Abstract
The important role of sialic acid in various biological phenomena is well-established. In order to further clarify the role of sialic acid in cell death induced by various stimuli, the present study compared the cell survival of the HBL-2 human diffuse large B-cell lymphoma cell line upon anticancer drug-induced cell death, with or without neuraminidase pretreatment: Cell survival was assessed using flow cytometry. Upon treatment with doxorubicin or etoposide, the HBL-2 cell viability decreased. In etoposide-induced cell death, the HBL-2 cells demonstrated nuclear fragmentation, which was consistent with morphologically apoptotic cells. In addition, a higher decrease in the cell viability of etoposide-treated HBL-2 cells was observed in cells pretreated with neuraminidase compared with cells that were not pretreated. Furthermore, the caspase-3, caspase-8 and caspase-9 activities in etoposide-induced apoptosis demonstrated a greater increase upon neuraminidase pretreatment compared with no neuraminidase pretreatment. In conclusion, cell surface sialylation appears to protect lymphoma cells from anticancer drug-induced apoptosis.
Keywords: human malignant lymphoma, sialic acid, drug-induced apoptosis
Introduction
The role of sialic acid as a biological mask of surface structures is well-documented (1,2). Sialic acid plays an important role in the biological behavior of tumor cells, with respect to cell recognition phenomena, metastasis (3–5) and cell adhesion (6), and influences the clinical outcome of patients (7,8). We have previously reported that sialylation of L-phyohemagglutinin (L-PHA) reactive oligosaccharides is associated with worse prognosis in patients with diffuse large B-cell lymphoma (7,8). Another study has demonstrated that differential cell surface sialylation of Burkitt lymphoma cell lines affects the cell adhesion to fibronectin and collagen type IV (9). In addition, differential cell surface sialylation is due to differences in the mRNA expression of UDP-GlcNAc2-epimerase, which is a key enzyme in sialic acid biosynthesis (9). Furthermore, Fas-induced apoptosis was found to be regulated by sialylation of the Fas molecule in the Jurkat T cell lymphoma cell line (10).
Doxorubicin is one of the chemotherapeutic agents used in the therapy of human malignant lymphoma (11). In addition, etoposide, a topoisomerase II inhibitor, is another chemotherapeutic agent used in malignant lymphoma (12). Etoposide-induced apoptosis has been reported to be regulated through caspase activation, mitochondrial damage and cytochrome c release (13–16). However, only a limited number of studies have investigated the association between cell surface sialylation and drug resistance in tumor cell lines (17). The aim of the present study was to investigated whether cell surface sialylation may regulate etoposide-induced apoptosis in a caspase-dependent manner in the HBL-2 human malignant lymphoma cell line.
Materials and methods
Cell line
The HBL-2 human lymphoma cell line was established in the laboratory of the Department of Diagnostic Pathology (Fukushima Medical University, Fukushima, Japan) from a patient suffering from diffuse large B-cell lymphoma. The study was approved by the Ethics Committee of Fukushima Medical University (Fukushima, Japan). The HBL-2 cells were grown in RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA) culture medium containing 15% fetal calf serum (FCS) in a 5%CO2 atmosphere at 37°C (18).
Reagents
Limax flavus agglutinin (LFA), a biotinylated lectin, was purchased from EY Laboratories, Inc. (San Mateo, CA, USA). Neuraminidase from Vibrio Cholerae was obtained from Roche Diagnostics GmbH, Mannheim, Germany.
Flow cytometry
HBL-2 cells (5×105 cells) were suspended in 100 µl phosphate-buffered saline (PBS), incubated at 4°C for 20 min with 5 µl biotinylated LFA lectin and washed twice with PBS. The cells were then incubated at 4°C for 20 min with 5 µl avidin-fluorescein isothiocyanate (Vector Laboratories, Inc., Burlingame, CA, USA) and washed twice with PBS. The fluorescent intensities were analyzed using a FACScan device (BD Biosciences, Mountain View, CA, USA). In order to analyze cell surface sialylation, 6×106 cells were incubated at 37°C for 30 min in 200 µl RPMI 1640 medium containing 15% FCS and 40 µl of 1 U/ml Vibrio Cholerae neuraminidase prior to incubation with biotinylated LFA lectin (9). Lectin reactivity was then analyzed by flow cytometry.
LFA is a sialic acid-specific lectin, reacts with the surfaces of HBL-2 cells, which was observed using flow cytometry. LFA lectin reactivity is completely eliminated by neuraminidase treatment, indicating that sialic acids on the cell surface are removed (19).
Doxorubicin or etoposide-induced cell death
Cell viability was assessed using a WST-1 Cell Proliferation Assay kit (Roche Diagnostics) (10). HBL-2 cells were grown for 2 days and then seeded in 96-well microtiter plates, at a density of 5×104 cells/well. Next, the cells were incubated for 24, 48 or 72 h at 37°C in 100 µl culture medium containing doxorubicin (D1515; Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 1.6 or 3.2 µM, or incubation with etoposide (40 µg/ml, for 24h; E1383; Sigma-Aldrich) at a final concentration of 10 or 30 µg/ml. Subsequent to the incubation, 10 µl WST-1 reagent was added to each well and the plates were incubated for a further 1 or 2 h at 37°C. Then the absorbance at 450 nm was measured with an i-MARK microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). To examine the extent of cell surface sialylation, 6×106 cells were incubated at 37°C for 30 min in 200 µl RPMI 1640 containing 15% FCS and 40 µl of 1 U/ml Vibrio Cholerae neuraminidase, prior to incubation with doxorubicin or etoposide (10).
Detection of apoptosis
The cultured cells were cytospun using a Cytospin2 (Thermo Fisher Scientific, Inc., Waltham, MA, USA), Japan. The cells which adhered to the slide glass were stained using Giemsa solution (Wako Pure Chemical Industries, Ltd., Chuo-ku, Japan), as to the manufacturer's instructions. The apoptosis-associated morphological changes, including nuclear fragmentation, condensation and membrane changes were evaluated by light microscope (BX51; Olympus Corporation, Tokyo, Japan). Giemsa-stained cytospin cell preparations were evaluated to detect any apoptosis-associated morphological changes, including nuclear fragmentation, condensation and membrane changes.
Caspase3, caspase-8 and caspase-9 activities
Upon induction of apoptosis with etoposide, the caspase-3, caspase-8 and caspase-9 activities were measured using a colorimetric caspase activity assay kit (Apopcyto™; Medical & Biological Laboratories Co., Ltd., Tokyo, Japan), according to the manufacturer's instructions (20).
Statistical analysis
P-values were calculated based on Student's t-test using Microsoft Office Excel software, version 2007 (Microsoft Corporation, Redmond, WA, USA), and P<0.05 was considered to indicate a statistically significant difference.
Results
Doxorubicin- and etoposide-induced cell death
HBL-2 cells were incubated with or without doxorubicin, at a concentration of 1.6 or 3.2 µM for the cell viability assay using WST reagent (Fig. 1): The cell viability of HBL-2 cells reduced in a dose- and time- dependent manner (Fig. 1A). The time dependent reduction of cell viability was observed with 1.6 µM (P=0.0002) and 3.2 µM (P<0.0001) doxorucin treatment. A dose dependent reduction in cell viability was observed at 48h (P=0.0001). In addition, HBL-2 cells were incubated with or without etoposide, at a concentration of 10 or 30 µg/ml. The cell viability of HBL-2 cells reducedd in a time-dependent manner, but not in a dose-dependent manner (Fig. 1B).
Figure 1.
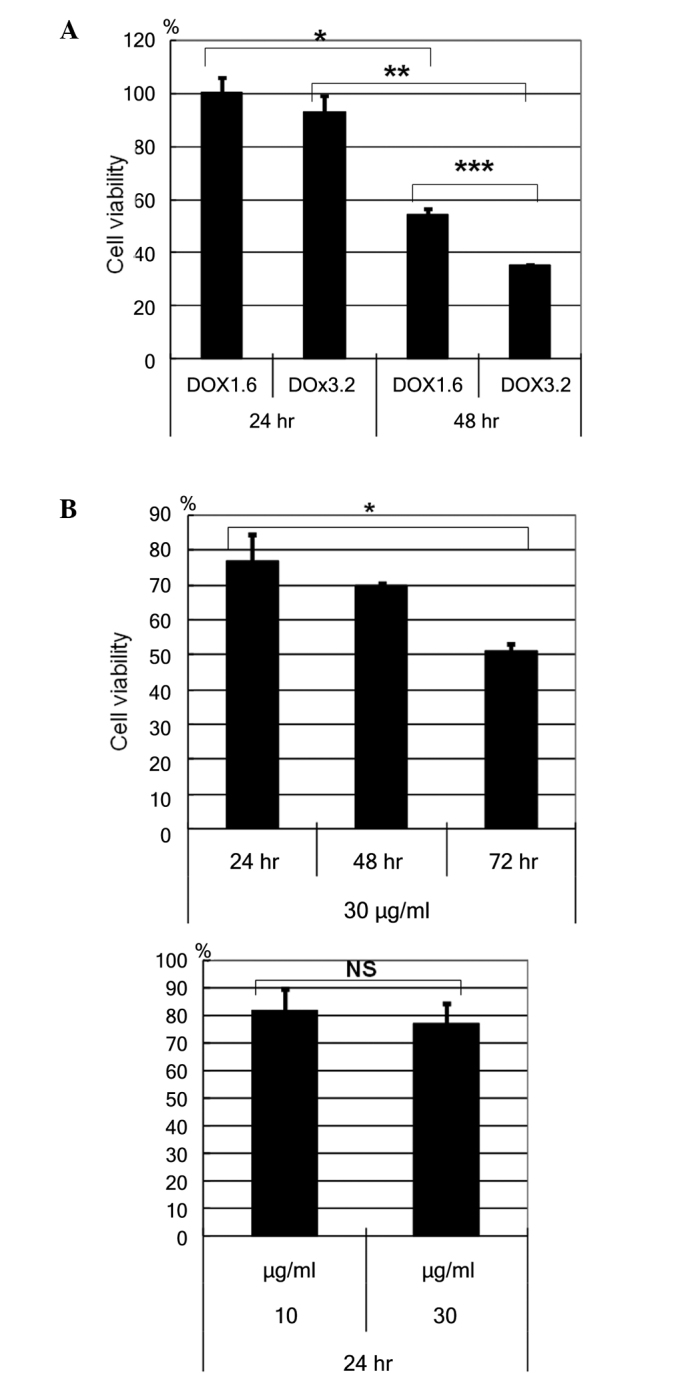
Doxorubicin- and etoposide-induced cell death. (A) HBL-2 cells were incubated with or without doxorubicin at a concentration of 1.6 or 3.2 µM, and their cell viability decreased in a dose- and time-dependent manner. *P=0.0002; **P<0.0001; ***P=0.0001. (B) HBL-2 cells were incubated with or without etoposide at a concentration of 10 or 30 µg/ml, and their cell viability decreased in a time- dependent manner, but not in a dose-dependent manner. *P=0.0044. Data are representative of two independent experiments. DOX, doxorubicin; NS, not significant.
Effect of neuraminidase treatment on doxorubicin- or etoposide-induced cell death
HBL-2 cells were incubated with etoposide at a concentration of 30 µg/ml (Fig. 2). After 72 h, the reduction in cell viability of neuraminidase-pretreated cells was greater when compared with cells that were not pretreated with neuraminidase (Fig. 2, P=0.0021).
Figure 2.
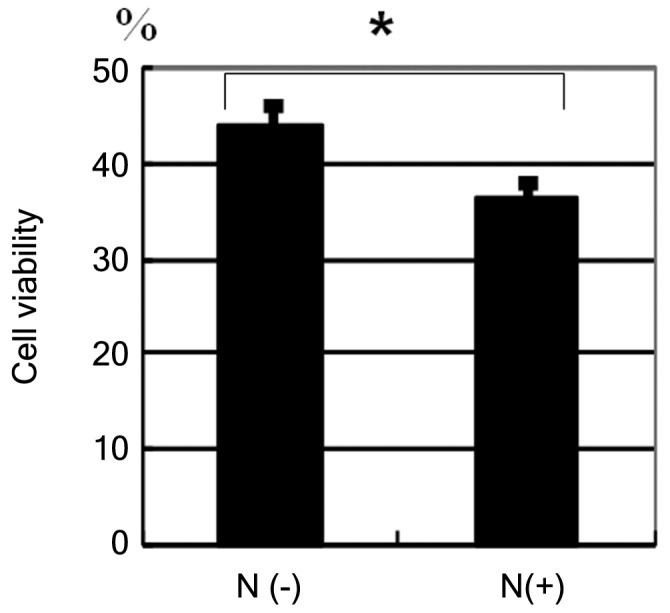
Cell surface sialylation and drug-induced cell death. HBL-2 cells were incubated with etoposide (30 µg/ml). After 72 h, cell viability decreased more in neuraminidase pretreated cells compared with not pretreated cells (*P=0.0021). Data are representative of two independent experiments. N(–), no neuraminidase pretreatment; N(+), neuraminidase pretreatment.
Detection of apoptosis
HBL-2 cells that were incubated with etoposide for 24 h demonstrated an apoptotic morphology (including nuclear fragmentation) on Giemsa-stained samples (Fig. 3A). The apoptotic morphology was also observed in HBL-2 cells pretreated with neuraminidase (Fig. 3B).
Figure 3.
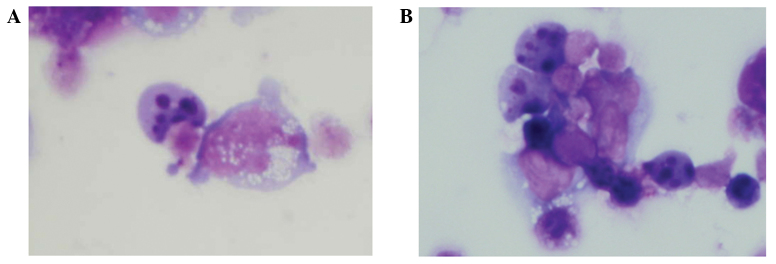
Detection of apoptosis. (A) Giemsa-stained HBL-2 cells that were incubated with etoposide for 24 h demonstrated an apoptotic morphology (including nuclear fragmentation). (B) Upon neuraminidase pretreatment, the HBL-2 cells also demonstrated an apoptotic morphology.
Caspase-3, caspase-8 and caspase-9 activities
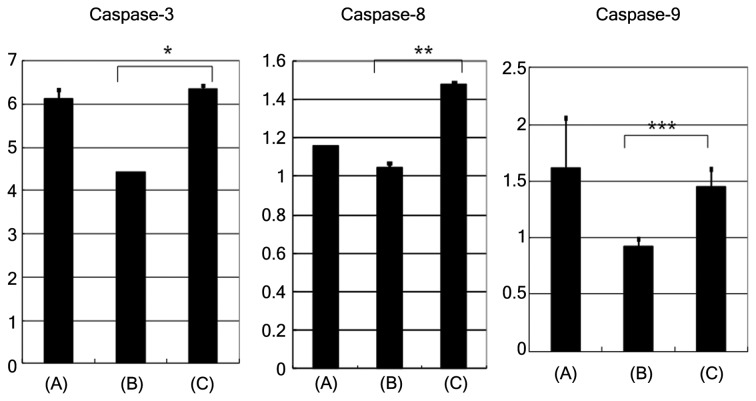
Upon induction of apoptosis with etoposide, the caspase-3, caspase-8 and caspase-9 activities (activity/h/mg of protein) were found to be higher in cells pretreated with neuraminidase, compared with cells that were not pretreated (Fig. 4; caspase-3, P=0.0011; caspase-8, P=0.0014; caspase-9, P>0.05). The removal of sialic acid by neuraminidase pretreatment resulted in enhancement of caspase-3, -8 and 9 activity compared with the absence of neuraminidase pre-treatment.
Figure 4.
Evaluation of caspase-3, caspase-8 and caspase-9 activities. Upon induction of apoptosis with etoposide, the caspase-3, caspase-8 and caspase-9 activities were more increased in neuraminidase pretreated cells compared with non-pretreated cells. Bars represent specific caspase activity (activity per hour per milligram of protein). Data are representative of two independent experiments. *P=0.0011; **P=0.0014; ***P=0.0504 (the difference in caspase-9 was not significant, but the P-value was marginal). (A), apoptosis in Jurkat cell line with agonistic anti-Fas monoclonal antibody; (B), apoptosis in HBL-2 induced by etoposide; (C), apoptosis in HBL-2 induced by etoposide with neuraminidase pretreatment.
Discussion
Etoposide is a topoisomerase II inhibitor that has been widely used to couple DNA damage to apoptosis (21). Topoisomerase II is able to unknot and untangle DNA molecules by passing an intact helix through a transient double-stranded break (13). Inhibitors, such as etoposide, stabilize the complex formed by topoisomerase II and form non-repairable DNA double-strand breaks. Subsequently, cells are able to recognize these DNA damages and eliminate the injured cells by apoptosis.
Etoposide-induced apoptosis has previously been found to be dependent on caspase activation and mediated by mitochondrial damage (22). Doxorubicin-induced apoptosis is also mediated by caspase-9 activation (23). Scaffidi et al identified two different types of Fas-induced apoptosis signaling pathways (24). Type I apoptosis is mediated by a death domain and activation of caspase-8 in various cell lines (24). By contrast, type II apoptosis is mediated by activation of caspase-9 and loss of mitochondrial membrane potential (Δψm). The activities of the death domain and caspase-8 in type I apoptosis are lower compared to those in type II apoptosis (24). In the present study, etoposide-induced apoptosis was found to be mediated by the activation of both caspase-8 and caspase-9. Therefore, although the detailed underlying mechanisms remain unclear, cell surface sialylation inhibited the pathway of apoptosis depending on the activation of caspase-8, caspase-9 and caspase-3. In addition, sialic acids may regulate both type I and type II apoptosis.
Cell surface sialylation in human lymphoma cells has been reported to be involved in modulating sensitivity towards Fas-mediated apoptotic cell death (10). Previous studies have suggested that alteration in the extent of sialylation in the cell surface of tumor cells appeared to be closely associated with cell adhesion, metastasis and the clinical outcome of the patients (7). Our previous study revealed that α2,6-sialic acid residues or sialylation in L-PHA reactive oligosaccharides appeared to be closely associated with worse prognosis in patients suffering from human diffuse large B-cell lymphoma (8). Differential cell surface sialylation regulates lymphoma cell adhesion to the extracellular matrix (9). In the present study, cell surface sialylation inhibited etoposide-induced apoptosis and this drug-induced apoptosis was caspase-3 dependent.
In conclusion, the results of the present study may provide new scientific foundation on the cell survival mechanism in drug-induced apoptosis and sialylation of cell surface glycans, which appeared to inhibit the anticancer drug effect in lymphoma. The current authors speculate that altered cell surface sialylation may be useful for more effective cell killing in human malignant lymphoma.
References
- 1.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 2.Kelm S, Schauer R. Sialic acids in molecular and cellular interactions. Int Rev Cytol. 1997;175:137–240. doi: 10.1126/science.7233237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altevogt P, Fogel M, Cheingsong-Popov R, Dennis J, Robinson P, Schirrmacher V. Different patterns of lectin binding and cell surface sialylation detected on related high- and low-metastatic tumor lines. Cancer Res. 1983;43:5138–5144. [PubMed] [Google Scholar]
- 4.Yogeeswaran G, Salk PL. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981;212:1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]
- 5.Abe M, Suzuki O, Tasaki K, Tominaga K, Wakasa H. Analysis of lectin binding properties on human Burkitt's lymphoma cell lines that show high spontaneous metastasis to distant organs in SCID mice: The binding sites for soybean agglutinin lectin masked by sialylation are closely associated with metastatic lymphoma cells. Pathol Int. 1996;46:977–983. doi: 10.1111/j.1440-1827.1996.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 6.Dennis J, Waller C, Timpl R, Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982;300:274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki O, Nozawa Y, Kawaguchi T, Abe M. Phaseolus vulgaris leukoagglutinating lectin-binding reactivity in human diffuse large B-cell lymphoma and its relevance to the patient's clinical outcome: Lectin histochemistry and lectin blot analysis. Pathol Int. 1999;49:874–880. doi: 10.1046/j.1440-1827.1999.00960.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki O, Nozawa Y, Kawaguchi T, Abe M. Alpha-2,6-sialylation of L-PHA reactive oligosaccharides and expression of N-acetylglucosaminyltransferase V in human diffuse large B cell lymphoma. Oncol Rep. 2003;10:1759–1764. [PubMed] [Google Scholar]
- 9.Suzuki O, Nozawa Y, Kawaguchi T, Abe M. UDP-GlcNAc2-epimerase regulates cell surface sialylation and cell adhesion to extracellular matrix in Burkitt's lymphoma. Int J Oncol. 2002;20:1005–1011. [PubMed] [Google Scholar]
- 10.Suzuki O, Nozawa Y, Abe M. Sialic acids linked to glycoconjugates of Fas regulate the caspase-9-dependent and mitochondria-mediated pathway of Fas-induced apoptosis in Jurkat T cell lymphoma. Int J Oncol. 2003;23:769–774. [PubMed] [Google Scholar]
- 11.Halaas JL, Moskowitz CH, Horwitz S, et al. ADR-CHOP-14 in patients with diffuse large B-cell lymphoma: Feasibility and preliminary efficacy. Leuk Lymphoma. 2005;46:541–547. doi: 10.1080/10428190400029932. [DOI] [PubMed] [Google Scholar]
- 12.Zinzani PL, Gherlinzoni F, Storti S, Zaccaria A, Pavone E, Moretti L, Gentilini P, Guardigni L, De Renzo A, Fattori PP, et al. Randomized trial of 8-week versus 12-week VNCOP-B plus G-CSF regimens as front-line treatment in elderly aggressive non-Hodgkin's lymphoma patients. Ann Oncol. 2002;13:1364–1369. doi: 10.1093/annonc/mdf208. [DOI] [PubMed] [Google Scholar]
- 13.Chen GL, Yang L, Rowe TC, Halligan BD, Tewey KM, Liu LF. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984;259:13560–13566. [PubMed] [Google Scholar]
- 14.Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:139–154. doi: 10.1016/S0167-4781(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 15.Karpinich NO, Tafani M, Rothman RJ, Russo MA, Farber JL. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. J Biol Chem. 2002;277:16547–16552. doi: 10.1074/jbc.M110629200. [DOI] [PubMed] [Google Scholar]
- 16.Mizumoto K, Rothman RJ, Farber JL. Programmed cell death (apoptosis) of mouse fibroblasts is induced by the topoisomerase II inhibitor etoposide. Mol Pharmacol. 1994;46:890–895. [PubMed] [Google Scholar]
- 17.Ma H, Zhou H, Song X, Shi S, Zhang J, Jia L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene. 2015;34:726–740. doi: 10.1038/onc.2014.7. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki O, Nozawa Y, Abe M. Regulatory roles of altered N-and O-glycosylation of CD45 in galectin-1-induced cell death in human diffuse large B cell lymphoma. Int J Oncol. 2005;26:1063–1068. [PubMed] [Google Scholar]
- 19.Suzuki O, Nozawa Y, Abe M. The regulatory roles of cell surface sialylation and N-glycans in human B cell lymphoma cell adhesion to galectin-1. Int J Oncol. 2006;28:155–160. [PubMed] [Google Scholar]
- 20.Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Tang MJ, Chang WC, Lin YS. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. J Biol Chem. 2004;279:40755–40761. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- 21.Boesen-de Cock JG, Tepper AD, de Vries E, van Blitterswijk WJ, Borst J. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999;274:14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- 22.Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis : Potential roles in cleaving mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24:6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamen S, Anel A, Perez-Galan P, Lasierra P, Johnson D, Pineiro A, Naval J. Doxorubicin treatment activates a Z-VAD-sensitive caspase, which causes deltapsim loss, caspase-9 activity, and apoptosis in Jurkat cells. Exp Cell Res. 2000;258:223–35. doi: 10.1006/excr.2000.4924. [DOI] [PubMed] [Google Scholar]
- 24.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]