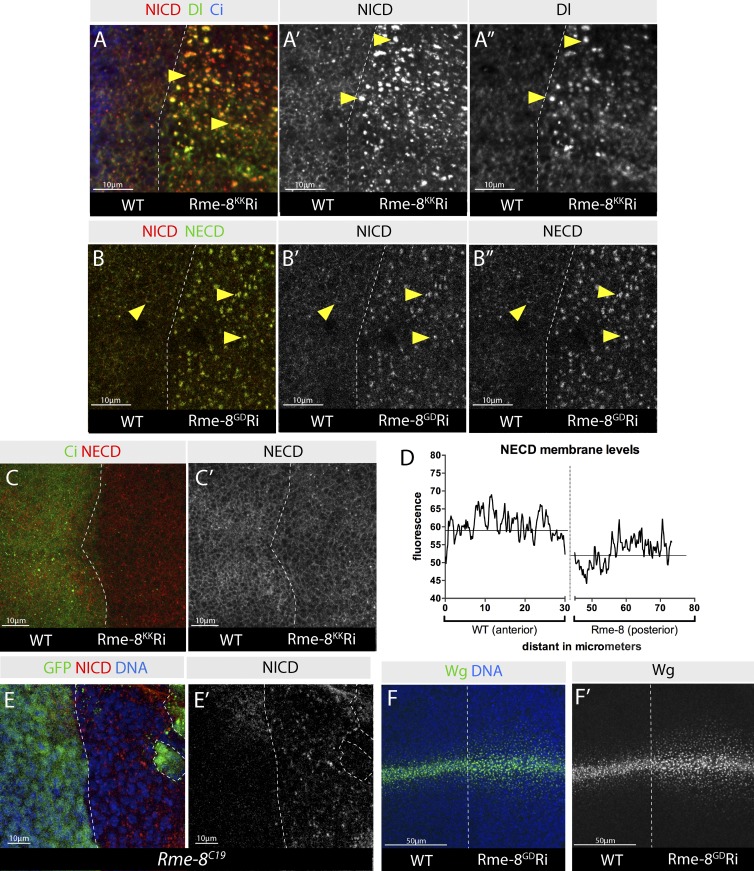
Figure 2.
Aberrant trafficking of Notch, Dl, and Wg in Rme-8–depleted cells. (A–B″) Effects of Rme-8 Ri on Notch (anti-NICD, red in A and B; anti-NECD, green in B) and Dl (green in A) trafficking. Both, full-length Notch and Dl accumulate together inside Rme-8-Ri cells (arrowheads). Depleted territory in this and subsequent figures is on the right side of dashed line, unless stated otherwise. (C and C′) NECD (staining without detergent) is depleted from the membrane when Rme-8 is knocked down (Rme-8KK). (D) Quantification of surface NECD levels from C. The data shown are from a single representative disc; n = 6 for the experiment shown; the experiment was repeated three times with similar results. Line shows mean fluorescence at the membrane level within the specified compartment. (E and E′) Intracellular Notch puncta accumulate in Rme-8C19 mutant cells (GFP negative, marked by dashed line; generated as in Fig 1 I). (F and F′) Wg is detected more broadly in the Rme-8-Ri tissue.
Although adult phenotypes were reminiscent of Notch loss of function, Rme-8 is likely to have broader effects on endocytosis. In agreement, Wg distribution was also altered in Rme-8–depleted discs. Notably, Wg was detected at a broad range from its site of synthesis (Fig. 2 F) and accumulated within cells at a distance from the Wg-producing cells (Fig. S1 D). These consequences were similar to those reported for Hrs loss of function (Piddini et al., 2005; Rives et al., 2006) and suggest that Wg trafficking within receiving cells becomes compromised when Rme-8 is depleted. In contrast, Wg secretion appeared relatively normal. First, the distribution of extracellular Wg in Rme-8–depleted tissue was similar to wild type (WT; Fig. S1 E). Second, senseless, a gene whose expression requires high levels of Wg signaling (Nolo et al., 2000) was still expressed in Rme-8–depleted cells (Fig. S1, F and G). These data argue that Wg secretion, which relies on retrograde recycling of its chaperone, Wntless, via a retromer-dependent route to the Golgi (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pan et al., 2008; Port et al., 2008; Yang et al., 2008; Harterink et al., 2011; Zhang et al., 2011), was still functional in Rme-8–depleted cells, although other aspects of Wg trafficking were perturbed. As the adult phenotypes from Rme-8 depletion are, however, most allied to deficits in Notch signaling, subsequent analysis was focused on the consequences on Notch trafficking and function.
Rme-8 regulates Notch trafficking
One strategy to investigate the stage at which trafficking is arrested is to follow the uptake of receptor-bound antibodies in live tissues (Le Borgne and Schweisguth, 2003). Using an antibody against NECD, we tracked the intracellular route by monitoring the location of antibody–Notch complexes at different times after the unbound antibody was washed out. First, we established mild conditions of Rme-8 knockdown, so that, at time 0, there were similar levels of the α-NECD antibody bound at the cell surface in control and Rme-8–depleted compartments (Fig. 3, A, D, and I). We then transferred tissues to RT and analyzed changes in distribution.
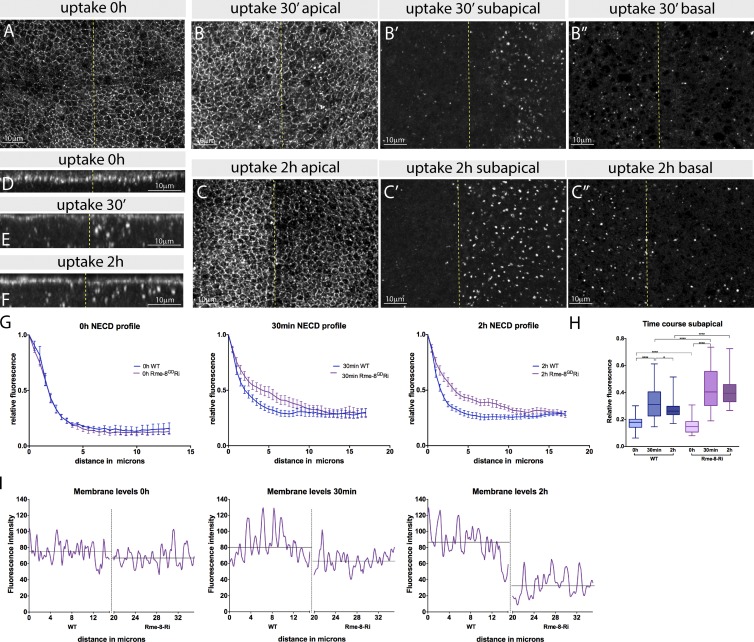
Figure 3.
Notch accumulates in subapical puncta in Rme-8–depleted cells. (A–C″) Antibody uptake assay to detect Notch trafficking (anti-NECD) in cultured wing discs. Different focal planes (apical [A, B, and C]; subapical [B′ and C′]; basal [B″ and C″]) from xy sections at 0 min (A), 30 min (B), or 2 h (C) after uptake. (D–F) xz cross sections of discs in A, B, and C, respectively. Notch–antibody complexes accumulate subapically in Rme-8-Ri (right) after 30 min but accumulate more basally in WT (left). (G) Quantification of fluorescence levels from Notch–antibody complexes according to distance from apical membrane in xz cross sections (apical levels were normalized to 1 for comparisons between samples). At 30 min and 2 h, more anti-NECD is detected in subapical regions with Rme-8-Ri compared with WT. Error bars represent the SEM. (H) Comparison between subapical levels of anti-NECD detected at different time points in wild-type (WT) and Rme-8 Ri tissue. Boxes indicate 25th to 75th percentiles, whiskers represent maximum and minimum values in the dataset, and lines show the median values. Asterisks indicate significance according to a one-way analysis of variance test; *, P = 0.05–0.01; ****, P < 0.0001. (I) Quantification of apical membrane anti-NECD in anterior (left, WT) versus posterior (right, Rme-8 Ri) of discs in A–C. The data shown are from a single representative disc; n = 10 for the experiment shown; the experiment was repeated two times with similar results. Lines show mean fluorescence at the membrane level within the specified compartment.
After 30-min internalization, large amounts of the Notch–antibody complex were already accumulated in subapical puncta in Rme-8–depleted regions (Fig. 3, B, E, G, and H). After 2 h, these subapical puncta were more pronounced (Fig. 3, C′ and F–H), and the amount of Notch–antibody complex at the cell surface was reduced in comparison to neighboring WT tissue (Fig. 3, C and I). At this time, the internalized complexes in WT tissue were enriched basally (Fig. 3, C″ and F) and most likely corresponded to a prelysosomal compartment. The results therefore demonstrate that Notch is internalized in Rme-8–depleted tissue and accumulates in an apical compartment. In support, accumulation of Notch was prevented by combined knockdown of Rme-8 with avalanche (Fig. S3 H), which is required at the earliest stages of endocytic trafficking (Lu and Bilder, 2005). The decrease in antibody-associated Notch at the membrane at 30 min and 2 h (Fig. 3 I) suggests either that Notch is endocytosed more rapidly in the absence of Rme-8 or that there is a defect in recycling of Notch back to the plasma membrane after endocytosis. Given the previously observed association of Rme-8 with endosomes (Chang et al., 2004; Freeman et al., 2014), the latter seems more likely.
Notch trafficking is regulated by E3-ubiquitin ligases including Dx and Suppressor of Dx (Su(dx); Hori et al., 2004; Wilkin et al., 2004; Yamada et al., 2011). For example, overexpression of Dx modifies the destination of Notch, causing ectopic activation detectable by ectopic NRE-GFP expression and tissue overgrowth (Fig. S2 A; Hori et al., 2004; Yamada et al., 2011). However, neither ectopic Notch activation nor overgrowth phenotypes were reduced when Rme-8 was depleted in Dx-overexpressing discs (Fig. S2 B), suggesting that they operate on different trafficking routes. In addition, Notch accumulations were detected throughout the cell, not just subapically as in Rme-8 knockdown (Fig. S2, C–F). In converse experiments, Dx knockdown led to membrane accumulation of Notch, whereas the phenotype in combination with Rme-8-Ri appeared to be an aggregate (Fig. S2, G–L). Likewise, similar experiments combining Rme-8-Ri with Su(dx) overexpression also yielded an aggregate of phenotypes (Fig. S2, M–P). Together, these results suggest that the pools of Notch modified by Dx and Su(dx) are likely to be separate from those trapped by Rme-8 knockdown.
Rme-8 depletion results in enlarged endosomes, where Notch accumulates in proximity to Rab4, and alters Notch dynamics at the membrane
To investigate where in the endocytic network Notch accumulated when Rme-8 was depleted, tissues were co-stained for Notch and endosomal markers (summarized in Fig. S3, G and K). First, Rab5 puncta showed little change in Rme-8–deficient cells, indicating that early endosomes form normally, and relatively few Rab5 puncta were associated with Notch (Fig. S3 A). Second, markers of maturing endosomes, Rab7 and Hrs, were present in larger aggregates than in WT (Fig. 4 A and Fig. S3 B) that were found in proximity to large Notch accumulations, although there was limited colocalization. Third, Notch puncta were also labeled by the phosphatidylinositol 3-phosphate sensor FYVE-GFP and by the FYVE domain–containing protein SARA (Smad anchor for receptor activation), indicating that Rab5-mediated recruitment of phosphatidylinositol-3-kinase had occurred (Fig. S3, C and E). The size of SARA puncta was also increased. Finally, the endolysosomal protein, Lamp1, was detected in enlarged structures, although showed relatively low colocalization with Notch (Fig. S3 F). These results indicate that loss of Rme-8 affects maturing endosomes and that, although Notch is associated with these, it is primarily confined to a domain distinct from those marked by Hrs or Rab7 (Fig. S3 K).
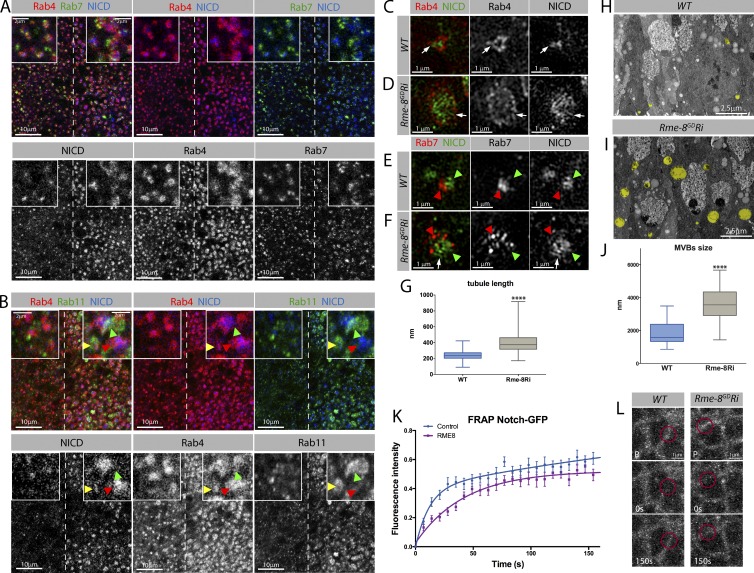
Figure 4.
Notch associates with enlarged endosomes and recovers more slowly at the membrane in Rme-8–depleted cells. (A) Enlarged puncta containing NICD, Rab4, and Rab7 are detected in Rme8-Ri, where NICD accumulates in proximity to Rab4 (see insets). (B) Enlarged puncta containing NICD, Rab4, and Rab11 were detected in Rme-8-Ri cells. Some puncta contain all three proteins (insets, yellow arrowheads), others contain Rab11 only (insets, green arrowheads) or Rab4+NICD (insets, red arrowheads). (C–F) Superresolution images of endosomes from WT (C and E) or Rme-8-Ri (D and F) wing disc cells stained for NICD and Rab4 (C and D) or Rab7 (E and F). Notch localizes adjacent to Rab4 in protrusions (arrows, C). In Rme-8–depleted cells, tubule-like protrusions appear more extensive (arrows, D). Rab7 labels a compartment adjacent to NICD accumulations, both in WT and Rme-8-Ri cells (arrowheads, E and F). (G) Measurements of protruding Rab4-labeled tubule-like structures on endosomes from WT and Rme-8 knockdown (≥50 tubules/disc; 5 discs per genotype). Asterisks indicate significance according to Mann–Whitney test: ****, P < 0.0001. (H and I) Sections from WT (H) or Rme-8-Ri (I) regions of a wing disc analyzed by TEM, with endosomes highlighted in yellow. (J) Quantification of endosome perimeters from TEM images. (WT n = 28; Rme-8-i+w-Ri n = 76.) Asterisks indicate significance according to t test; ****, P < 0.0001. (K and L) FRAP experiments, measuring recovery of Notch-GFP after photobleaching. (K) Plot of averaged recovery curves (mean ± SEM) with best-fit curves as solid lines. Boxes indicate 25th to 75th percentiles, whiskers represent maximum and minimum values in the dataset, and lines show the median values. (L) Single FRAP examples with red circles showing bleach spots. (P, prebleach). The recovery curves could be described by a biexponential equation (WT, P < 0.0001; Rme-8, P = 0.02; Bulgakova et al., 2013; see Materials and methods) suggestive of two recovery processes, fast diffusion within the membrane and slower endocytic trafficking. Both appear affected by Rme-8 depletion.
Other endosomal subdomains include those involved in Rab4- and Rab11-dependent recycling to the plasma membrane (Sönnichsen et al., 2000; Li et al., 2008; Grant and Donaldson, 2009). As with other markers, Rme-8 depletion resulted in enlarged Rab4 and Rab11 puncta, which largely segregated into distinct domains (Fig. 4 B). The most marked effect was with Rab4, which also exhibited the most intimate relationship with Notch (Fig. 4, A and B). The retromer Vps26 subunit showed a similar colocalization (Fig. S3 D). Altogether, the results support a model in which the absence of Rme-8 generates maturing endosomes that are comprised of an array of enlarged subdomains. The preferential relationship between Notch, Rab4, and Vps26 in this enlarged structure suggests that the receptor is trapped in a recycling compartment (Fig. S3 K).
Using 3D structured illumination microscopy, it was possible to further resolve the endosomal structures containing Notch. In WT cells, Notch associated with Rab4-labeled endosomal structures with a perimeter of ∼2.5–3.5 µm (Fig. 4 C). Elongated Rab4- and/or Notch-labeled structures extended out from and across each endosome, consistent with them forming part of the tubular endosomal network (e.g., Fig. 4 C, arrows). Punctate Rab7 was normally segregated into an adjacent domain, although occasionally Rab7 and Notch puncta were directly juxtaposed (Fig. 4 E, arrowheads). After Rme-8 depletion, Notch and Rab4 were present in enlarged structures ∼4–6 µm in perimeter (Fig. 4 D). A punctuated circumferential ring of Rab4 was usually evident but was larger in Rme-8–depleted tissues, and the protrusions, likely tubules, were more extensive (Fig. 4, D and G). Alternating segments of Rab4 and Notch were detected in several of these, suggesting they are present in the same protrusions (Fig. 4 D, arrows). Rab7 was also associated with the same large structures but was largely segregated from Notch (Fig. 4 F, arrowheads). Altogether, these results indicate that Notch accumulates preferentially in Rab4 positive compartments and show that the endosomes detected after Rme-8 depletion are enlarged with more extensive tubule-like structures than in WT. A similar increase in highly branched endosomal tubules occurred in Rme-8–depleted HeLa cells (Freeman et al., 2014).
Enlarged maturing endosomes were also detected by transmission EM (TEM; Fig. 4, H–J; and Fig. S4). Sectioning the discs to directly compare WT and Rme-8–depleted tissue showed that the mean size of MVBs (identified by the presence of ILVs) was 2–3× greater than in WT (Fig. 4 J and Fig. S4, D, H, and I). Although the TEM did not capture the tubular morphology suggested by the superresolution images, the size of the structures observed was similar using the two methods (2.5–4-µm perimeter). Thus, it appears that in the absence of Rme-8, the vacuolar endosome and associated tubular network become greatly enlarged.
The localization experiments show that a significant proportion of Notch associates with Rab4 when Rme-8 is reduced. One explanation is that Rme-8 is necessary for normal recycling of Notch and that, when Rme-8 is missing, Notch is trapped in a recycling domain of the sorting endosome. To investigate further, we monitored the dynamics of Notch protein at the membrane via FRAP (Fig. 4, K and L) using a functional Notch-GFP fusion protein (Couturier et al., 2012). Small circular regions along apical cellular junctions were bleached, (Fig. 4 L, red circles) and the reappearance of unbleached Notch-GFP was measured in WT and Rme-8–depleted cells. The recovery with time was significantly altered in Rme-8–depleted cells compared with WT controls (Fig. 4 K). These data demonstrate that the dynamics of Notch proteins at the membrane are reduced. Furthermore, the Notch-GFP recovery was also curtailed by treating the tissues with the dynamin inhibitor dynasore, indicating that endocytic trafficking contributes to the recovery (Fig. S3, I and J). However, these results do not unequivocally demonstrate that recycling is affected in the Rme-8–depleted cells, as several factors can contribute to recovery and the experiments are not able to distinguish direct from indirect effects. Nevertheless, as Notch becomes trapped with Rab4 and Vps26 in the Rme-8–depleted cells, it is likely that the reduced recovery of Notch-GFP reflects, in part, a defect in endocytosis-mediated recycling
Combined knockdown of Vps26 and Rme-8 leads to ectopic Notch activity
Many recycling pathways involve the retromer complex, including the Rab4-associated route to the plasma membrane (Temkin et al., 2011; Seaman, 2012; Seaman et al., 2013; Steinberg et al., 2013; Wang et al., 2014). Furthermore, Rme-8 localizes at places where retromer-mediated sorting occurs (Popoff et al., 2009; Shi et al., 2009; Freeman et al., 2014). To investigate whether Notch trafficking defects in Rme-8–depleted cells involve retromer, we analyzed the consequences from depleting Vps26.
First, we monitored consequences on NRE-mCherry when Vps26 was depleted either alone or together with Rme-8. Vps26 single knockdown had little impact on NRE-mCherry expression (Fig. 5 A), indicating that Notch activity was not substantially changed by retromer depletion as reported previously (Franch-Marro et al., 2008). Strikingly, however, the combined knockdown of Vps26+Rme-8 resulted in NRE expression throughout the domain, indicating that this combination results in strong ectopic Notch activation (Fig. 5 B). The reduced Vps26 protein levels (Fig. S5 A) along with the accumulation of Wg (Fig. 5 C), whose secretion depends on retrograde transport of Wntless in a retromer-dependent manner, confirmed the efficacy of Vps26 knockdown (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pan et al., 2008; Port et al., 2008; Yang et al., 2008; Harterink et al., 2011; Zhang et al., 2011). The slight broadening of Wg expression in Vps26+Rme-8 combined knockdowns is consistent with the ectopic NRE-mCherry expression in this genotype (Fig. 5 D) because wg is a Notch target. Mutations affecting a second component of the retromer complex, Vps35, had a similar effect to Vps26 Ri. Thus, Rme-8 knockdown in Vps35 mutant cells resulted in ectopic expression of deadpan, a HES (Hairy and Enhancer of split) gene directly regulated by Notch in this tissue (Fig. S5, B–D).
Figure 5.
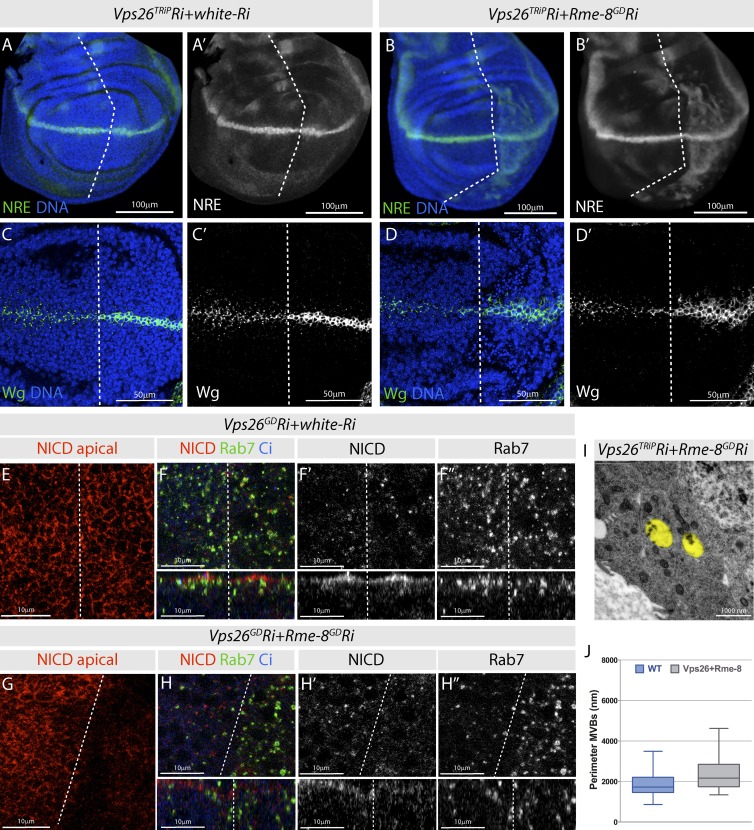
Combined depletion of Rme-8 with Vps-26 results in ectopic Notch activation. (A–B′) NRE-mCherry is ectopically expressed in Vps26-Ri;Rme8-Ri (B) compared with Vps26-Ri;w-Ri (A). (C–D′) Wg accumulates inside both producing and receiving cells in Vps26-Ri;Rme8-Ri (D) but only in producing cells in Vps26-Ri;w-Ri (C). (E–H″) NICD and Rab7 distribution in Vps26-Ri;w-Ri (E and F) or Vps26-Ri;Rme-8-Ri (G and H) wing discs. Apical (E and G) and subapical focal planes (F and H) with xz cross sections from discs in F and H beneath. (I and J) TEM images of Vps26-Ri;Rme8-Ri (I) with endosomes colored in yellow, and quantification of endosomal perimeter from TEM images (J; WT n = 83; Vps26-Ri+Rme-8-Ri n = 30). Boxes indicate 25th to 75th percentiles, whiskers represent maximum and minimum values in the dataset, and lines show the median values.
In agreement with the minimal effects on NRE-mCherry expression, there was little consequence on Notch intracellular distribution or Notch membrane levels after depletion of Vps26 alone (Fig. 5, E and F). However, membrane Notch levels were reduced in Vps35 mutant cells, suggesting that retromer does contribute to Notch trafficking (Fig. S5 E). In addition, Vps26 depletion had pronounced effects in the context of reduced Rme-8 (Fig. 5, G and H). The enlarged Notch puncta, characteristic of Rme-8 depletion, were absent in the combined Rme-8; Vps26 knockdown (Fig. 5 H). The levels of apical, membrane-associated, Notch were also very strongly reduced in this condition (Fig. 5 G). Moreover, the Rab7 puncta localized more basally, although they were still slightly enlarged compared with WT (Fig. 5 H″). Finally, the TEM suggests that the Vps26 depletion partially suppressed the MVB enlargement caused by loss of Rme-8 (Fig. 5, I and J; and Fig. S4, G–I).
In the absence of Vps26 and Rme-8, it appears that Notch is neither recycled to the membrane nor trapped in enlarged endosomes. This fits with the cargo-sorting role of Vps26 and suggests that, in its absence, Notch fails to become concentrated into a retromer-dependent recycling domain. Instead, Notch must be redirected into other endosomal routes, possibly the endolysosomal pathway where ectopic activation can occur if Notch remains in the limiting membrane (Wilkin et al., 2004, 2008; Hori et al., 2011).
Removal of ESCRT-0 components in combination with Rme-8 results in ectopic Notch activation and hyperplasic growth
Proteins destined for lysosomal degradation become segregated into ILVs through the activity of four ESCRT complexes (Hurley and Emr, 2006). The first, the ESCRT-0 complex containing Hrs (hepatocyte growth factor–regulated tyrosine kinase substrate) and Stam (signal transducing adaptor molecule), sorts ubiquitinylated cargoes destined for ILVs into clathrin-coated patches. In addition, Hrs has been suggested to act antagonistically toward complexes containing Rme-8 and retromer to coordinate sorting functions on early endosomes (Popoff et al., 2009). We therefore analyzed the consequences when Hrs or Stam were depleted in combination with Rme-8.
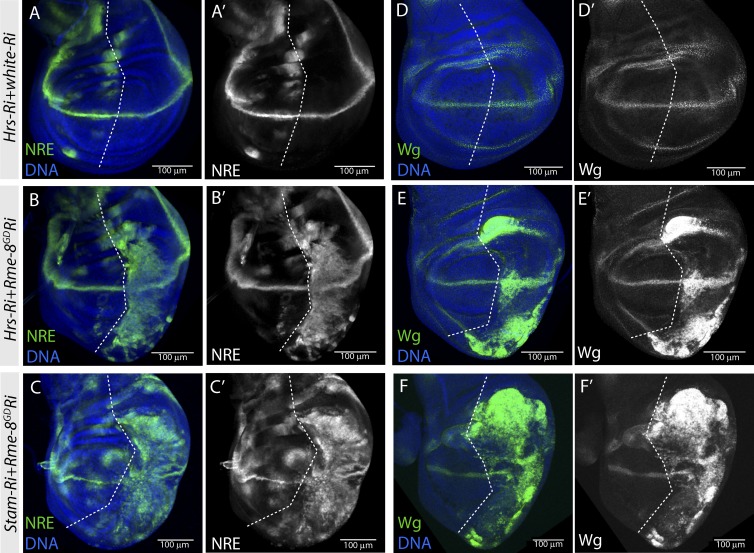
First, we tested effects on NRE-GFP expression. As reported (Vaccari et al., 2008), this showed little change after knockdown of either ESCRT-0 (Fig. 6 A). Surprisingly, when either Hrs or Stam were depleted together with Rme-8, NRE expression was strongly up-regulated, indicative of extensive ectopic Notch activation (Fig. 6, B and C). This was even greater than that seen with the Vps26 combination and was accompanied by concomitant tissue overgrowth, similar to the hyperplasia observed when other endocytic regulators were compromised (Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Childress et al., 2006; Morrison et al., 2008; Vaccari et al., 2008, 2009). In general, the effects were stronger in the Stam;Rme-8 combination than in Hrs;Rme-8 (Fig. 6, B and C), possibly reflecting differences in depletion efficiencies for Stam and Hrs Ri lines, although the latter was nevertheless sufficient to cause substantial reduction in Hrs protein levels (Fig. S5 F).
Figure 6.
Combined depletion of Rme-8 with ESCRT-0 results in ectopic Notch activation and tissue overgrowth. (A–F′) NRE-GFP (A–C) and Wg (D–F) expression are strongly up-regulated in Hrs+Rme-8 double knockdown (B and E) and Stam+Rme8 double knockdown (C and F), in contrast to the Hrs single knockdown where expression is unaffected (A and D, Hrs-Ri;w-Ri).
The outcome on wg expression was similar to that on NRE. In Hrs or Stam single knockdown, more Wg accumulated inside receiving cells, suggesting that steady-state levels are affected by the amount of protein routed to lysosomes. However, there was no expansion of the expression domain (Fig. 6 D). In contrast, when Rme-8 was depleted together with Hrs or Stam, there was widespread ectopic expression of Wg, similar to NRE (Fig. 6, E and F). Finally, ectopic NRE-GFP expression was also seen when Rme-8 Ri was expressed in Hrs mutant clones (Fig. S5 G). Thus, the removal of Rme-8 uncovers a profound requirement for ESCRT-0 to prevent ectopic Notch activation, which is not evident under normal conditions.
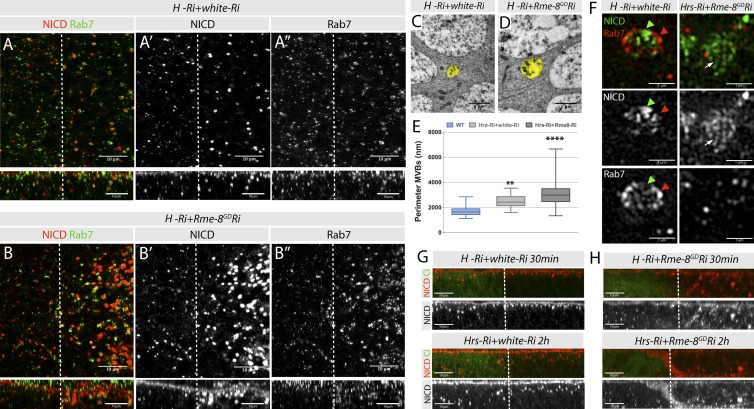
Several studies show that ectopic activation can occur when Notch is trapped at the limiting membrane of mature endosomes marked by Rab7 (Wilkin et al., 2004, 2008; Hori et al., 2011; Schneider et al., 2013). As reported previously, in cells depleted for Hrs alone, Notch accumulated in large Rab7 puncta (Fig. 7, A and F). When Hrs and Rme-8 were knocked down simultaneously, Notch puncta size increased (Fig. 7 B), and larger Notch-stained structures were detected by superresolution microscopy (Fig. 7 F). However, the Notch-enriched regions in the combined Hrs;Rme-8 knockdown were not more coincident with Rab7 (Fig. 7 B) than in the WT or Hrs-depleted tissue (Fig. 4, A and E; and Fig. 7 A), and the superresolution images from Hrs;Rme8 tissues did not reveal any increase in overlap between Rab7 and the large Notch conglomerates. Indeed, Rab7 was frequently present in dispersed clusters of diffuse small puncta (Fig. 7 F). Finally, the MVBs detected by TEM in this combination were larger than with Hrs knockdown alone (Fig. 7, C–E), but the mean perimeter was not significantly different from Rme-8-Ri alone (Fig. S4), suggesting that the MVB is not further enlarged in the double combination. One possibility is that, although the vacuolar endosome/MVB is not enlarged, the Notch tubulated region is more extensive in Hrs-Ri+Rme-8-Ri than in Rme-8-Ri alone, as suggested by the size of the Notch-stained structures in the superresolution images (Fig. 7 F, arrows).
Figure 7.
Effects on Notch trafficking of combined Rme-8 and ESCRT-0 depletion. (A–B″) Distribution of NICD and Rab7. xy section of discs at the subapical plane (top) and xz cross sections of the same specimens (bottom). Notch accumulates in enlarged Rab7 structures upon Hrs knockdown (A; Hrs-Ri+w-Ri) but not in double knockdown (B; Hrs-Ri+Rme8-Ri). (C and D) TEM images of Hrs-Ri;w-Ri (C) or Hrs-Ri;Rme8-Ri (D) with endosomes colored in yellow. (E) Quantification of endosome perimeters from TEM images (WT n = 83, Hrs-Ri+w-Ri n = 33, and Hrs-Ri+Rme-8Ri n = 42). Boxes indicate 25th to 75th percentiles, whiskers represent maximum and minimum values in the dataset, and lines show the median values. Asterisks indicate significance according to one-way analysis of variance test; **, P = 0.01–0.001; ****, P < 0.0001. (F) Superresolution images of single endosomes from Hrs-Ri;w-Ri (left)– or Hrs-Ri;Rme8-Ri (right)–expressing discs, stained for NICD and Rab7, which appear to localize in the same vesicular compartment in Hrs knockdown (arrowheads, left), but not in the Rme-8-Hrs double knockdown, where Notchlocalizes in long tubules (arrows, right). (G and H) Antibody uptake assay for Notch in cultured wing imaginal discs expressing Hrs-Ri;w-Ri (G) or Hrs-Ri;Rme8-Ri (H).
One difference between the Notch accumulations detected in Hrs alone compared with the combined knockdown was their apical–basal position as detected in uptake assays. In Hrs knockdown, internalized Notch remained trapped close to the apical surface, even after 2 h (Fig. 7 G). In contrast, when both Hrs and Rme-8 were perturbed, large Notch accumulations were present in basal regions (Fig. 7 H). This result suggests that, when both Hrs and Rme-8 are reduced, Notch becomes trapped at a later stage in the endosomal pathway. In addition, more Notch accumulated than in either single knockdown, consistent with multiple different trafficking routes being perturbed by this combination.
Ectopic Notch activation in the Rme-8 combinations is not dependent on Kuzbanian (Kuz)
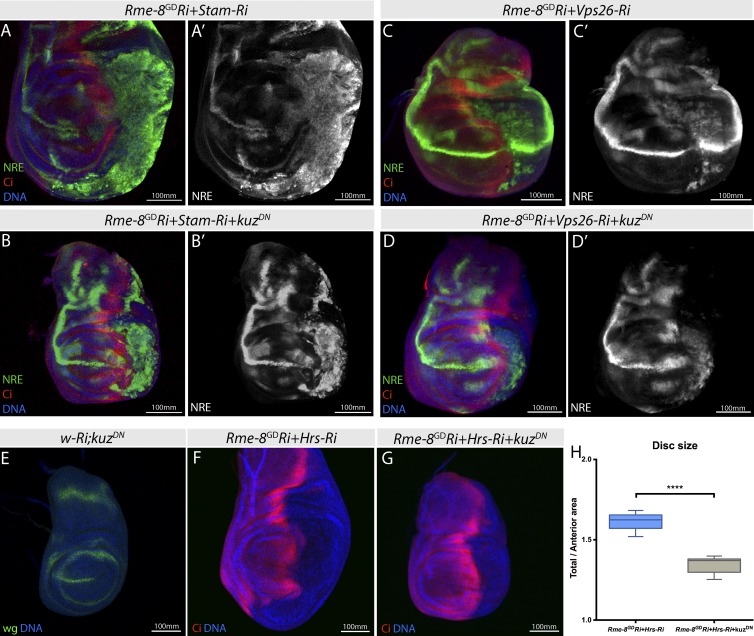
In many instances in which Notch becomes activated in the endocytic network, it does not require ligands (Hori et al., 2004; Thompson et al., 2005; Childress et al., 2006; Jaekel and Klein, 2006; Vaccari et al., 2008). To assess whether this might the case in the combinations with Rme-8, we tested whether activation could be prevented by expression of dominant-negative ADAM 10/kuz, the protease mediating the ligand-dependent S2 cleavage of Notch. Expression of KuzDN in either Vps26;Rme-8 or ESCRT-0;Rme-8 double knockdown was not able to suppress ectopic NRE expression (nor ectopic expression of another Notch target, deadpan) although it blocked the normal D/V expression of NRE (Fig. 8, A–D). Moreover, expression of KuzDN alone prevented D/V boundary expression of wg, in which it is dependent on Notch activity, without affecting hinge expression hinge, where it is Notch independent (Fig. 8 E). The former confirmed that KuzDN was effectively inhibiting ligand dependent signaling, suggesting that the residual NRE expression when kuzDN is combined with Vps26;Rme-8 or ESCRT-0;Rme-8 arises through ligand-independent activation. However, expression of KuzDN did rescue the overgrowth produced by depletion of ESCRT-0 and Rme-8 (Fig. 8, F–H). One possible explanation is the positive feedback loop that occurs in this tissue, whereby expression of ligands is positively up-regulated in response to Notch activation (de Celis and Bray, 1997). Hence, any ligand-independent activity might be amplified by subsequent induction of ligand expression. Inhibiting Kuz function would prevent this amplification, limiting the ectopic Notch activity and tissue overgrowth to that from the ligand-independent route. Alternatively, Kuz might affect another growth regulatory pathway independent of Notch. Despite these secondary effects, it is evident that a significant proportion of ectopic Notch activation remained in the presence of KuzDN, suggesting it arose via a ligand-independent mechanism.
Figure 8.
Ectopic Notch activation observed in combined depletions is not prevented by KuzDN. (A–D′) NRE-GFP expression shows that ectopic Notch activation in StamRi+Rme-8-Ri (A) or Vps26-Ri+Rme-8-Ri (C) is not rescued by kuzDN coexpression (B and D). (E) Wg D/V expression is lost in discs expressing kuzDN alone. (F and G) kuzDN coexpression rescues overgrowth caused by Hrs-Ri+Rme-8-Ri. (H) Quantification of wing disc size for genotypes shown in F and G. Boxes indicate 25th to 75th percentiles, whiskers represent maximum and minimum values in the dataset, and lines show the median values. Asterisks indicate significance according to t test; ****, P < 0.0001.
Discussion
Cells continuously internalize Notch, where much of it is routed to lysosomes for degradation. This process must be carefully regulated, not only to ensure steady-state levels of surface expression but also to prevent inappropriate activation within the endosomal pathway (Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Childress et al., 2006; Morrison et al., 2008; Vaccari et al., 2008, 2009). Our analysis of Rme-8 function reveals the importance of another aspect of trafficking, endocytic recycling, in maintaining sufficient levels of Notch at the membrane for signaling to occur correctly. As previous studies have not detected any requirement for retromer in normal Notch pathway function (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pocha et al., 2011), we were surprised to find that Notch pathway activity was sensitive to depletion of Rme-8. In addition, the consequences from combined knockdown of Rme-8 with Hrs, Stam, or Vps26 show that Rme-8 is important to prevent inappropriate Notch activation when these cargo-sorting complexes are disrupted. Combined knockdowns resulted in high levels of ectopic Notch signaling and tissue hyperplasia, which could be of significance in the context of recently identified disease-linked mutations in Rme-8 and other retromer members (Vilariño-Güell et al., 2011, 2014; Zimprich et al., 2011; McGough et al., 2014; Miura et al., 2014; Zavodszky et al., 2014).
In Rme-8–deficient tissues, Notch accumulated with Rab4 in enlarged endosomal structures and was depleted from the cell surface. Structured illumination microscopy suggested that it was trapped in Rab4-positive tubular structures, which have not previously been detected in Drosophila, although tubular Rab5 plasma membrane invaginations have been observed (Fabrowski et al., 2013). FRAP measurements were compatible with the hypothesis that Notch recycling was compromised under these conditions, making it likely that Rme-8 is necessary for Rab4-mediated recycling and that recycling is important to maintain membrane Notch levels. Notably, however, signaling was only detectably impeded when apical levels of Notch were significantly compromised. This is surprising given that Notch activity is dosage sensitive. One possible explanation is that signaling depends critically on the relative levels of Notch and Dl (de Celis and Bray, 1997; Sprinzak et al., 2010) and that, because trafficking of both proteins was perturbed in the Rme-8 knockdown, the impact may have been lessened. Another possibility is that the Notch pool engaged in functional signaling is localized away from the apical surface, where it is protected from defects in apical recycling. For example, some studies have suggested that signaling occurs between basal filopodia (de Joussineau et al., 2003; Cohen et al., 2010) and others have indicated that different receptor pools exist depending on modification state and/or presence of adaptors (Wilkin et al., 2008).
Notch accumulation in Rme-8–depleted tissue appeared strictly dependent on Vps26, a key component of sorting retromer, whereas reducing the latter alone had only minor effects on membrane Notch levels. This raises the possibility that another recycling pathway requiring Rme-8 can partially compensate, via the activity of other sorting complexes. One candidate for such alternative sorting could be Hrs, which appears to be involved in sorting cargoes for a fast recycling route as part of the CART complex (actinin-4, Brain-Expressed RING finger protein, myosinV; Hanyaloglu et al., 2005; Yan et al., 2005; Millman et al., 2008). Conversely, the Snx3-retromer–dependent pathway, which recycles Wntless to the Golgi (Harterink et al., 2011; Zhang et al., 2011), appears less sensitive to reductions in Rme-8 than in Vps26, as Wg secretion still occurred in Rme-8–depleted tissue. This fits with the model that there is spatial separation of SNX3 and SNX–BAR (containing a Bin/Amphiphysin/Rvs domain) retromer complexes, which may consequently involve different mechanisms (Mari et al., 2008; Harterink et al., 2011; Burd and Cullen, 2014). Recent data have shown that Rme-8 associates with the WASH complex (Freeman et al., 2014), which is involved in the formation/scission of endosomal tubules as well as in cargo recruitment (Seaman et al., 2013; Burd and Cullen, 2014). Our high-resolution microscopy further suggests that Rme-8 could be involved in tubulation of the sorting endosome in a developmental context. Hence, Rme-8 may be specifically required for endosomal routes involving combined actions of retromer and WASH. The possibility that other recycling routes occur independently from Rme-8 would explain why recycling of some receptors, such as the transferrin receptor, still occurs normally when Rme-8 is knocked down in HeLa cells (Girard and McPherson, 2008).
The ectopic Notch activity in Rme-8;Vps26 double knockdown was unexpected because neither single knockdown resulted in receptor activation. The activity was independent of Kuz/Adam10, implying that it was ligand independent, as seen for other conditions that result in ectopic Notch activation. Such activation is thought to occur when the receptor remains in the limiting membrane of acidifying endosomes where it can be proteolysed to release NICD (Wilkin et al., 2004, 2008; Hori et al., 2011; Schneider et al., 2013). With the combined depletion of Vps26 and Rme-8, all endocytosed Notch may thus have been routed toward the endolysosomal pathway where some could fail to be incorporated into ILVs, either because it lacked the appropriate modifications or because the volume exceeded the cargo sorting capacity. No such ectopic Notch activity was seen in the absence of Vps26 alone, suggesting either that the volume of misrouted Notch is higher in the Rme-8;Vps26–depleted conditions, for example, if Notch can also be retrieved by Vps26-independent recycling routes that become nonfunctional when Rme-8 is missing, or that sorting into ILVs is more disrupted when both are depleted.
More dramatic levels of Notch activation occurred when either ESCRT-0 components, Stam or Hrs, were perturbed in Rme-8–depleted cells. Although ESCRT-0 is implicated in sorting ubiquitinylated cargo in preparation for its sequestration into ILVs, no ectopic Notch activation was seen when either or both ESCRT-0 components were missing on their own (Vaccari et al., 2008; Tognon et al., 2014). Furthermore, Hrs removal can even suppress ectopic Notch activity caused by some other trafficking defects (Thompson et al., 2005; Childress et al., 2006; Gallagher and Knoblich, 2006; Jaekel and Klein, 2006; Yamada et al., 2011; Troost et al., 2012). How can these observations be reconciled with the high level of Notch activation in the combined knockdown of Rme-8 with Hrs or Stam? One possibility is that, as with the recycling pathway, there are additional Rme-8–dependent mechanisms that sort cargoes into ILVs besides Hrs/Stam. One candidate is SARA, a FYVE domain protein that forms a complex with RNF11 and that has been reported to function in cargo sorting with ESCRT-0 (Kostaras et al., 2013). SARA partially colocalized with Notch in the Rme-8–depleted cells and has been associated with Notch activity in some lineages (Coumailleau et al., 2009; Montagne and González-Gaitan, 2014). Another candidate is CD63, a tetraspanin family member implicated in ESCRT-independent sorting of cargoes into ILVs, as well as exosomes (van Niel et al., 2011). Although the function of CD63 homologues has not been explored in Drosophila, several tetraspanins affected Notch pathway trafficking or activity in general screens (Dornier et al., 2012; Le Bras et al., 2012). If failure in these, or alternate complexes, can account for the defects brought about by Rme-8 depletion, it will be interesting to ascertain whether their deployment also involves the WASH complex, making a link between different Rme-8–dependent routes.
Posttranslational modifications also influence ligand-independent Notch activity in the endocytic pathway. In particular, Dx-mediated Notch ubiquitinylation promotes ligand-independent activation by favoring Notch retention in the limiting membrane of MVBs (Hori et al., 2004, 2011; Wilkin et al., 2008; Yamada et al., 2011). However, Rme-8 depletion did not modify the Dx overexpression phenotypes. This makes it likely that a different pool of Notch is affected and further suggests that not all endolysosomal trafficking routes depend on Rme-8. This may explain the differing consequences of Rme-8 depletion on other receptor recycling.
Recent discoveries highlight the importance of Rme-8 as a regulatory node in endocytic trafficking. Notably, mutations in Rme-8 are found in some patients with familial Parkinson’s disease where defects in endocytic trafficking are associated with Lewy bodies (Vilariño-Güell et al., 2014). Rme-8 is also among a small cohort of intracellular transport proteins up-regulated in mammal-infective trypanosomes, suggesting it has a role in controlling the copy number of surface proteins (Koumandou et al., 2013). Here, we have demonstrated its importance in Notch receptor recycling, to maintain adequate levels for signaling, which may also be of relevance for clinical conditions. Furthermore, when combined with defects in Vps26 or ESCRT-0, reductions in Rme-8 enable high levels of ectopic Notch activation, causing tissue hyperplasia and highlighting the potential hazards when such combinatorial mutations arise.
Materials and methods
Drosophila genetics
Further details of the stocks used are provided in Table S1. Knockdown of gene expression was achieved using upstream activation sequence (UAS) Ri lines (Dietzl et al., 2007; Ni et al., 2011) as follows: UAS-Rme-8-Ri (Vienna Drosophila Resource Center [VDRC] 22671, 107706), UAS-w-Ri (Bloomington Drosophila Stock Center [BL] 35573), UAS-Su(H)-Ri (BL 28900), UAS-Dx-Ri (VDRC 7795, 106086), UAS-Vps26-Ri (VDRC 18396; BL 38937), UAS-Hrs-Ri (BL 33900, 34086), and UAS-Stam-Ri (VDRC 22497). Additionally, UAS-KuzDN (BL 6578), UAS-dx (Matsuno et al., 2002), and UAS-Su(dx) (Cornell et al., 1999) were used for protein expression. Expression of UAS constructs was driven by en-GAL4,Tub-GAL80ts, and larvae were shifted to 30°C 24 h after egg laying, except that Rme-8;ESCRT0 and Rme-8;Vps26 double knockdowns were shifted to 30°C 72 h after egg laying to avoid lethality. For adult phenotypes, salEPv-GAL4 (Cruz et al., 2009) was used, and crosses were maintained at 25°C until adult eclosion.
Mitotic clones were generated using the FRT/FLP system, in which the FLP recombinase induces homologue recombination between FRT (FLP recognition target) sites (Xu and Rubin, 1993). Clones lacking Rme-8 were obtained using previously characterized x ray–induced loss-of-function alleles whose phenotypes were reportedly rescued by expression of Rme-8 (Chang et al., 2004). Clones were generated by crossing FRT42D Rme-8C19/CyO, FRT42D Rme-8A9/CyO, or Vps35[MH20] FRT42D/CyO (Franch-Marro et al., 2008) to hsp-FLP122, tub-Gal4, UAS-GFP;tub-Gal80, FRT42D GFP/CyO or to FRT42D PCNA, Ubi-GFP;hh-flp. To generate Hrs mutant clones, HrsD28 FRT40A (Lloyd et al., 2002) was crossed to hsp-FLP122, tub-Gal4, UAS-GFP;tub-Gal80, FRT40A GFP/CyO;NRE-mCherry. Other lines used include Gbe+Su(H)-GFP (NRE-GFP) and Gbe+Su(H)-mCherry (NRE-mCherry; Housden et al., 2012), tub-Rab4-RFP (Yousefian et al., 2013), UAS-FYVE-GFP (Wucherpfennig et al., 2003), UAS-Lamp1-GFP (BL 42714), and Notch-GFP (Couturier et al., 2012).
Immunostaining and microscopy
Wing imaginal discs of third-instar larvae were dissected in PBS and fixed in 4% formaldehyde for 20 min. Discs were washed in PBS-DT (0.3% sodium deoxycholate and 0.3% Triton X-100 in PBS) and blocked in BBT (PBS-DT + 1% BSA) before incubation with primary antibodies overnight at 4°C. The following antibodies were used (for further details see Table S2): mouse anti-NICD (1:20; Developmental Studies Hybridoma Bank [DSHB]; Fehon et al., 1990), mouse anti-NECD (1:20; DSHB; Diederich et al., 1994), rat anti-NECD (1:500; Fehon et al., 1990; a gift from S. Artavanis-Tsakonas, Harvard Medical School, Boston, MA), mouse anti-Wg (1:20; DSHB; van den Heuvel et al., 1989), guinea pig anti-Dl (1:3,000; Huppert et al., 1997), rat anti–Cubitus interruptus (Ci; 1:20; DSHB; Motzny and Holmgren, 1995), rabbit anti-GFP (1:1,000; Invitrogen), rabbit anti-dsRed (1:200; Takara Bio Inc.), rabbit anti-Rab7 (1:3,000), and rabbit anti-Rab11 (1:8,000; Tanaka and Nakamura, 2008), guinea pig anti-Hrs (1:1,000; Lloyd et al., 2002; a gift from H. Bellen, Baylor College of Medicine, Houston, TX), rabbit anti-Lva (1:500; Sisson et al., 2000), rabbit anti-Avl (1:1,000; Lu and Bilder, 2005; a gift from D. Bilder, University of California, Berkeley, Berkeley, CA), rabbit anti-Rab5 (1:500; Abcam), guinea pig anti-Vps26 (1:1,000; Wang et al., 2014; a gift from H. Bellen), and rabbit anti-SARA (1:500; Coumailleau et al., 2009). After several washes in PBS-DT, the discs were incubated with fluorescently labeled secondary antibodies, (Jackson ImmunoResearch Laboratories, Inc.; details provided in Table S3), for 1–2 h at RT followed by washing in PBS-DT and labeling with the nuclear marker Hoechst 33342. The samples were mounted in Vectashield (Vector Laboratories) for imaging with D-Eclipse C1 (Plan Fluor 40×, 1.30 NA oil; Plan Apochromat VC 60×, 1.40 NA oil objective lenses; Nikon) or SP2 (63×, 1.40 NA oil objective lens; Leica) confocal microscopes. Samples for high resolution microscopy were stained using Alexa Fluor secondary antibodies and imaged using an OMX-V3 microscope designed by J.W. Sedat (University of California, San Francisco, San Francisco, CA) and built by Applied Precision (Gustafsson et al., 2008). This system uses structured illumination whereby the sample is illuminated with light that has passed through a grating to generate a structured illumination pattern in X, Y, and Z. Three cameras oriented in different angles with respect to the sample collect the emitted light (in total, 15 images/focal plane were acquired). The use of postacquisition algorithms allows reconstruction of the image, giving a resolution below the Abbe limit (∼120 nm in X,Y and 350 nm in Z). After acquisition, analysis was performed using ImageJ (National Institutes of Health), a continuous structure over ≥2 focal planes that extended from the main circumference of the endosome was considered to be a “tubule,” and the lengths were measured accordingly.
Notch uptake assay
Imaginal discs were dissected in Schneider’s Drosophila medium and cultured for 1 h at 4°C in the presence of the anti-NECD antibody (1:50; DSHB C458.2H, ascites). After three washes with warm media, discs were incubated at 25°C in Schneider’s medium with 200 µM Chloroquine to block lysosomal degradation (100 mM stock in ddH2O; Sigma-Aldrich). Discs were fixed in 4% formaldehyde at the indicated time points and then incubated with anti-Ci and with the appropriate secondary antibodies to reveal protein localization.
TEM
Wing discs were fixed in 2.5% glutaraldehyde in 100 mM phosphate buffer washed in 100 mM phosphate buffer and postfixed in 2% osmium tetroxide in phosphate buffer for 1 h on ice. After contrasting en bloc in 2% uranyl acetate, the specimens were dehydrated in EtOH and embedded in araldite using acetone as an intermediate solvent. Thin sections were stained with 2% uranyl acetate and lead citrate. Sections were observed under a microscope (EM 902; Carl Zeiss) at 80 kV.
FRAP
For FRAP experiments, WT or Rme-8–depleted wing discs expressing Ni-GFP were dissected in culture media (Schneider’s Drosophila medium supplemented with 2% FBS, 0.5% penicillin-streptavidin, and 0.1 µg/ml Ecdysone (20-hydroxyecdysone; Sigma-Aldrich). Unharmed discs were placed in an observation chamber as previously described (Aldaz et al., 2010) and covered with viscous media (culture media + 2.5× wt/vol methyl-cellulose [Sigma-Aldrich]). For dynasore treatment (Sigma-Aldrich), discs were incubated in Schneider’s media containing 100 µM dynasore for 30 min before imaging. Image acquisition was performed using a confocal microscope (FV1000; Olympus) with 100×/1.40 NA oil UPlan-S Apochromat objective lens. Stacks of five z sections spaced by 0.4 µm at a speed of 8 µs/pixel were acquired before and after bleaching and then at 7-s intervals afterward. In each half disc, five circular regions of 0.35-µm radius were photobleached at apical junctions so that there was only one bleach event per cell. Photobleaching was performed with 10 scans at 8 µs/pixel, using 100% 488-nm laser power. For each field, three background, five control regions, and five bleached regions were measured over time, and intensities were calculated using ImageJ. Mean fluorescent intensities from background were subtracted from the mean control and mean bleached datasets, and, to normalize for fluorescence changes during imaging, the bleached regions were then normalized to controls. At least two fields of view were analyzed per disc, and 10–12 discs were analyzed per dataset. As described previously for experiments analyzing Cadherin (Bulgakova et al., 2013), each dataset was fitted to a biexponential of the form f(t) = 1 − Fim − A1 × e−t/Tfast − A2 × e−t/Tslow and to a single exponential of the form f(t) = 1 − Fim − A1 × e−t/Tfast, where Fim is a size of the immobile pool of the protein, Tfast and Tslow are the half-times, and A1 and A2 are the amplitudes of the fast and slow components of the recovery. An F test was used to assess which of the equations better described each dataset. For recovery curves in WT or Rme-8–depleted tissues, the biexponential equation gave the better fit, and the resulting p-values are indicated.
Online supplemental material
Fig. S1 shows Rme-8 Ri knockdown and phenotypes. Fig. S2 shows that Rme-8 appears to regulate Notch trafficking independently of Dx and Su(dx). Fig. S3 shows that Notch associates with enlarged endosomes. Fig. S4 shows TEM analysis of Rme-8, Hrs, and Vps26 knockdowns. Fig. S5 shows retromer and ESCRT-0 Ri validation and phenotypes. Table S1 gives details of Drosophila strains. Table S2 gives details of primary antibodies. Table S3 gives details of secondary antibodies. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201411001/DC1.
Supplementary Material
Acknowledgements
We thank N. Bulgakova for assistance with FRAP experiments, N. Lawrence for help with OMX-V3, T. Vaccari (l'Istituto FIRC di Oncologia Molecolare, Milan, Italy) for guidance with the uptake assay, H. Bellen (Baylor College of Medicine, Houston, TX), D. Bilder (University of California, Berkeley, Berkeley, CA), J.-P. Vincent (National Institute for Medical Research, London, England, UK), and S. Artavanis-Tsakonas (Harvard Medical School, Boston, MA) for sharing reagents. We thank TRiP (Transgenic RNAi Project) at Harvard Medical School (National Institutes of Health/National Institute of General Medical Sciences R01-GM084947) and VDRC (Vienna, Austria) for transgenic Ri flies used in this study.
This work was funded by a Medical Research Council program grant (G0800034) to S.J. Bray. L.A. Snowden was the recipient of a Biotechnology and Biological Sciences Research Council PhD studentship. E. Seib and T. Klein were funded by the Deutsche Forschungsgemeinschaft (Sachbeihilfe KL 1028/5-1).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- Ci
- Cubitus interruptus
- Dl
- Delta
- D/V
- dorsoventral
- Dx
- Deltex
- en
- engrailed
- ESCRT
- endosomal sorting required for transport
- ILV
- intraluminal vesicle
- Kuz
- Kuzbanian
- MVB
- multivesicular body
- NECD
- Notch extracellular domain
- NICD
- Notch intracellular domain
- NRE
- Notch responsive element
- Ri
- RNAi
- Su(dx)
- Suppressor of Dx
- TEM
- transmission EM
- UAS
- upstream activation sequence
- Wg
- Wingless
- WT
- wild type
References
- Aldaz S., Escudero L.M., and Freeman M.. 2010. Live imaging of Drosophila imaginal disc development. Proc. Natl. Acad. Sci. USA. 107:14217–14222. 10.1073/pnas.1008623107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. 2012. Endocytic routes to Notch activation. Semin. Cell Dev. Biol. 23:437–442. 10.1016/j.semcdb.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Belenkaya T.Y., Wu Y., Tang X., Zhou B., Cheng L., Sharma Y.V., Yan D., Selva E.M., and Lin X.. 2008. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell. 14:120–131. 10.1016/j.devcel.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Bray S.J. 2006. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7:678–689. 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- Brou C. 2009. Intracellular trafficking of Notch receptors and ligands. Exp. Cell Res. 315:1549–1555. 10.1016/j.yexcr.2008.09.010 [DOI] [PubMed] [Google Scholar]
- Bulgakova N.A., Grigoriev I., Yap A.S., Akhmanova A., and Brown N.H.. 2013. Dynamic microtubules produce an asymmetric E-cadherin–Bazooka complex to maintain segment boundaries. J. Cell Biol. 201:887–901. 10.1083/jcb.201211159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C., and Cullen P.J.. 2014. Retromer: a master conductor of endosome sorting. Cold Spring Harb. Perspect. Biol. 6:6 10.1101/cshperspect.a016774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.C., Hull M., and Mellman I.. 2004. The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J. Cell Biol. 164:1055–1064. 10.1083/jcb.200311084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress J.L., Acar M., Tao C., and Halder G.. 2006. Lethal giant discs, a novel C2-domain protein, restricts notch activation during endocytosis. Curr. Biol. 16:2228–2233. 10.1016/j.cub.2006.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M., Georgiou M., Stevenson N.L., Miodownik M., and Baum B.. 2010. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev. Cell. 19:78–89. 10.1016/j.devcel.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Cornell M., Evans D.A., Mann R., Fostier M., Flasza M., Monthatong M., Artavanis-Tsakonas S., and Baron M.. 1999. The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics. 152:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumailleau F., Fürthauer M., Knoblich J.A., and González-Gaitán M.. 2009. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature. 458:1051–1055. 10.1038/nature07854 [DOI] [PubMed] [Google Scholar]
- Couturier L., Vodovar N., and Schweisguth F.. 2012. Endocytosis by Numb breaks Notch symmetry at cytokinesis. Nat. Cell Biol. 14:131–139. 10.1038/ncb2419 [DOI] [PubMed] [Google Scholar]
- Cruz C., Glavic A., Casado M., and de Celis J.F.. 2009. A gain-of-function screen identifying genes required for growth and pattern formation of the Drosophila melanogaster wing. Genetics. 183:1005–1026. 10.1534/genetics.109.107748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis J.F., and Bray S.. 1997. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development. 124:3241–3251. [DOI] [PubMed] [Google Scholar]
- de Joussineau C., Soulé J., Martin M., Anguille C., Montcourrier P., and Alexandre D.. 2003. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature. 426:555–559. 10.1038/nature02157 [DOI] [PubMed] [Google Scholar]
- Diederich R.J., Matsuno K., Hing H., and Artavanis-Tsakonas S.. 1994. Cytosolic interaction between deltex and Notch ankyrin repeats implicates deltex in the Notch signaling pathway. Development. 120:473–481. [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 448:151–156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Dornier E., Coumailleau F., Ottavi J.F., Moretti J., Boucheix C., Mauduit P., Schweisguth F., and Rubinstein E.. 2012. TspanC8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J. Cell Biol. 199:481–496. 10.1083/jcb.201201133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrowski P., Necakov A.S., Mumbauer S., Loeser E., Reversi A., Streichan S., Briggs J.A., and De Renzis S.. 2013. Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nat. Commun. 4:2244 10.1038/ncomms3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon R.G., Kooh P.J., Rebay I., Regan C.L., Xu T., Muskavitch M.A.T., and Artavanis-Tsakonas S.. 1990. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila . Cell. 61:523–534. 10.1016/0092-8674(90)90534-L [DOI] [PubMed] [Google Scholar]
- Fortini M.E., and Bilder D.. 2009. Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19:323–328. 10.1016/j.gde.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X., Wendler F., Guidato S., Griffith J., Baena-Lopez A., Itasaki N., Maurice M.M., and Vincent J.-P.. 2008. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat. Cell Biol. 10:170–177. 10.1038/ncb1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C.L., Hesketh G., and Seaman M.N.J.. 2014. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J. Cell Sci. 127:2053–2070. 10.1242/jcs.144659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi A., Taguchi T., Misaki R., Ohtani M., Dohmae N., Takio K., Yamada M., Gu J., Yamakami M., Fukuda M., et al. 2008. Human RME-8 is involved in membrane trafficking through early endosomes. Cell Struct. Funct. 33:35–50. 10.1247/csf.07045 [DOI] [PubMed] [Google Scholar]
- Gallagher C.M., and Knoblich J.A.. 2006. The conserved c2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila . Dev. Cell. 11:641–653. 10.1016/j.devcel.2006.09.014 [DOI] [PubMed] [Google Scholar]
- Girard M., and McPherson P.S.. 2008. RME-8 regulates trafficking of the epidermal growth factor receptor. FEBS Lett. 582:961–966. 10.1016/j.febslet.2008.02.042 [DOI] [PubMed] [Google Scholar]
- Girard M., Poupon V., Blondeau F., and McPherson P.S.. 2005. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J. Biol. Chem. 280:40135–40143. 10.1074/jbc.M505036200 [DOI] [PubMed] [Google Scholar]
- Grant B.D., and Donaldson J.G.. 2009. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10:597–608. 10.1038/nrm2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M.G., Shao L., Carlton P.M., Wang C.J., Golubovskaya I.N., Cande W.Z., Agard D.A., and Sedat J.W.. 2008. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94:4957–4970. 10.1529/biophysj.107.120345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu A.C., McCullagh E., and von Zastrow M.. 2005. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 24:2265–2283. 10.1038/sj.emboj.7600688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harterink M., Port F., Lorenowicz M.J., McGough I.J., Silhankova M., Betist M.C., van Weering J.R.T., van Heesbeen R.G.H.P., Middelkoop T.C., Basler K., et al. 2011. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 13:914–923. 10.1038/ncb2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., and Emr S.D.. 2011. The ESCRT pathway. Dev. Cell. 21:77–91. 10.1016/j.devcel.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Hori K., Fostier M., Ito M., Fuwa T.J., Go M.J., Okano H., Baron M., and Matsuno K.. 2004. Drosophila deltex mediates suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development. 131:5527–5537. 10.1242/dev.01448 [DOI] [PubMed] [Google Scholar]
- Hori K., Sen A., Kirchhausen T., and Artavanis-Tsakonas S.. 2011. Synergy between the ESCRT-III complex and Deltex defines a ligand-independent Notch signal. J. Cell Biol. 195:1005–1015. 10.1083/jcb.201104146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housden B.E., Millen K., and Bray S.J.. 2012. Drosophila reporter vectors compatible with ΦC31 integrase transgenesis techniques and their use to generate new Notch reporter fly lines. G3 (Bethesda). 2:79–82. 10.1534/g3.111.001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert S.S., Jacobsen T.L., and Muskavitch M.A.. 1997. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development. 124:3283–3291. [DOI] [PubMed] [Google Scholar]
- Hurley J.H., and Emr S.D.. 2006. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 35:277–298. 10.1146/annurev.biophys.35.040405.102126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel R., and Klein T.. 2006. The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev. Cell. 11:655–669. 10.1016/j.devcel.2006.09.019 [DOI] [PubMed] [Google Scholar]
- Jovic M., Sharma M., Rahajeng J., and Caplan S.. 2010. The early endosome: a busy sorting station for proteins at the crossroads. Histol. Histopathol. 25:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostaras E., Sflomos G., Pedersen N.M., Stenmark H., Fotsis T., and Murphy C.. 2013. SARA and RNF11 interact with each other and ESCRT-0 core proteins and regulate degradative EGFR trafficking. Oncogene. 32:5220–5232. 10.1038/onc.2012.554 [DOI] [PubMed] [Google Scholar]
- Koumandou V.L., Boehm C., Horder K.A., and Field M.C.. 2013. Evidence for recycling of invariant surface transmembrane domain proteins in African trypanosomes. Eukaryot. Cell. 12:330–342. 10.1128/EC.00273-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R., and Schweisguth F.. 2003. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell. 5:139–148. 10.1016/S1534-5807(03)00187-4 [DOI] [PubMed] [Google Scholar]
- Le Bras S., Rondanino C., Kriegel-Taki G., Dussert A., and Le Borgne R.. 2012. Genetic identification of intracellular trafficking regulators involved in Notch-dependent binary cell fate acquisition following asymmetric cell division. J. Cell Sci. 125:4886–4901. 10.1242/jcs.110171 [DOI] [PubMed] [Google Scholar]
- Li H., Li H.-F., Felder R.A., Periasamy A., and Jose P.A.. 2008. Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J. Biomed. Opt. 13:031206 10.1117/1.2943286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T.E., Atkinson R., Wu M.N., Zhou Y., Pennetta G., and Bellen H.J.. 2002. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 108:261–269. 10.1016/S0092-8674(02)00611-6 [DOI] [PubMed] [Google Scholar]
- Lobry C., Oh P., and Aifantis I.. 2011. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J. Exp. Med. 208:1931–1935. 10.1084/jem.20111855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., and Bilder D.. 2005. Endocytic control of epithelial polarity and proliferation in Drosophila . Nat. Cell Biol. 7:1232–1239. 10.1038/ncb1324 [DOI] [PubMed] [Google Scholar]
- Mari M., Bujny M.V., Zeuschner D., Geerts W.J.C., Griffith J., Petersen C.M., Cullen P.J., Klumperman J., and Geuze H.J.. 2008. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic. 9:380–393. 10.1111/j.1600-0854.2007.00686.x [DOI] [PubMed] [Google Scholar]
- Matsuno K., Ito M., Hori K., Miyashita F., Suzuki S., Kishi N., Artavanis-Tsakonas S., and Okano H.. 2002. Involvement of a proline-rich motif and RING-H2 finger of Deltex in the regulation of Notch signaling. Development. 129:1049–1059. [DOI] [PubMed] [Google Scholar]
- McGough I.J., and Cullen P.J.. 2011. Recent advances in retromer biology. Traffic. 12:963–971. 10.1111/j.1600-0854.2011.01201.x [DOI] [PubMed] [Google Scholar]
- McGough I.J., Steinberg F., Jia D., Barbuti P.A., McMillan K.J., Heesom K.J., Whone A.L., Caldwell M.A., Billadeau D.D., Rosen M.K., and Cullen P.J.. 2014. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr. Biol. 24:1670–1676. 10.1016/j.cub.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman E.E., Zhang H., Zhang H., Godines V., Bean A.J., Knoll B.J., and Moore R.H.. 2008. Rapid recycling of β-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb. Traffic. 9:1958–1971. 10.1111/j.1600-0854.2008.00813.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura E., Hasegawa T., Konno M., Suzuki M., Sugeno N., Fujikake N., Geisler S., Tabuchi M., Oshima R., Kikuchi A., et al. 2014. VPS35 dysfunction impairs lysosomal degradation of α-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol. Dis. 71:1–13. 10.1016/j.nbd.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Moberg K.H., Schelble S., Burdick S.K., and Hariharan I.K.. 2005. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell. 9:699–710. 10.1016/j.devcel.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Montagne C., and González-Gaitan M.. 2014. Sara endosomes and the asymmetric division of intestinal stem cells. Development. 141:2014–2023. 10.1242/dev.104240 [DOI] [PubMed] [Google Scholar]
- Morrison H.A., Dionne H., Rusten T.E., Brech A., Fisher W.W., Pfeiffer B.D., Celniker S.E., Stenmark H., and Bilder D.. 2008. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol. Biol. Cell. 19:4167–4176. 10.1091/mbc.E08-07-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzny C.K., and Holmgren R.. 1995. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 52:137–150. 10.1016/0925-4773(95)00397-J [DOI] [PubMed] [Google Scholar]
- Ni J.Q., Zhou R., Czech B., Liu L.P., Holderbaum L., Yang-Zhou D., Shim H.S., Tao R., Handler D., Karpowicz P., et al. 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods. 8:405–407. 10.1038/nmeth.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolo R., Abbott L.A., and Bellen H.J.. 2000. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila . Cell. 102:349–362. 10.1016/S0092-8674(00)00040-4 [DOI] [PubMed] [Google Scholar]
- Pan C.L., Baum P.D., Gu M., Jorgensen E.M., Clark S.G., and Garriga G.. 2008. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev. Cell. 14:132–139. 10.1016/j.devcel.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddini E., Marshall F., Dubois L., Hirst E., and Vincent J.P.. 2005. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development. 132:5479–5489. 10.1242/dev.02145 [DOI] [PubMed] [Google Scholar]
- Pocha S.M., Wassmer T., Niehage C., Hoflack B., and Knust E.. 2011. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr. Biol. 21:1111–1117. 10.1016/j.cub.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Popoff V., Mardones G.A., Bai S.-K., Chambon V., Tenza D., Burgos P.V., Shi A., Benaroch P., Urbé S., Lamaze C., et al. 2009. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic. 10:1868–1880. 10.1111/j.1600-0854.2009.00993.x [DOI] [PubMed] [Google Scholar]
- Port F., Kuster M., Herr P., Furger E., Bänziger C., Hausmann G., and Basler K.. 2008. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat. Cell Biol. 10:178–185. 10.1038/ncb1687 [DOI] [PubMed] [Google Scholar]
- Rives A.F., Rochlin K.M., Wehrli M., Schwartz S.L., and DiNardo S.. 2006. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev. Biol. 293:268–283. 10.1016/j.ydbio.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Troost T., Grawe F., Martinez-Arias A., and Klein T.. 2013. Activation of Notch in lgd mutant cells requires the fusion of late endosomes with the lysosome. J. Cell Sci. 126:645–656. 10.1242/jcs.116590 [DOI] [PubMed] [Google Scholar]
- Seaman M.N.J. 2012. The retromer complex - endosomal protein recycling and beyond. J. Cell Sci. 125:4693–4702. 10.1242/jcs.103440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N.J., Gautreau A., and Billadeau D.D.. 2013. Retromer-mediated endosomal protein sorting: all WASHed up! Trends Cell Biol. 23:522–528. 10.1016/j.tcb.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi A., Sun L., Banerjee R., Tobin M., Zhang Y., and Grant B.D.. 2009. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J. 28:3290–3302. 10.1038/emboj.2009.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson J.C., Field C., Ventura R., Royou A., and Sullivan W.. 2000. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J. Cell Biol. 151:905–918. 10.1083/jcb.151.4.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., De Renzis S., Nielsen E., Rietdorf J., and Zerial M.. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149:901–914. 10.1083/jcb.149.4.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzak D., Lakhanpal A., Lebon L., Santat L.A., Fontes M.E., Anderson G.A., Garcia-Ojalvo J., and Elowitz M.B.. 2010. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 465:86–90. 10.1038/nature08959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J., Tavaré J.M., and Cullen P.J.. 2013. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 15:461–471. 10.1038/ncb2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., and Nakamura A.. 2008. The endocytic pathway acts downstream of Oskar in Drosophila germ plasm assembly. Development. 135:1107–1117. 10.1242/dev.017293 [DOI] [PubMed] [Google Scholar]
- Temkin P., Lauffer B., Jäger S., Cimermancic P., Krogan N.J., and von Zastrow M.. 2011. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 13:715–721. 10.1038/ncb2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.J., Mathieu J., Sung H.-H., Loeser E., Rørth P., and Cohen S.M.. 2005. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell. 9:711–720. 10.1016/j.devcel.2005.09.020 [DOI] [PubMed] [Google Scholar]
- Tognon E., Wollscheid N., Cortese K., Tacchetti C., and Vaccari T.. 2014. ESCRT-0 is not required for ectopic Notch activation and tumor suppression in Drosophila. PLoS ONE. 9:e93987 10.1371/journal.pone.0093987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troost T., Jaeckel S., Ohlenhard N., and Klein T.. 2012. The tumour suppressor Lethal (2) giant discs is required for the function of the ESCRT-III component Shrub/CHMP4. J. Cell Sci. 125:763–776. 10.1242/jcs.097261 [DOI] [PubMed] [Google Scholar]
- Vaccari T., and Bilder D.. 2005. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell. 9:687–698. 10.1016/j.devcel.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Vaccari T., Lu H., Kanwar R., Fortini M.E., and Bilder D.. 2008. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180:755–762. 10.1083/jcb.200708127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Rusten T.E., Menut L., Nezis I.P., Brech A., Stenmark H., and Bilder D.. 2009. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 122:2413–2423. 10.1242/jcs.046391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse R., Johnston P., and Lawrence P.A.. 1989. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 59:739–749. 10.1016/0092-8674(89)90020-2 [DOI] [PubMed] [Google Scholar]
- van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., Marks M.S., Rubinstein E., and Raposo G.. 2011. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell. 21:708–721. 10.1016/j.devcel.2011.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C., Wider C., Ross O.A., Dachsel J.C., Kachergus J.M., Lincoln S.J., Soto-Ortolaza A.I., Cobb S.A., Wilhoite G.J., Bacon J.A., et al. 2011. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89:162–167. 10.1016/j.ajhg.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C., Rajput A., Milnerwood A.J., Shah B., Szu-Tu C., Trinh J., Yu I., Encarnacion M., Munsie L.N., Tapia L., et al. 2014. DNAJC13 mutations in Parkinson disease. Hum. Mol. Genet. 23:1794–1801. 10.1093/hmg/ddt570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Tan K.L., Agosto M.A., Xiong B., Yamamoto S., Sandoval H., Jaiswal M., Bayat V., Zhang K., Charng W.-L., et al. 2014. The retromer complex is required for rhodopsin recycling and its loss leads to photoreceptor degeneration. PLoS Biol. 12:e1001847 10.1371/journal.pbio.1001847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin M.B., Carbery A.-M., Fostier M., Aslam H., Mazaleyrat S.L., Higgs J., Myat A., Evans D.A.P., Cornell M., and Baron M.. 2004. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 14:2237–2244. 10.1016/j.cub.2004.11.030 [DOI] [PubMed] [Google Scholar]
- Wilkin M., Tongngok P., Gensch N., Clemence S., Motoki M., Yamada K., Hori K., Taniguchi-Kanai M., Franklin E., Matsuno K., and Baron M.. 2008Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev. Cell. 15:762–772. 10.1016/j.devcel.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Bräuninger M., and González-Gaitán M.. 2003. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161:609–624. 10.1083/jcb.200211087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., and Rubin G.M.. 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 117:1223–1237. [DOI] [PubMed] [Google Scholar]
- Yamada K., Fuwa T.J., Ayukawa T., Tanaka T., Nakamura A., Wilkin M.B., Baron M., and Matsuno K.. 2011. Roles of Drosophila deltex in Notch receptor endocytic trafficking and activation. Genes Cells. 16:261–272. 10.1111/j.1365-2443.2011.01488.x [DOI] [PubMed] [Google Scholar]
- Yan Q., Sun W., Kujala P., Lotfi Y., Vida T.A., and Bean A.J.. 2005. CART: an Hrs/actinin-4/BERP/myosin V protein complex required for efficient receptor recycling. Mol. Biol. Cell. 16:2470–2482. 10.1091/mbc.E04-11-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P.T., Lorenowicz M.J., Silhankova M., Coudreuse D.Y., Betist M.C., and Korswagen H.C.. 2008. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev. Cell. 14:140–147. 10.1016/j.devcel.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Yousefian J., Troost T., Grawe F., Sasamura T., Fortini M., and Klein T.. 2013. Dmon1 controls recruitment of Rab7 to maturing endosomes in Drosophila. J. Cell Sci. 126:1583–1594. 10.1242/jcs.114934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodszky E., Seaman M.N., Moreau K., Jimenez-Sanchez M., Breusegem S.Y., Harbour M.E., and Rubinsztein D.C.. 2014. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 5:3828 10.1038/ncomms4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Grant B., and Hirsh D.. 2001. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol. Biol. Cell. 12:2011–2021. 10.1091/mbc.12.7.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wu Y., Belenkaya T.Y., and Lin X.. 2011. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res. 21:1677–1690. 10.1038/cr.2011.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A., Benet-Pagès A., Struhal W., Graf E., Eck S.H., Offman M.N., Haubenberger D., Spielberger S., Schulte E.C., Lichtner P., et al. 2011. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89:168–175. 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.