Abstract
Hypoxia promotes pancreatic cancer progression by triggering cancer cell invasion. However, the mechanism underlying this process remains unclear, hindering the development of effective therapies. The present study aimed to delineate the molecular mechanisms underlying the prometastatic effect of hypoxia in pancreatic cancer cells. The expression of microRNA-150 (miRNA-150) was detected using reverse transcription-quantitative polymerase chain reaction in pancreatic cancer samples and in the hypoxia-induced CaPan2 human pancreatic cancer cell line. The target gene was identified using bioinformatics and a luciferase reporter assay. Inhibition of the expression of C-X-C chemokine receptor type 4 (CXCR4) by miRNA-150 was confirmed using transfection with miRNA-150 mimics. The prometastatic effect of hypoxia was detected using migration assays. The expression of miRNA-150 was shown to be downregulated in pancreatic cancer samples compared with that in normal pancreatic tissue samples. Furthermore, its expression was reduced in hypoxia-induced CaPan2 cells, compared with that in control cells. Bioinformatics and the results of the luciferase reporter assay, demonstrated that miRNA-150 inhibited the expression of CXCR4 by directly targeting the 3′ untranslated region of CXCR4 mRNA. The results of the migration assay showed that hypoxia promotes cell migration and invasion. However, this prometastatic effect was reversed by transfection with miRNA-150 mimics. The present results suggest that hypoxia promotes pancreatic cancer migration by downregulating miRNA-150.
Keywords: hypoxia, pancreatic cancer, miRNA-150, C-X-C chemokine receptor type 4
Introduction
Pancreatic cancer (PC) is among the most lethal malignancies, with a high incidence and rate of metastasis (1). At the time of diagnosis, the majority of patients are at an advanced stage of disease, with multi-organ metastasis, which indicates a poor prognosis for digestive system tumors (2). It is challenging to diagnose PC at an early stage, which results in a low 5-year survival rate; only 4% of patients diagnosed with pancreatic cancer survive after 5 years in China (3). Therefore, the identification of novel gene targets, which are differentially expressed in PC and functionally involved in the development of malignant phenotypes, is required in order to enable early diagnosis and the development of effective therapeutic strategies.
Hypoxia is an important characteristic of solid tumors. As a tumor increases in size, it quickly outgrows its blood supply, leaving regions of the tumor, in which the oxygen concentration is significantly lower than that in normal tissues. In order to support tumor growth and proliferation within hypoxic environments, the expression of a number of genes is altered, and changes in metabolism also occur (4). Recently, a novel class of endogenous small non-coding regulatory RNAs, termed microRNAs (miRNAs), has received increasing attention. These small molecules exert their regulatory effects by base pairing with partially complementary messenger RNAs (mRNAs). They act via one of two mechanisms: Degradation of target mRNA or inhibition of its translation. It has been shown that miRNAs are involved in the development of cancer via alteration of the expression of oncogenes or tumor suppressor genes. Increasing evidence, accumulated using microarray technology, has indicated a number of miRNAs that are differentially expressed in response to hypoxia. For example, miRNA-210, -155, -372/373 and -10b were shown to be upregulated, whereas miR-20b and -200b were found to be downregulated in response to hypoxia (5–8). A recent study suggested that miRNA-150, a hypoxia-sensitive miRNA, is involved in cancer metastasis via regulation of its target genes (9).
The present study investigated the role of hypoxia in the regulation of miRNA-150 and the expression of its target gene, C-X-C chemokine receptor type 4 (CXCR4), in primary PC tissues and PC cells. The findings demonstrated that hypoxia downregulates the expression of miRNA-150 in PC cells. Furthermore, it was shown that miRNA-150 directly targets the 3′ untranslated region (UTR) of CXCR4 mRNA, thereby suppressing its expression. Downregulation of miRNA-150 by hypoxia also led to a concomitant increase in CXCR4 expression. The present findings also demonstrated that miRNA-150 overexpression leads to increased migration and invasion of hypoxia cultured PC cells. In conclusion, the present study suggests a novel mechanism underlying the hypoxia-induced promotion of tumor invasion.
Materials and methods
Clinical samples and cell line
A total of 15 pancreatic tissue samples were obtained from patients who had undergone pancreatoduodenectomy at Urumqi General Hospital (Urumqi, China) between 2008 and 2012. None of these patients received radiotherapy or chemotherapy prior surgery. Three parts of pancreatic tissues from each of 15 patients with PC were collected: Tumor tissues, adjacent non-tumor pancreatic tissues within 2 cm, and tumor free tissues 5 cm distance from the tumor edge. All PC and normal pancreatic tissue samples were histologically confirmed. Tissues were snap frozen in liquid nitrogen following surgical resection, prior to use. Written informed consent conforming to the tenets of the Declaration of Helsinki, was obtained from each participant prior to the study. The institutional review boards of the Chinese PLA Urumqi General Hospital Ethics Committee (Urumqi, China) approved the current study.
The CaPan2 human pancreatic cancer cell line was obtained from the State Key Laboratory of Cancer Biology at Fourth Military Medical University (Xi'an, China). The cell line was maintained in RPMI-1640, supplemented with 10% FBS. For the induction of hypoxia, cells were incubated in temperature-controlled hypoxic culture chambers with 1% O2, 5% CO2 and 94% N2. Hypoxia induced cells were collected following incubation for 0, 8, 12 and 24 h respectively, and the miRNA-150 expression was detected at these different time points.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was extracted using a mirVanamiR isolation kit (Life Technology, USA), according to the manufacturer's instructions. Following extraction, all RNAs were treated with DNase in order to remove genomic DNA. The first strand cDNA was synthesized using RT2miRNA First Strand kit (Qiagen China, Shanghai, China) and specific miRNA-150 primers (Qiagen China) were used for RT-qPCR. The sequence of the primers were as follows: F 5′-TCT CCC AAC CCT TGT ACC-3′ and R 5′-CGA GGA AGA AGA CGG AAG AAT-3′. The PCR cycling conditions used were: 35 cycles of 2 sec at 92°C and 10 s at 70°C. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control, by which to calculate relative target gene expression levels. Relative expression was calculated using the comparative Ct method (2−ΔΔCt) (10,11). All PCR reactions were performed in triplicate.
miRNA-150 mimic or inhibitor transfection
The miRNA-150 mimic (miRNA-150-agomir) and inhibitor (miRNA-150-antagomir) were obtained from GenePharma Company (Shanghai, China). The day prior to transfection, CaPan2 cells were seeded in antibiotic-free medium. Transfections were conducted using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. In order to monitor transfection efficiency, fluorescein (FAM) siRNA (GenePharma Company) was used as a control. Successfully transfected cells were observed using a fluorescence microscope (BX51, Olympus Corporation, Tokyo, Japan).
Western blot analysis
CaPan2 cells or tissue samples were lysed in lysis buffer [50 mmol/l Tris (pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP-40, 0.5% Triton X-100, 2.5 mmol/l sodium orthovanadate, 10 µl/ml protease inhibitor cocktail and 1 mmol/l PMSF] by incubating for 20 min at 4°C. Whole cell protein extracts were quantified using a bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL, USA). Total proteins were concentrated and separated using 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis. The proteins were transferred to polyvinylidene difluoride membranes (AmershamBiosciences, Pittsburgh, PA, USA) and sequentially incubated with rabbit anti-human antibody to CXCR4 (cat. no. ab1047, 1:500; Abcam, Cambridge, MA, USA) for overnight at 4°C. Following this incubation the membrane was rinsed and incubated in a horseradish peroxidase (HRP)-conjugated mouse anti-rabbit monoclonal antibody (cat. no. ZB5301, 1:1,000; Zhongshan Goldenbridge, Ltd., China) for 1 h at room temperature. A HRP-conjugated rabbit anti-GAPDH polyclonal antibody (cat no. sc-25778 HRP, 1:1,000; SantaCruz Biotechnology, Dallas TX, USA) was used for the analysis of protein loading. Bands were developed using enhanced chemiluminescence western blotting detection reagents (Thermo Fisher, Rockford, IL, USA).
Migration assay
Cell invasion was analyzed with Matrigel-coated Transwell™ cell culture chambers (8-µm pore size; Millipore, Billerica, MA, USA). Briefly, differently treated cells (5×104 cells/well) were serum starved for 24 h at 37°C and plated in the upper insert of a 24-well chamber in a serum-free medium. A medium containing 10% serum as a chemoattractant was added to the wells. Cells were incubated for 24 h. Cells on the upper side of the filters were mechanically removed by scrubbing with a cotton swab, following which the membrane was fixed with 4% formaldehyde for 10 min at room temperature and stained with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 10 min. Finally, invasive cells were counted using a light microscope (E100T HD, Nikon Corporation, Japan) at x200 magnification in 6 different fields from each filter (12).
Statistical analysis
All data are presented as the mean ± standard deviation. Comparisons between two mean values were made using an unpaired Student two-tailed t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
miRNA-150 expression was downregulated in PC tissues and hypoxia-induced CaPan2 cells
In order to investigate the association between miRNA-150 and hypoxia, the expression of miRNA-150 was evaluated in PC samples from 15 patients. As demonstrated by RT-qPCR analysis, miRNA-150 expression was significantly reduced in PC tissues compared with tumor free pancreatic tissues (P<0.05). In addition, there was a trend (P=0.073) towards reduced expression in adjacent non-tumor tissues compared with tumor free pancreatic tissues. The expression of miRNA-150 in hypoxia-cultured CaPan2 cells was subsequently examined. The expression of miRNA-150 was downregulated following culture in hypoxic conditions for 8 h. These results demonstrated that hypoxia may downregulate the expression of miRNA-150 (Fig. 1).
Figure 1.
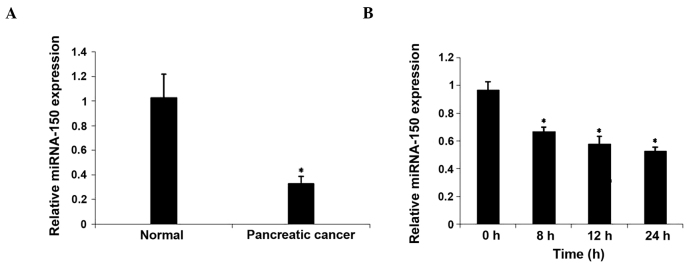
Expression of miRNA-150 was downregulated in pancreatic cancer tissues and hypoxia induced CaPan2 cells. Pancreatic cancer cell lines were incubated in 1% O2 for the durations indicated, and examined for the induction of gene expression. (A) RT-qPCR measurement of miRNA-150 expression in pancreatic cancer and normal tissues. *P<0.05 compared with tumor free tissue. (B) RT-qPCR measurement of miRNA-150 expression at various time points (0, 8, 12 and 24 h). *P<0.05 compared with 0 h. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; miRNA, microRNA.
miRNA-150 directly targets the 3′UTR of CXCR4 mRNA
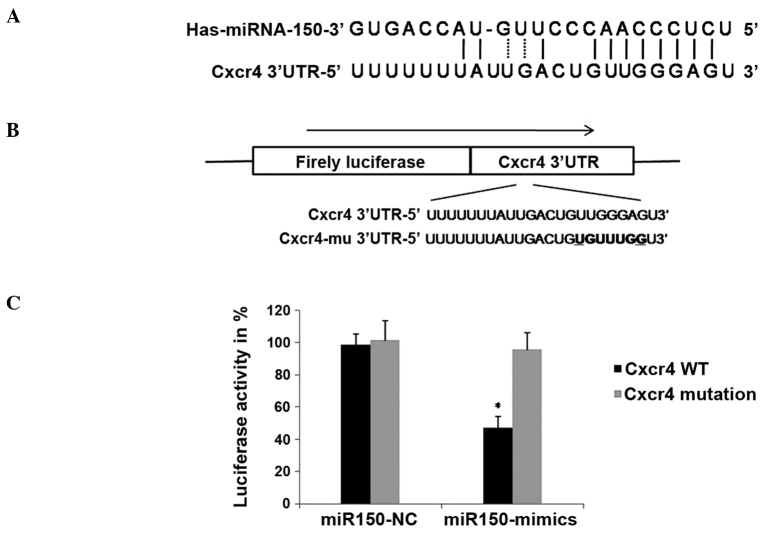
Bioinformatics was employed to identify potential targets of miRNA-150, using TargetScan (www.targetscan.org) and miRanda (www.microrna.org). Over 100 genes with sequence matching for the mature miRNA-150 sequence were identified. Given that miRNAs frequently target multiple genes post-transcriptionally, miRNA-150 may exert its effects by negatively regulating genes involved in cell migration and invasion (Fig. 2A). Therefore, CXCR4, which is known to be involved in the migration of a number of types of cancer (13), was selected as a candidate.
Figure 2.
CXCR4 is a target of miRNA150. (A) Predicted duplex formation between human CXCR4 3′-UTR and miRNA-150. (B) Luciferase report vector: The miRNA-150 binding site in the 3′-UTR and mutated sites in the 3′-UTR of CXCR4 were cloned into a luciferase report vector. (CXCR4-3′-UTR-pIS0/mu-CXCR4-3′-UTR-pIS0). (C) Luciferase activity of CaPan2 cells cotransfected with CXCR4-3′-UTR-pIS0 or mu-CXCR4-3′-UTR-pIS0, and miR-150 mimics or NC. *P<0.05 compared with CaPan2 cells cotransfected with CXCR4-3′UTR-pIS0 miRNA-150 mimics. CXCR4, C-X-C chemokine receptor type 4; UTR, untranslated region; miRNA, microRNA; NC, normal control; WT, wild type.
In order to confirm direct targeting of CXCR4 by miRNA-150, a DNA fragment containing the region of the CXCR4 3′UTR, in which the miRNA-150 target site is located, was integrated into a luciferase reporter vector (Fig. 2B), and cotransfected in CaPan2 cells with the miRNA-150 mimic or miRNA-NC (non-targeting control) (14). As a control, a vector containing a CXCR4 3′UTR with a mutation in the miRNA-150 target region in order to disrupt its binding, was generated and cotransfected into CaPan2 cells with the miRNA-150 mimics or miRNA-NC. Luciferase activity was measured after 24 h of transfection. The results showed that miRNA-150 overexpression significantly inhibited the expression of luciferase in the vector containing a wild type miRNA-150 binding site, compared with the NC group (Fig. 2C). This inhibition was reversed by mutations in the seed complementary sites of the CXCR4 3′UTR. These results suggested that miRNA-150 may negatively regulate the expression of CXCR4 by directly targeting the 3′UTR of CXCR4 mRNA.
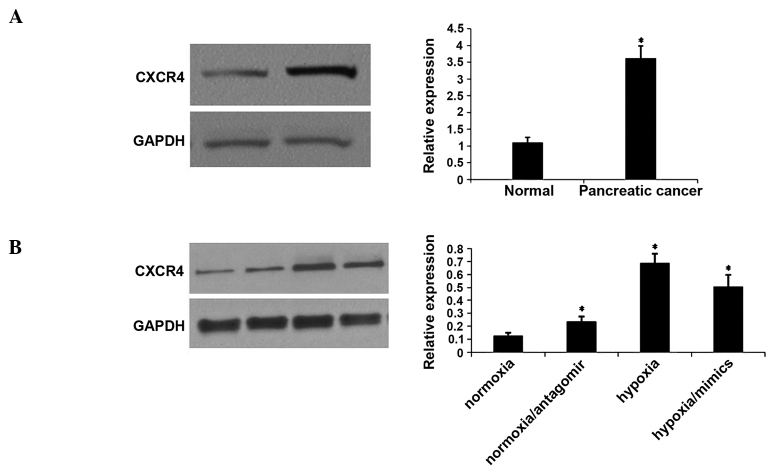
Hypoxia promotes CXCR4 expression through downregulation of miRNA-150
In order to investigate the effect of hypoxia on CXCR4 expression, the level of the CXCR4 protein was measured in tissue samples and hypoxia-induced CaPan2 cells. Western blotting demonstrated that CXCR4 protein expression was significantly increased in PC tissues compared with distant normal tissues (Fig. 3A). The expression of the CXCR4 protein was upregulated in hypoxia-induced CaPan2 cells. However, miRNA-150 mimics reversed the upregulation of CXCR4, which had been induced by hypoxia. By contrast, the miRNA-150 inhibitor increased the expression of CXCR4 under normoxic conditions (Fig. 3B).
Figure 3.
Hypoxia promotes the expression of CXCR4 via downregulation of miRNA-150. A western blot assay was used to analyze the expression of CXCR4 in (A) Pancreatic and tumor free tissues (normal) *P<0.05 compared with tumor free tissues. (B) The expression of CXCR4 in CaPan2 cells.*P<0.05 compared with normoxia-treated CaPan2 cells. Data are presented as the mean ± standard deviation. CXCR4, C-X-C chemokine receptor type 4; miRNA, microRNA.
Hypoxia promotes CaPan2 cell invasion and migration, through its effects on miRNA-150 and CXCR4
The aggressiveness of a cancer cell is determined by its capacity to invade through the basement membrane. CXCR4 is a well-established mediator of metastasis in numerous types of cancer (13,15.16). The present study investigated whether hypoxia promotes cancer cell migration and invasion through its effects on miRNA-150 and CXCR4. The results demonstrated that hypoxia leads to increased cell migration and invasion. However, transfection of the miRNA-150 mimic reversed this prometastatic effect. Furthermore, the miRNA-150 inhibitor promoted cell migration and invasion under normoxic conditions (Fig. 4). These results suggested that hypoxia may promote migration and invasion via its effects on miRNA-150 and CXCR4.
Figure 4.
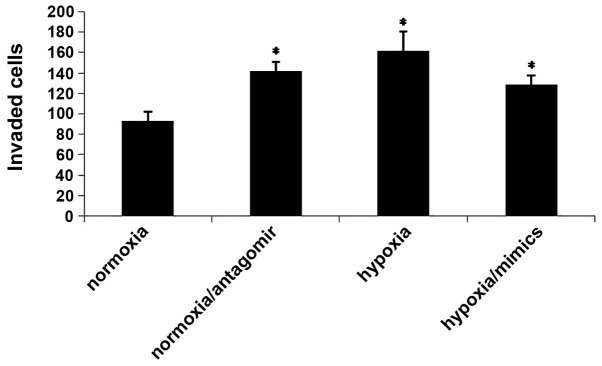
Hypoxia promotes migration and invasion via its effects on miRNA-150 and CXCR4. Following various treatment of CaPan2 cells, the invasion and migration capacities were measured using Transwell cell culture chambers. CaPan2 cells were treated with 50 mol/l antagomir or NC. Data are presented as the mean ± standard deviation. *P<0.05, compared with non-treated CaPan2 cells. CXCR4, C-X-C chemokine receptor type 4; miRNA, microRNA; PC, pancreatic cancer; NC, normal control.
Discussion
In order to adapt to hypoxia, tumors develop alterations in a number of functions, such as metabolism, migration, progress through the cell cycle and gene expression. The expression of certain genes is involved in various biological processes, including survival, migration and apoptosis. There is accumulating evidence that dysregulation of the expression of particular miRNAs occurs following exposure to hypoxia. These miRNAs have been implicated in a broad range of biological processes including cell proliferation, apoptosis, differentiation, metabolism, migration and invasion (17,18). A previous study confirmed that miR-210 is induced by hypoxia in hepatocellular carcinoma cells and may promote cancer cell metastasis (19). Vacuole membrane protein 1 (VMP1) has been identified as a direct target of miR-210, and downregulation of VMP1 by hypoxia has been shown to increase cancer cell migration (19,20). A separate study suggested that miRNA-103, miRNA-107, miRNA-372 and miRNA-373 may be upregulated in hypoxic conditions, through the transcriptional regulation of hypoxia inducible factor-1α. These miRNAs affect tumor behavior by decreasing the expression of their respective target genes (2). However, certain miRNAs, such as miRNA-20b and miRNA-200b, have also been shown to be downregulated in response to hypoxia (4).
The present study measured the expression of miRNA-150 in pancreatic cancer tissues and hypoxia-induced CaPan2 cells. The results indicated that miRNA-150 was significantly downregulated in primary tumors and hypoxia-induced cell lines. This suggests that hypoxia may downregulate miRNA-150 in PC cells.
Using bioinformatics, the results of the current study demonstrated that CXCR4 is a potential target gene of miRNA-150. miRNA-150 binds the 3′UTR of CXCR4 mRNA though partial complementary elements. The regulation of the expression of CXCR4 by miRNA-150, was confirmed using a luciferase assay and transfection with an miRNA-150 mimic. Over recent years, upregulation of CXCR4 and its unique ligand, stromal cell-derived-factor-1 (SDF-1), has been reported as an independent prognostic factor for disease relapse and survival in patients with PC (13,16). In order to further examine the mechanism underlying the effect of miRNA-150 in PC cells, the expression of CXCR4 in PC tissue samples and cultured cell lines was measured. The results demonstrated that CXCR4 was overexpressed in PC tissues and hypoxia-induced cells. By contrast, this increase in the expression of the CXCR4 protein was reversed when cells were transfected with the miRNA-150 mimics. These results suggested that hypoxia may promote CXCR4 expression via the downregulation of miRNA-150.
In conclusion, the present results suggest that hypoxia may promote CXCR4 expression and PC cancer cell migration via the downregulation of miRNA-150 expression. To the best of our knowledge, the current study identified, for the first time, a hypoxia/miRNA-150/CXCR4/SDF-1 axis in human pancreatic cells that may be responsible for cancer cell migration and invasion.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant no's. 81300596 and 81201000).
References
- 1.Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Surg Oncol. 2013;107:1–7. doi: 10.1002/jso.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D, Abbruzzese JL. New strategies in pancreatic cancer: Emerging epidemiologic and therapeutic concepts. Clin Cancer Res. 2010;16:4313–4318. doi: 10.1158/1078-0432.CCR-09-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Li B, Chen D, Liu L, Huang C, Lu Z, Lun L, Wan X. miR-139 and miR-200c regulate pancreatic cancer endothelial cell migration and angiogenesis. Oncol Rep. 2015 doi: 10.3892/or.2015.3945. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Esencay M, Sarfraz Y, Zagzag D. CXCR7 is induced by hypoxia and mediates glioma cell migration towards SDF-1α. BMC Cancer. 2013;13:347. doi: 10.1186/1471-2407-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen G, Li X, Jia YF, Piazza GA, Xi Y. Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin. 2013;34:336–341. doi: 10.1038/aps.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noman MZ, Buart S, Romero P, et al. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 7.Pocock R. Invited review: Decoding the microRNA response to hypoxia. Pflugers Arch. 2011;461:307–315. doi: 10.1007/s00424-010-0910-5. [DOI] [PubMed] [Google Scholar]
- 8.Bruning U, Cerone L, Neufeld Z, et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1 alpha activity during prolonged hypoxia. Mol Cell Biol. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu ZY, Bai YN, Luo LX, Wu H, Zeng Y. Expression of microRNA-150 targeting vascular endothelial growth factor-A is downregulated under hypoxia during liver regeneration. Mol Med Rep. 2013;8:287–293. doi: 10.3892/mmr.2013.1493. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley J, Roberts D, Bond A, Keys D, Chen C. Stem-loop RT-qPCR for microRNA expression profiling. Methods Mol Biol. 2012;822:33–52. doi: 10.1007/978-1-61779-427-8_3. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF. The down-regulation of Notch1 inhibits the invasion and migration of hepatocellular carcinoma cells by inactivating the cyclooxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci. 2013;58:1016–1025. doi: 10.1007/s10620-012-2434-7. [DOI] [PubMed] [Google Scholar]
- 13.Oh YS, Kim HY, Song IC, Yun HJ, Jo DY, Kim S, Lee HJ. Hypoxia induces CXCR4 expression and biological activity in gastric cancer cells through activation of hypoxia-inducible factor-1α. Oncol Rep. 2012;28:2239–2246. doi: 10.3892/or.2012.2063. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava SK, Bhardwaj A, Singh S, et al. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikand K, Slaibi JE, Singh R, Slane SD, Shukla GC. miR 488* inhibits androgen receptor expression in prostate carcinoma cells. Int J Cancer. 2011;129:810–819. doi: 10.1002/ijc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Fei M, Xue G, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: New insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–259. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas S, Roy S, Banerjee J, et al. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci USA. 2010;107:6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying Q, Liang L, Guo W, et al. Hypoxia-inducible microRNA-210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology. 2011;54:2064–2075. doi: 10.1002/hep.24614. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Zhao L, Chen W, Li Z, Hou H, Ding L, Li X. Inactivation of von Hippel-Lindau increases ovarian cancer cell aggressiveness through the HIF1α/miR-210/VMP1 signaling pathway. Int J Mol Med. 2014;33:1236–1242. doi: 10.3892/ijmm.2014.1661. [DOI] [PubMed] [Google Scholar]