Abstract
Shark liver oil (SLO) has long been used as a traditional health food, with a particular benefit for vascular health, in Japan. The aim of this study was to assess the effect of dietary supplementation with SLO on arterial stiffness and peripheral microvascular function in otherwise healthy middle-aged and older males with slightly increased arterial stiffness. A randomized, double-blind, placebo-controlled, parallel study design was used to assign 41 healthy males with a mean age of 59.0±4.0 years (range, 45–69 years) to either SLO (n=21) or placebo (n=20) treatment for eight weeks. The effects on arterial stiffness and peripheral microvascular function were assessed by the cardio-ankle vascular index (CAVI) and by measurement of hand blood flow to cutaneous tissues using a laser Doppler perfusion imaging (LDPI) technique, respectively. Although the magnitude of the changes in the CAVI value during the eight-week intervention for the SLO group did not significantly differ from that for the placebo group, the changes in the CAVI value for the former group were significantly associated (r=0.575, P<0.01) with age. It was also found that the LDPI values at week 8 were significantly lowered (P<0.05) compared with the baseline values in the placebo group, while no change was observed in the SLO group, resulting in a significant difference in the changes between the two groups (P=0.002). Neither SLO supplementation-related adverse side-effects nor any abnormal changes in routine laboratory tests, including lipid profiles and anthropometric and haemodynamic parameters, were observed throughout the intervention. SLO may have the potential to safely improve vascular health in middle-aged and elderly males.
Keywords: shark liver oil, blood flow, cardio-ankle vascular index, laser Doppler perfusion imaging, arterial stiffness
Introduction
Shark liver oil (SLO) has long been used as a dietary supplement with health-promoting activities, particularly for cardiovascular health, in Japan. At present, several SLO-containing supplements are commercially available and their dietary consumption is increasing among the Japanese middle-aged and elderly populations, most likely due to the fact that there has been considerable interest in alternative therapies.
SLO is known to contain large quantities of squalene and, thus, to be considered as the richest source of the compound. Squalene received its name as a result of its first isolation from liver oil of sharks (Squalus spp.) (1). Later, squalene was found in variety of vegetable oils, including olive, palm, wheat-germ and rice bran oils. Chemically, squalene is a polyprenyl compound, having a structural similarity with β-carotene, coenzyme Q10 and vitamins A, E and K (2). Among these squalene-related compounds, vitamin E, known as the most potent lipid-soluble antioxidant in vivo, has been most intensively studied for its effects on cardiovascular health; there have been a number of studies reporting that vitamin E is effective in decreasing arterial stiffening in overweight hypertensive patients (3,4), and others reporting that vitamin E supplementation improves peripheral vascular diseases due to diabetes mellitus and atherosclerosis-associated intermittent claudication (5–7). It may be, therefore, that this antioxidative vitamin has a beneficial effect on arterial stiffness, which has been demonstrated to be associated with an increased risk of cardiovascular events and mortality (8–10), as well as on peripheral microvascular function.
These results obtained with vitamin E, a squalene-related antioxidant compound, led to our hypothesis that supplementation with SLO rich in squalene may also have the potential to exert a similar central and/or peripheral vascular modification. To the best of our knowledge, no data regarding the vascular effects of SLO preparations or their major constituent, squalene, in humans have been reported. The aim of the present study is therefore to investigate the efficacy, as well as tolerance, compliance and safety, of supplementation with a commercially available SLO preparation in otherwise healthy middle-aged and elderly males with marginal arterial stiffness.
Subjects and methods
Subjects
Healthy, male, non-overweight Japanese participants without hypertension or hyperlipidaemia, aged 45–69 years, were recruited for inclusion in this study. Participants with a higher cardio-ankle vascular index (CAVI) were given inclusion preference. Subjects were excluded if they were receiving any dietary supplement, cosmetics or medicines rich in SLO or its major constituents (squalene and alkylglycerols). Subjects were also excluded if they currently suffered from any diagnosed diseases requiring medical treatment or had a past history of such medical conditions, or if they had stopped smoking within the six months prior to the study run-in period. The other exclusion criteria were as follows: i) Known allergies to SLO or any other major components of the study supplement, including gelatin, glycerin and processed starch; ii) participation in another clinical study within the month before the initiation of the present study; and iii) the presence of any medical condition judged by the investigator to preclude the participant's inclusion in the study. Written informed consent was obtained from all participants prior to their enrolment in the study.
Study design
A randomized, double-blind, placebo-controlled study was designed to assess the efficacy and safety of supplementation with SLO for improving the arterial stiffness and peripheral microvascular function in enrolled participants when compared with placebo. The study was performed between August and November 2012. The study protocol was approved by the Tana Orthopedic Surgery Institutional Review Board (Yokohama, Japan), and the study was conducted in accordance with the principles of the Declaration of Helsinki (1995; as revised in Edinburgh, 2000) and the Ethical Guidelines for Epidemiological Research (enacted by the Japanese Government in 2008). The overall design of the study consisted of an eight-week intervention period preceded by a three-week run-in period, during which eligible subjects were screened.
Intervention and subject assessment
The test supplement was a commercially available product manufactured by Egao Co., Ltd. (Kumamoto, Japan) in a form of 400-mg capsule. The supplement contained 250 mg SLO and 150 mg vehicle (consisting of 97 mg gelatin, 34 mg glycerol and 19 mg processed starch) and was referred to as the SLO capsule. In the placebo capsule, SLO was substituted by the same amount of safflower oil. Chemical analysis of the SLO preparation used in this study revealed that it was almost free of n-3 long-chain fatty acids and mainly composed of (w/w) squalene (38.8%) and alkylglycerols (43.6%), indicating that a daily dose of the SLO capsule contained squalene at 582 mg in weight. Safflower oil used for preparing the placebo capsule was virtually free of squalene (<0.004%). The SLO and placebo capsules were similar in colour and packaging. Subjects were assigned to receive six SLO capsules per day or six placebo capsules per day and were instructed to take the allocated capsules once daily following a meal (either breakfast, lunch or dinner) with the aid of a cup of drinking water during the eight-week intervention period.
Each participant was instructed to maintain a study diary, covering the time from the start of the run-in period to the end of the intervention period, describing their allocated capsule intake, dietary composition, any adverse effects, physical activity and all medication and therapy received. Participants were also requested to maintain their body weight and to continue with their normal exercise, eating and drinking habits. At the completion of the run-in period, the eligible subjects were sequentially assigned to receive one of the two masked study capsules (SLO or placebo) and instructed to take six of the allocated capsules once daily, following a meal. Non-compliance was defined as administration of <80% of the total course of the allocated study capsule. All non-compliant subjects were excluded from the efficacy assessment.
Measurement of the CAVI
To determine the extent of the arterial stiffness, a relatively new stiffness diagnostic parameter, known as the CAVI, was used. The CAVI was selected as it is can be measured easily and non-invasively and is not influenced by blood pressure changes during measurement (11,12). Furthermore, it has been reported to be independent of blood pressure levels (13–15). In principle, the CAVI is measured from an electrocardiogram, a phonocardiogram and brachial artery and ankle arterial waveforms and calculated using a specific algorithm (13). An epidemiological study on the clinical interpretation of CAVI values in a large Japanese population suggested that values <8.0 can be considered within the normal range and that those ≥9.0 are indicative of suspected atherosclerosis (16).
In the present study, measurement of the CAVI was performed with a VaSera VS-1500 vascular screening system (Fukuda Denshi Co., Ltd., Tokyo, Japan) using the method described by Yambe et al (13). The subjects were placed in a supine position in a room kept at a room temperature and cuffs were applied to the bilateral upper arms and ankles. Subsequent to the subject having rested for ≥10 min, automatic measurements were performed twice for the right and left extremities. Averages of the right and left CAVIs measured in the run-in period were compared with each other, and the side with a higher CAVI value was selected as the target for CAVI measurement and data analysis.
Measurement of laser Doppler perfusion imaging (LDPI)
Peripheral microvascular function was assessed on the basis of hand blood flow to surface tissues measured by an LDPI technique. The examination was performed in a room with a constant temperature (~24°C) and humidity (~50%) and the subjects were acclimatized for ≥20 min. The cutaneous blood flow on the dorsum of the left hand was measured with a laser Doppler perfusion imager (Periscan PIM-II; Perimed AB, Stockholm, Sweden) (17) and is expressed in arbitrary perfusion units (PU) (18).
Measurement of haematochemical, haematological, anthropometric and haemodynamic parameters
Total cholesterol, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, triglycerides (TG) and other routine haematochemical laboratory test variables were measured in serum samples collected from individual subjects following an overnight fast at baseline (week 0) and at eight weeks after the start of intervention (week 8). Haematological variables, including red blood cell, white blood cell and platelet counts, as well as haemoglobin and haematocrit, were measured in whole blood. Several anthropometric and haemodynamic parameters, including weight, body mass index (BMI), blood pressure (BP) and heart rate, were also measured at weeks 0 and 8.
Safety assessment
Safety was assessed based on the incidence of intervention-related adverse events recorded during the eight-week intervention period, as well as on abnormal changes observed in the haematochemical and haematological test variables and anthropometric and haemodynamic parameters.
Statistical analyses
All analyses were performed using Microsoft Excel [Microsoft Corporation (2003), Redmond, WA, USA] and PASW Statistics (version 18; SPSS Inc., Chicago, IL, USA). Comparisons between the baseline characteristics of the placebo and SLO groups were conducted using unpaired t-tests, and paired t-tests were utilized for intragroup comparisons for all variables. The respective changes within the intervention groups were compared using analysis of covariance, taking the baseline (week 0) value as the covariate. The simple linear regression model was used to examine the associations. P<0.05 was considered to indicate a statistically significant difference. Values in the text are presented as the mean ± standard error of the mean or as the change from week 0.
Results
Enrolment and baseline characteristics
A total of 42 subjects were enrolled in the study, and 21 subjects were randomly assigned to each of the placebo and the SLO supplement interventions (placebo and SLO groups, respectively). A total of 41 subjects completed all components of the study protocol, resulting in a retention rate of 98%. One subject (placebo group) withdrew due to unrelated personal reasons during the study. There was no incident of unmasking of subject assignment.
Table I shows the baseline characteristics of the 41 subjects who completed the study. Their mean age at enrolment was 59.0±4.0 years (range, 45–69 years). The mean values of all the anthropometric, haemodynamic and haematochemical parameters tested, as well as the CAVI, were within the normal range for clinical measurements, although the mean CAVI value was marginally high (8.63±0.10). Almost all the subjects were judged to be free from hypertension, hyperlipidaemia or atherosclerosis and to not be overweight.
Table I.
Baseline characteristics of the subjects who completed the study (n=41).
| Variable | Value |
|---|---|
| Age (years) | 59.0±4.0 |
| Weight (kg) | 65.5±4.8 |
| BMI (kg/m2) | 22.8±2.6 |
| Smoking habit (smokers, n/non-smokers, n) | 10/31 |
| CAVI | 8.63±0.10 |
| LDPI (PU) | 0.73±0.05 |
| Systolic BP (mmHg) | 132±6.7 |
| Diastolic BP (mmHg) | 83±5.1 |
| Heart rate (beats/min) | 70±4.8 |
| Serum total cholesterol (mg/dl) | 222±9.1 |
| Serum LDL-cholesterol (mg/dl) | 142±8.5 |
| Serum HDL-cholesterol (mg/dl) | 61±6.0 |
| Serum TG (mg/dl) | 119±12 |
| Serum glucose (mg/dl) | 92±4.0 |
Data (with the exception of smoking habits) are presented as the mean ± standard error of the mean. BMI, body mass index; CAVI, cardio-ankle vascular index; LDPI, laser Doppler perfusion imaging; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; PU, perfusion units.
Effects on main outcome measures
Table II shows the effects of SLO supplementation on the anthropometric, haemodynamic, haematochemical and vascular characteristics in comparison with those of the placebo over the eight-week intervention. No significant group difference in the variables was observed at week 0, with the exception of the LDPI, for which the value for the placebo group was significantly higher than that for the SLO group (0.76±0.02 versus 0.69±0.02 PU, P<0.05). The magnitude of the changes in the CAVI values over the eight weeks for the SLO group was not significantly different from that for the placebo group; however, there are a substantial number of reports demonstrating that the CAVI as an arterial stiffness parameter is strongly correlated with age (11,12,19–22). This finding raised the possibility that the potential effect of the SLO supplementation on the CAVI may be modified by the age of the study subjects; therefore, the association between the magnitude of the change in the CAVI and the age of subjects who received supplementation with SLO or placebo was examined. As shown in Fig. 1, the univariate linear regression analysis revealed that the changes (decreases) in the CAVI after the eight-week supplementation period were significantly correlated with the age in the SLO group (r=0.575, P<0.01), while such a significant correlation was not noted in the placebo group (r=0.242, P=0.303). Different from this, the baseline value of the CAVI was not associated with the changes in the CAVI for subjects receiving the SLO supplementation for eight weeks (r=0.179, P=0.438) nor for those receiving the placebo for eight weeks (r=0.099, P=0.678).
Table II.
Values of vascular, anthropometric, haemodynamic and haemobiochemical parameters for the placebo and SLO groups at weeks 0 and 8.
| Placebo (n=20) | SLO (n=21) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Week 0 | Week 8 | Change | Week 0 | Week 8 | Change | P-valueb |
| CAVI | 8.66±0.14 | 8.49±0.17 | −0.18±0.10 | 8.60±0.14 | 8.38±0.16 | −0.21±0.11 | 0.800 |
| LDPI (PU) | 0.76±0.02 | 0.68±0.02a | −0.08±0.02 | 0.69±0.02 | 0.70±0.02 | 0.00±0.01 | 0.002 |
| Weight (kg) | 65.4±1.9 | 65.3±1.8 | −0.1±0.3 | 65.7±2.3 | 66.1±2.3 | 0.4±0.2 | 0.150 |
| BMI (kg/m2) | 22.8±0.6 | 22.7±0.6 | −0.02±0.09 | 22.8±0.7 | 22.9±0.7 | 0.15±0.08 | 0.165 |
| Systolic BP (mmHg) | 132±4 | 128±4 | −3.0±2.1 | 133±4 | 127±4 | −5.3±3.2 | 0.563 |
| Diastolic BP (mmHg) | 82±2 | 82±2 | −0.2±1.4 | 83±2 | 81±3 | −2.6±2.0 | 0.319 |
| Heart rate (beats/min) | 69±2 | 67±3 | −2.0±1.8 | 71±2 | 71±2 | −0.1±1.1 | 0.369 |
| Serum total cholesterol (mg/dl) | 217±10 | 217±10 | −0.6±4.3 | 226±5 | 226±9 | −0.4± 8.9 | 0.923 |
| Serum LDL-cholesterol (mg/dl) | 138±8 | 138±8 | 0.1±3.8 | 147±5 | 148±8 | 1.5±8.3 | 0.874 |
| Serum HDL-cholesterol (mg/dl) | 62±3 | 64±3 | 1.7±1.4 | 59±4 | 59±3 | 0.5±1.6 | 0.582 |
| Serum TG (mg/dl) | 112±14 | 99±9 | −13.1±13.7 | 125±13 | 121±12 | −3.7±9.2 | 0.570 |
| Serum glucose (mg/dl) | 92±2 | 93±2 | 1.1±1.4 | 92±1 | 93±2 | 1.2±1.5 | 0.945 |
All values are expressed as the mean ± standard error of the mean.
Within-group difference from week 0 (P<0.05).
P-value refers to the comparison of within-group changes between the two intervention groups as assessed by analysis of covariance. SLO, shark liver oil; CAVI, cardio-ankle vascular index; LDPI, laser Doppler perfusion imaging; BMI, body mass index; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; PU, perfusion units.
Figure 1.
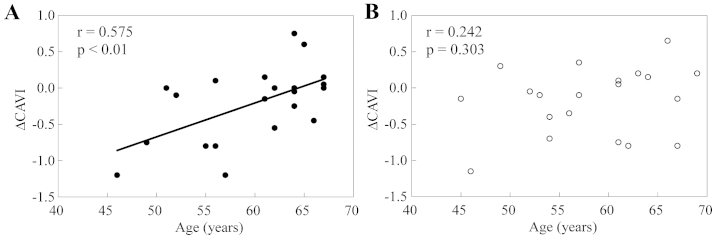
Association between the changes in the CAVI (ΔCAVI) and the age of the subjects during the eight-week supplementation with (A) SLO (SLO group, n=21) and (B) placebo (placebo group, n=20). A significant association between the changes in the CAVI and the age of the subjects can be noted in the SLO group but not in the placebo group. CAVI, cardio-ankle vascular index.
Regarding the cutaneous blood flow or peripheral microvascular function, the LDPI value for the placebo group at week 8 was significantly lower than that at week 0 (0.68±0.02 versus 0.76±0.02, P<0.05). This reduction may have been due to the seasonal drop in temperature during the eight-week intervention period starting in August to September and ending in October to November. It is well known that superficial blood flow is closely associated with the skin surface temperature (23). Despite this, such a reduction in LDPI values did not occur in the SLO group, leading to a significant difference in the change from the baseline value between the two groups (P=0.002, Table II). It is, therefore, suggested that SLO supplementation is effective in enhancing peripheral blood flow.
Effects on vascular health-related confounding factors
Table II also shows the changes in the mean values of weight, BMI, BP, serum lipids and serum glucose over the eight-week intervention, with comparisons between the placebo and SLO groups. No significant differences were observed in any parameters within or between the groups. No subject reported a significant change in dietary habit, physical activity or smoking behaviour during the study period, and no subject developed a noteworthy concurrent illness or was given any medication.
Safety assessment
The incidence and pattern of adverse events was virtually equivalent in the two groups. In the end-point study diary, 19% (4/21) of subjects taking the placebo and 29% (6/21) taking the SLO supplement reported minor adverse events (P=0.469), the most common being gastrointestinal upset with or without eruption. There was no intervention-related untoward side-effect with clinical significance. No abnormality in the routine laboratory test parameters was found in any subject in the two groups.
Discussion
In this study, the effect of eight weeks of dietary supplementation with 1,500 mg SLO (582 mg as squalene) daily was compared with that of the placebo on the CAVI and LDPI values, which are indexes of central arterial stiffness and peripheral microvascular function, respectively. The dose of 1,500 mg SLO was selected as it was the dose found to be well tolerated in our preliminary toxicological studies. It is also the dose that is most often taken by individuals using this SLO-containing supplement.
One of main findings of this study was the presence of a correlation between the SLO supplementation-induced changes in arterial stiffness and the age; lower ages were associated with greater decreases in arterial stiffness after the eight-week SLO supplementation period. No difference, however, was found in the mean changes of arterial stiffness between the placebo and SLO groups. This may be due to the wide and unmatched distribution of the age of the subjects in the two groups. To obtain conclusive results, a similar study conducted with several different, narrow-ranging age groups is therefore required.
Another main finding of this study was that eight weeks of SLO supplementation significantly increased the peripheral blood flow measured by an LDPI technique; however, the changes in the LDPI values for the SLO group were not correlated with the changes in the CAVI values. It is, therefore, likely that the effects of SLO on each of these two vascular parameters may be independent. Although the active components of SLO responsible for the improvement of central arterial stiffness and peripheral microvascular function by supplementation with SLO remain to be explored, they warrant discussion.
The SLO supplement used in the present study contained, in a daily dose, 582 mg squalene and 654 mg alkylglycerols as the two major constituents. Information on the biological or pharmacological activity of alkylglycerols, the largest constituent of SLO in quantity, in animals or humans is extremely limited. While the immunostimulatory and haematopoiesis-stimulating effects or anti-tumor and anti-metastasis activities of alkylglycerols have been reported (24,25), no data are available, to the best of our knowledge, on their central arterial or peripheral microvascular effects. Squalene, the second largest constituent of SLO, is a polyunsaturated triterpene containing six isoprene units and thus has a structural similarity with various naturally occurring polyprenyl compounds, including vitamin E (2). In vitro experimental evidence indicates that squalene is a unique antioxidant molecule exhibiting highly effective oxygen-scavenging activity (26,27). Furthermore, in previous studies utilizing an isoprenaline-induced myocardial infarction rat model it was shown that continuous oral administration of squalene produced antioxidant and cardioprotective effects by maintaining the levels of endogenous antioxidant molecules, such as vitamins A, C and E, and by blocking the induction of lipid peroxidation (28–30). It is, therefore, likely that squalene, as a polyprenyl antioxidant compound, has the structural and functional similarity with vitamin E that has been reported to be effective in improving arterial stiffness and microvascular function following continuous oral administration (13,14,16,17). These findings and the relatively high squalene content of SLO suggest that squalene is the most likely contributing factor to the beneficial vascular effects of SLO observed in the present study.
Orally administered squalene is absorbed well (60–85%) and is distributed to various tissues (31–33). Although the underlying mechanisms remain to be elucidated, the rapidity with which oral doses of SLO improve vascular structures and/or functions indicates a direct effect on the physiological components rather than the structural components that regulate the elasticity of the arterial wall, as was observed for vitamin E (34), as well as for fish oil (35,36) and n-3 long-chain fatty acids (37,38).
In the present study, supplementation with SLO and thus squalene did not show clinically significant effects on weight, BP and heart rate, or on serum levels of lipids, glucose or any other laboratory test parameters. Among these biological effects the major focus was on the effect on lipid profiles, as squalene is well-known as a biochemical precursor of cholesterol, leading to the possibility that orally administered squalene may increase serum levels of cholesterol; however, this possibility is challenged by a substantial number of existing in vitro and in vivo studies demonstrating that exogenous squalene exhibits feedback inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme functioning in cholesterol biosynthesis (39–41). Furthermore, Strandberg et al (31), who conducted a human study in which volunteers received a dietary supplement of squalene (900 mg/day for 7–30 days), reported that, while serum squalene levels were increased 17-fold, there was no significant change in serum TG or cholesterol levels, and that this was likely due to a significant increase in the faecal excretion of cholesterol synthesised from squalene, as well as of bile acids converted from squalene. These results support the findings of the present study showing that orally administered SLO, a dietary source of squalene, did not increase serum levels of cholesterol nor TG.
No significant adverse events that could be attributed to the SLO supplement were noted in this study. As neither SLO nor squalene have been extensively studied in humans, information on their toxicity and side-effects is limited. Kamimura et al (42), who conducted experiments in animals (rats and dogs), reported that no appreciable side-effects or toxic signs were observed in animals receiving squalene for three months. Despite this, the long-term effects and safety of extreme squalene are not known (43). Since SLO, as a dietary source of squalene, is of biological origin and frequently used as a dietary supplement, it appears likely that, at reasonable supplemental levels, such as those used in this study, SLO is safe for prolonged administration in humans.
To the best of our knowledge, there is no previous study that has investigated the effect of SLO on either arterial stiffness or peripheral blood flow or both in humans; however, as the present study has a limitation that the sample size was small, the findings, particularly those on the apparently age-related benefits of SLO supplementation on arterial stiffness, require confirmation in a larger population with a narrower range of ages.
In conclusion, SLO supplementation may have the potential to improve central arterial elasticity and peripheral microvascular function in otherwise healthy middle-aged and elderly males with slightly increased arterial stiffness. Further study is required to confirm the age-related beneficial vascular effect of SLO supplementation on arterial stiffness.
References
- 1.Tsujimoto M. A highly unsaturated hydrocarbon in shark liver oil. Ind Eng Chem. 1916;8:889–896. doi: 10.1021/i500010a005. [DOI] [Google Scholar]
- 2.Reddy LH, Couvreur P. Squalene: A natural triterpene for for use in disease management and therapy. Adv Drug Deliv Rev. 2009;61:1412–1426. doi: 10.1016/j.addr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Mottram P, Shiqe H, Nestel P. Vitamin E improves arterial compliance in middle-aged men and women. Atherosclerosis. 1999;145:399–404. doi: 10.1016/S0021-9150(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 4.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392–397. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Jörneskog G, Brismar K, Fagrell B. Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia. 1995;38:474–480. doi: 10.1007/BF00410286. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson L, Apelqvist J, Edvinsson L. Effects of alpha-trinositol on peripheral circulation in diabetic patients with critical limb ischaemia. A pilot study using laser Doppler fluxmetry, transcutaneous oxygen tension measurements and dynamic capillaroscopy. Eur J Vasc Endovasc Surg. 1998;15:331–336. doi: 10.1016/S1078-5884(98)80037-3. [DOI] [PubMed] [Google Scholar]
- 7.Collins EG, Edwin Langbein W, Orebaugh C, Bammert C, Hanson K, Reda D, Edwards LC, Littooy FN. PoleStriding exercise and vitamin E for management of peripheral vascular disease. Med Sci Sports Exerc. 2003;35:384–393. doi: 10.1249/01.MSS.0000053658.82687.FF. [DOI] [PubMed] [Google Scholar]
- 8.Gatzka CD, Cameron JD, Kingwell BA, Dart AM. Relation between coronary artery disease, aortic stiffness, and left ventricular stiffness in a population sample. Hypertension. 1998;32:575–578. doi: 10.1161/01.HYP.32.3.575. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Health ABC Study: Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Kubozono T, Miyata M, Ueyama K, Nagaki A, Otsuji Y, Kusano K, Kubozono O, Tei C. Clinical significance and reproducibility of new arterial distensibility index. Circ J. 2007;71:89–94. doi: 10.1253/circj.71.89. [DOI] [PubMed] [Google Scholar]
- 12.Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A, Matsuzaki M. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J. 2007;71:1710–1714. doi: 10.1253/circj.71.1710. [DOI] [PubMed] [Google Scholar]
- 13.Yambe T, Yoshizawa M, Saijo Y, Yamaguchi T, Shibata M, Konno S, Nitta S, Kuwayama T. Brachio-ankle pulse wave velocity and cardio-ankle vascular index (CAVI) Biomed Pharmacother 58 Suppl. 2004;1:S95–S98. doi: 10.1016/S0753-3322(04)80015-5. [DOI] [PubMed] [Google Scholar]
- 14.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13:101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 15.Matsui Y, Kario K, Ishikawa J, Eguchi K, Hoshide S, Shimada K. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res. 2004;27:851–857. doi: 10.1291/hypres.27.851. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Ishizuka N, Makoto M, et al. Establishment of reference values of CAVI and disease characterisitics. In: Omori H, Saito Y, editors. From Bench to Bedside: CAVI as a Novel Indicator of Vascular Function. Nikkei Medical Custom Publishing, Inc.; Tokyo: 2009. pp. 34–42. [Google Scholar]
- 17.Wådell K, Jakobsson A, Nilsson G. Laser Doppler perfusion imaging by dynamic light scattering. IEEE Trans Biomed Eng. 1993;40:309–316. doi: 10.1109/10.222322. [DOI] [PubMed] [Google Scholar]
- 18.Bornmyr S, Svensson H, Lilja B, Sundkvist G. Skin temperature changes and changes in skin blood flow monitored with laser Doppler flowmetry and imaging: a methodological study in normal humans. Clin Physiol. 1997;17:71–81. doi: 10.1046/j.1365-2281.1997.01313.x. [DOI] [PubMed] [Google Scholar]
- 19.Okura T, Watanabe S, Kurata M, Manabe S, Koresawa M, Irita J, Enomoto D, Miyoshi K, Fukuoka T, Higaki J. Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res. 2007;30:335–340. doi: 10.1291/hypres.30.335. [DOI] [PubMed] [Google Scholar]
- 20.Kadota K, Takamura N, Aoyagi K, Yamasaki H, Usa T, Nakazato M, Maeda T, Wada M, Nakashima K, Abe K, Takeshima F, Ozono Y. Availability of cardio-ankle vascular index (CAVI) as a screening tool for atherosclerosis. Circ J. 2008;72:304–308. doi: 10.1253/circj.72.304. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72:598–604. doi: 10.1253/circj.72.598. [DOI] [PubMed] [Google Scholar]
- 22.Sakane K, Miyoshi T, Doi M, Hirohata S, Kaji Y, Kamikawa S, Ogawa H, Hatanaka K, Kitawaki T, Kusachi S, Yamamoto K. Association of new arterial stiffness parameter, the cardio-ankle vascular index, with left ventricular diastolic function. J Atheroscler Thromb. 2008;15:261–268. doi: 10.5551/jat.E576. [DOI] [PubMed] [Google Scholar]
- 23.Mirbod SM, Yoshida H, Jamail M, Miyashita K, Takeda H, Inaba R, Iwata H. Finger skin temperature and laser-Doppler finger blood flow in subjects exposed to hand-arm vibration. Ind Health. 1998;36:171–178. doi: 10.2486/indhealth.36.171. [DOI] [PubMed] [Google Scholar]
- 24.Hallgren B, Niklasson A, Ställberg G, Thorin H. On the occurrence of 1-O-alkylglycerols and 1-O-(2-methoxyalkyl)glycerols in human colostrum, human milk, cow's milk, sheep's milk, human red bone marrow, red cells, blood plasma and a uterine carcinoma. Acta Chem Scand B. 1974;28:1029–1034. doi: 10.3891/acta.chem.scand.28b-1029. [DOI] [PubMed] [Google Scholar]
- 25.Deniau AL, Mosset P, Pédrono F, Mitre R, LeBot D, Legrand AB. Multiple beneficial health effects of natural alkylglycerols from shark liver oil. Mar Drugs. 2010;8:2175–2184. doi: 10.3390/md8072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saint-Leger D, Bague A, Cohen E, Chivot M. A possible role for squalene in the pathogenesis of acne. I. In vitro study of squalene oxidation. Br J Dermatol. 1986;114:535–542. doi: 10.1111/j.1365-2133.1986.tb04060.x. [DOI] [PubMed] [Google Scholar]
- 27.Kohno Y, Egawa Y, Itoh S, et al. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim Biophys Acta. 1995;1256:52–56. doi: 10.1016/0005-2760(95)00005-W. [DOI] [PubMed] [Google Scholar]
- 28.Sabeena Farvin KH, Surendraraj A, Anandan R. Protective effect of squalene on endogenous antioxidant vitamins in experimentally induced myocardial infarction in rats. Asian J Biochem. 2009;4:133–139. doi: 10.3923/ajb.2009.133.139. [DOI] [Google Scholar]
- 29.Sabeena Farvin KH, Kumar SHS, Anandan R, Mathew S, Sankar TV, Nair PGV. Supplementation of squalene attenuates experimentally induced myocardial infarction in rats. Food Chem. 2007;105:1390–1395. doi: 10.1016/j.foodchem.2007.05.034. [DOI] [Google Scholar]
- 30.Sabeena Farvin KH, Anandan R, Kumar SH, Shiny KS, Sankar TV, Thankappan TK. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol Res. 2004;50:231–236. doi: 10.1016/j.phrs.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Strandberg TE, Tilvis RS, Miettinen TA. Metabolic variables of cholesterol during squalene feeding in humans: comparison with cholestyramine treatment. J Lipid Res. 1990;31:1637–1643. [PubMed] [Google Scholar]
- 32.Miettinen TA, Vanhanen H. Serum concentration and metabolism of cholesterol during rapeseed oil and squalene feeding. Am J Clin Nutr. 1994;59:356–363. doi: 10.1093/ajcn/59.2.356. [DOI] [PubMed] [Google Scholar]
- 33.Gylling H, Miettinen TA. Postabsorptive metabolism of dietary squalene. Atherosclerosis. 1994;106:169–178. doi: 10.1016/0021-9150(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 34.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Tsunoda R, Moriyama Y, Miyao Y, Yoshimura M, Ogawa H, Yasue H. Vitamin E administration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J Am Coll Cardiol. 1998;32:1672–1679. doi: 10.1016/S0735-1097(98)00447-1. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Ma AQ, Song SW, Quan QH, Zhao XF, Zheng XH. Fish oil supplementation improves large arterial elasticity in overweight hypertensive patients. Eur J Nutr. 2008;62:1426–1431. doi: 10.1038/sj.ejcn.1602886. [DOI] [PubMed] [Google Scholar]
- 36.Fahs CA, Yan H, Ranadive S, Rossow LM, Agiovlasitis S, Wilund KR, Fernhall B. The effect of acute fish-oil supplementation on endothelial function and arterial stiffness following a high-fat meal. Appl Physiol Nutr Metab. 2010;35:294–302. doi: 10.1139/H10-020. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siasos G, Kioufis S, Maniatis K, Miliou A, Siasou Z. Effects of Ω-3 fatty acids on endothelial function, arterial wall properties, inflammatory and fibrinolytic status in smokers: a cross-over study. Int J Cardiol. 2013;116:340–346. doi: 10.1016/j.ijcard.2011.10.081. [DOI] [PubMed] [Google Scholar]
- 39.Sawada M, Matsuo M, Hagihara H, Tenda N, Nagayoshi A, Okumura H, Washizuka K, Seki J, Goto T. Effect of FR194738, a potent inhibitor of squalene epoxidase on cholesterol metabolism in HepG2 cells. Eur J Pharmacol. 2001;431:11–16. doi: 10.1016/S0014-2999(01)01411-X. [DOI] [PubMed] [Google Scholar]
- 40.Newmark HL. Squalene, olive oil, and cancer risk. Review and hypothesis. Ann NY Acad Sci. 1999;889:193–203. doi: 10.1111/j.1749-6632.1999.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 41.Smith TJ. Squalene: potential chemopreventive agent. Expert Opin Investig Drugs. 2000;9:1841–1848. doi: 10.1517/13543784.9.8.1841. [DOI] [PubMed] [Google Scholar]
- 42.Kaminura H, Koga N, Oguri K, Yoshimura H, Inoue H, Sato K, Ohkubo M. Studies on distribution, excretion and subacute toxicity of squalene in dogs. Fukuoka Igaku Zasshi. 1989;80:269–280. (In Japanese) [PubMed] [Google Scholar]
- 43.Sotiroudis TG, Kyrtopoulos SA. Anticarcinogenic compounds of olive oil and related biomarkers. Eur J Nutr. 2008;47(Suppl 2):69–72. doi: 10.1007/s00394-008-2008-9. [DOI] [PubMed] [Google Scholar]


