Abstract
The aim of the present study was to evaluate the efficacy and safety of a neoadjuvant gemcitabine and nedaplatin chemotherapy regimen, followed by concurrent chemoradiotherapy or radiotherapy alone, in locoregionally advanced nasopharyngeal carcinoma (NPC). Eighty-six patients with stage III, IVA or IVB NPC, who received neoadjuvant chemotherapy [gemcitabine, 1,000 mg/m2 on day 1 (d1) and d5; nedaplatin, 25 mg/m2 on d 1–3] every 3 weeks for at least two cycles, followed by intensity-modulated radiotherapy every 3 weeks, with or without concurrent nedaplatin (25 mg/m2, d1-3) between September 2010 and December 2013, were retrospectively analyzed. By comparing pretreatment and post-treatment MRI images, it was shown that seven patients achieved a complete response (8.5%), while 66 achieved a partial response (80.5%), following completion of neoadjuvant chemotherapy (combined response rate, 89.0%). Grade 3–4 toxicities following neoadjuvant chemotherapy included neutropenia (29.1%), leukopenia (11.6%), liver dysfunction (9.3%), thrombocytopenia (9.3%) and nausea/vomiting (8.1%). The median follow-up was 18 months (range, 5–44 months). The 2-year relapse-free survival, distant metastasis-free survival, progression-free survival and overall survival rates were 96.6, 85.4, 83.3 and 96.1%, respectively. Compared with alternative neoadjuvant chemotherapy regimens in combination with radiotherapy or concurrent chemoradiotherapy, the present gemcitabine and nedaplatin did not provide additional survival benefit and led to a higher frequency of liver dysfunction. Therefore, neoadjuvant gemcitabine and nedaplatin should be used with caution in locoregionally advanced NPC.
Keywords: nasopharyngeal carcinoma, gemcitabine, nedaplatin, induction chemotherapy, chemoradiation
Introduction
Nasopharyngeal carcinoma (NPC) is a squamous cell malignancy that is common in South East Asia, particularly in Southern China (1). The Epstein-Barr virus infection is strongly associated with the development of NPC in endemic and non-endemic areas (2,3). Globally, NPC accounted for an estimated 84,400 incident cases, and was the cause of 51,600 mortalities in 2008 (4). The majority of patients (75–90%) with NPC, present with locoregionally advanced disease, frequently with cervical node metastases (5,6). Despite improvements in radiotherapy technology, such as intensity-modulated radiotherapy (IMRT), the 5 year overall survival (OS) rate for patients with locally advanced NPC treated by radiotherapy alone is <50% (7,8), with local recurrence and distant metastasis the most common patterns of failure (8).
Concurrent chemoradiotherapy (CCRT) is frequently used, with the aim of reducing local recurrence and distant metastasis in a range of tumor types. CCRT that is based on cisplatin (CDDP)-containing regimes, is the current standard therapy for locally advanced NPC (9,10). Whilst this approach has improved the 3 year OS and disease-free survival (DFS) rates to ~80% and 70%, respectively (9–14), patients remain at a high risk of developing distant metastases following treatment (15–19% for all patients) (15–17), and CCRT is insufficient for patients with extensive nodal disease or bulky tumors.
A number of recent studies have been performed in order to evaluate the efficacy of neoadjuvant chemotherapy (NACT), or adjuvant chemotherapy in combination with CCRT, in the treatment of locoregionally advanced NPC. However, the majority of these studies failed to demonstrate that adjuvant chemotherapy or NACT significantly reduced locoregional recurrence or distant metastasis, and the improvements in OS were minimal (8,18,19). Only a small number of studies have reported that NACT improves OS in NPC. In 2009, Hui et al (20) reported a 3-year OS rate of 94.1% for the neoadjuvant arm, compared with 67.7% for the concurrent CDDP-radiotherapy alone arm. Recently, a meta-analysis conducted by OuYang et al (21), indicated that NACT provided an absolute overall survival gain of 5.13% and also significantly reduced the rate of distant metastasis at 3 years. However, no reduction in the locoregional recurrence rate was observed (21). Therefore, the value of NACT in locoregionally advanced NPC remains uncertain.
Gemcitabine (GEM) is a novel nucleoside antimetabolite that inhibits DNA synthesis. GEM-containing regimens have demonstrated tolerable toxicity profiles and encouraging efficacy in bladder cancer, breast cancer, non-small cell lung cancer and pancreatic cancer (22). GEM-based regimens are also commonly used in metastatic and recurrent NPC (23–25), and exhibit a moderately high activity with tolerable toxicity profiles in patients with NPC who are resistant to CDDP (26). CDDP is an important component of chemotherapy regimens for NPC. However, CDDP commonly induces kidney dysfunction and nausea/vomiting. Due to the risk of renal damage and digestive tract disorders following treatment with this agent, Kurita et al (27) developed nedaplatin (NDP), a novel platinum complex with a different molecular structure but similar mechanism of action to that of CDDP, which is associated with a lower frequency of digestive symptoms and renal toxicity. Additionally, NDP has been shown to be as effective, or more effective, than CDDP in the treatment of head and neck cancers (28), and the combination of NDP with GEM, synergistically inhibited the growth of human lung cancer cells in a xenograft model (29).
Relatively few studies (30–33) have examined the efficacy and toxicity profile of CDDP-GEM (GP regimen) or carboplatin (CBP)-GEM (GC regimen) as NACT, or during CCRT in locoregionally advanced NPC. These regimens exhibited good efficacy and tolerable toxicity profiles. However, they failed to significantly improve OS compared with neoadjuvant docetaxel + CDDP + 5-FU (5-fluorouracil; TPF regimen) or neoadjuvant docetaxel + CDDP (TP regimen) (34–36). However, compared with other regimes, such as neoadjuvant TP or neoadjuvant TPF, the benefits of the GP regimen remain unclear, as the majority of studies had a relatively short follow-up time and small sample size (32–38).
To date, the efficacy and toxicity of neoadjuvant GEM + NDP followed by radiotherapy or CCRT has not been evaluated in locoregionally advanced NPC. It was hypothesized that a neoadjuvant GEM + NDP regimen would result in an improved prognosis with lower toxicity. Therefore, a retrospective analysis was performed in order to examine the treatment-related toxicities and efficacy of neoadjuvant GEM + NDP, followed by radiotherapy or CCRT with NDP, in patients with locoregionally advanced NPC.
Patients and methods
Patients
The following enrollment criteria were used: Stage III–IVB NPC [according to the 2010 American Joint Committee on Cancer (AJCC) staging system for NPC] (39), without distant metastasis; availability of complete medical data (including gender, age and blood type); patients were treated with definitive intent; adequate hematological, renal and hepatic function; and a Karnofsky Score ≥70 (40). This study was approved by the Research Ethics Committee of Zhejiang Cancer Hospital (Hangzhou, China) and was performed in accordance with the Helsinki Declaration of 1975 (and 1983 revision). All patients provided written informed consent.
Pretreatment evaluation
Prior to treatment, all patients underwent a complete physical examination and medical history review, including nasopharyngoscopy and biopsy, full blood count, comprehensive serum chemistry profile, chest X-ray or computed tomography (CT), electrocardiogram, echocardiography (if necessary), ultrasonography of the abdomen, bone scan (N2/3 patients) and a magnetic resonance imaging (MRI) scan of the nasopharynx and neck.
Chemotherapy
The neoadjuvant GEM + NDP regimen, comprised GEM [1,000 mg/m2, 30 min, on day (d)1 and d5, every 3 weeks] and NDP (25 mg/m2, 60 min, on d1-3, every 3 weeks) prior to the administration of radiotherapy. All patients completed at least two cycles of NACT. If necessary, the dose was modified according to interim toxicity effects and the nadir blood counts during the preceding cycle. If the platelet count decreased to ≤25,000/ml or the leukocyte count decreased to ≤1,000/ml, the doses of NDP and GEM were reduced by 25% in the subsequent cycle.
During CCRT, NDP was administered every 3 weeks at a dose of 25 mg/m2 by intravenous infusion on d1-3, and given ~60 min prior to radiation. The decision to administer adjuvant chemotherapy and the regimen selected, were based on the tumor response to the preceding treatment, risk factors for recurrence (bulky or extensive nodal disease), in addition to the patient's general condition, full blood count, and hepatic and renal function. The adjuvant chemotherapy regimens included NDP/tegafur (PF), CBP/GEM (GC), and NDP/GEM (GP). For the PF regimen, NDP and tegafur were administered every 3 weeks at a dose of 25 mg/m2 and 600 mg/m2, respectively, by intravenous administration for 60 min on d1-3. For the GC regimen, CBP was administered every 3 weeks at a dose of 275 mg/m2 by intravenous infusion for 60 min on d1 and GEM was administered every 3 weeks at a dose of 800 mg/m2 by intravenous infusion for 30 min on d1 and 5. For the GP regimen, NDP was administered every 3 weeks at a dose of 25 mg/m2 by intravenous infusion for 60 min on d1-3 and GEM was administered every 3 weeks at a dose of 800 mg/m2 by intravenous infusion for 30 min on d1 and 5.
Radiotherapy
Patients received IMRT using a 6 MV X-ray. The gross tumor volume (GTV) included the primary tumor, and involved lymph nodes observed on clinical and imaging examinations. The clinical target volume (CTV) included the entire nasopharyngeal cavity, the anterior third of the clivus, pterygoid plates, parapharyngeal space, inferior sphenoid sinus, posterior third of the nasal cavity and maxillary sinus, and the drainage of the upper neck (levels II, III and Va) in N0 disease. In N1-N3 disease, levels IV and Vb were also included. A total dose of 69 Gy in 30 fractions over 6 weeks, was prescribed to the planning target volume of the primary tumor (PTVg), defined as the GTV with a 0.3–0.5 cm margin. A total dose of 63 Gy in 30 fractions over 6 weeks, was prescribed to the planning target volume of the metastatic nodes (PTVnd) defined as the GTVnd with a 0.3–0.5 cm margin. The PTV60 (high-risk clinical target volume) was defined as the CTV and a 0.3–0.5 cm margin, and was prescribed a dose of 60 Gy over 30 fractions. The PTV54 (low-risk clinical target volume) was prescribed a dose of 54 Gy over 30 fractions. In T3 and T4 disease, the aim was to cover the entire clivus and sphenoid sinus. All patients received one fraction daily, for 5 days each week.
Response to treatment, follow-up and adverse affects
The primary endpoint of the present study was the response rate (RR). The secondary endpoints were treatment-related toxicities and the rates of progression-free survival (PFS), relapse-free survival (RFS), distant metastasis-free survival (DMFS) and OS. Following each cycle of NACT, patients underwent a complete physical examination, their general condition and symptoms were reviewed, and a full blood count and comprehensive serum chemistry profile test were performed. At the end of the NACT and radiotherapy, a follow-up MRI was performed to evaluate the tumor response to treatment. At the completion of all therapy, a comprehensive scan, including chest CT, and ultrasonography or intensive CT of the abdomen, were performed in addition to the aforementioned examinations. Tumor response following NACT, and radiotherapy or CCRT, was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (41). Treatment-related toxicities were graded using the National Cancer Institute - Common Toxicity Criteria Version 3.0 (42).
Outpatient check-ups were the primary follow-up method. Clinical examination, full blood count, comprehensive serum chemistry profiles, MRI, and intensive CT, abdominal ultrasonography or nasopharyngoscopy, were performed every 3 months for the first two years following treatment, and every 6 months thereafter while patients were alive. Bone scans were performed when bone metastases were suspected. Other tests were conducted at the discretion of the treating physician.
Statistical analysis
RFS and DMFS were defined as the interval from the date of pathological diagnosis to the date of diagnosis of relapse or distant metastasis. PFS was defined as relapse or metastasis following the completion of all treatment. OS was defined as the interval from the date of pathological diagnosis to the date of death or the last known date alive. Associations between clinicopathological features and tumor response were analyzed using the Pearson χ2 test of independence. The Kaplan-Meier method was used to calculate survival rates.
Results
Patients
Between September 2010 and December 2013, a total of 99 patients with locoregionally advanced NPC received neoadjuvant GEM + NDP at Zhejiang Cancer Hospital. Thirteen patients, who refused additional cycles of NACT after the first course, were excluded from further analysis. The primary reasons for refusal were treatment-related toxicities, including grade 3–4 neutropenia (6 patients) and grade 3–4 thrombocytopenia (5 patients), as well as cost (2 patients). The remaining 86 patients were included.
Sixty-three (73.3%) of the 86 patients were male, and the median patient age was 55 years (range, 17–77). According to the World Health Organization (WHO) histological classification (43), 84 (97.6%) of the patients had WHO type II disease, while 2 patients (2.3%) had type 1 disease. According to the 2010 edition of the AJCC staging system for NPC, 58 (67.4%) patients had Stage III disease, 21 (24.4%) had Stage IVA disease and 7 (8.1%) had Stage IVB disease.
Table II summarizes the tumor responses following NACT and radiotherapy, and their association with the clinicopathological characteristics of the patients. No significant correlation was detected between any of the clinicopathological parameters and the rate of complete remission (CR) or partial remission (PR) following NACT, or the rate of CR following radiotherapy.
Table II.
Tumor response and association with clinicopathological characteristics.
| Characteristic | CR+PR following NACT | SD+PD following NACT | P-value | CR following RT | PR+SD+PD following RT | P-value |
|---|---|---|---|---|---|---|
| Age (years), n | 1.000 | 0.959 | ||||
| ≥55 | 37 | 4 | 27 | 17 | ||
| <55 | 36 | 5 | 26 | 16 | ||
| Gender, n | 0.420 | 0.556 | ||||
| Male | 51 | 8 | 40 | 23 | ||
| Female | 22 | 1 | 13 | 10 | ||
| Blood type, n | – | – | ||||
| A | 25 | 4 | 22 | 8 | ||
| B | 17 | 2 | 11 | 9 | ||
| AB | 9 | 1 | 6 | 4 | ||
| O | 22 | 2 | 13 | 12 | ||
| T stage, n | 1.000 | 1.000 | ||||
| T1+T2 | 27 | 3 | 20 | 12 | ||
| T3+T4 | 46 | 6 | 33 | 21 | ||
| N stage, n | 0.762 | 1.000 | ||||
| N0+N1 | 9 | 2 | 8 | 5 | ||
| N2+N3 | 64 | 7 | 45 | 28 | ||
| Overall stage, n | 0.624 | 0.286 | ||||
| III | 51 | 5 | 38 | 20 | ||
| IVA and IVB | 22 | 4 | 15 | 13 | ||
| Smoker, n | 0.236 | 0.127 | ||||
| No | 44 | 3 | 33 | 15 | ||
| Yes | 29 | 6 | 20 | 18 | ||
| Alcohol consumption, n | 0.815 | 0.557 | ||||
| No | 48 | 5 | 32 | 22 | ||
| Yes | 25 | 4 | 21 | 11 | ||
| NACT courses, n | 0.944 | 1.000 | ||||
| 2 | 62 | 7 | 45 | 28 | ||
| 3–4 | 11 | 2 | 8 | 5 |
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; NACT, neoadjuvant chemotherapy; RT, radiotherapy.
Efficacy and survival
Tumor response following NACT was evaluated in 82 patients (Table III), while toxicity was evaluated in all 86 patients (Table IV). Tumor response was not evaluated in the 4 (4.9%) patients, who refused a CT or MRI scan following NACT. All response outcomes are based on clinical examination, and CT or MRI of the nasopharynx and neck at the end of NACT and 3 months after radiotherapy. According to the RECIST criteria, responses were classified as CR, PR, stable disease (SD) or progressive disease (PD).
Table III.
Tumor response following NACT and RT (intention-to-treat analysis).
| Number of patients | Response of primary tumor (%) | Response of nodes (%) | Response of primary tumor and nodes (%) |
|---|---|---|---|
| Following NACT (n=82) | |||
| Complete response | 11 (13.4) | 29 (35.4) | 7 (8.5) |
| Partial response | 67 (81.7) | 45 (54.9) | 66 (80.5) |
| Stable disease | 4 (4.9) | 7 (8.5) | 8 (9.8) |
| Progressive disease | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Following radiotherapy (n=86) | |||
| Complete response | 62 (72.1) | 68 (79.1) | 53 (61.6) |
| Partial response | 21 (24.4) | 15 (17.4) | 28 (32.6) |
| Stable disease | 3 (3.5) | 3 (3.5) | 5 (5.8) |
| Progressive disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
NACT, neoadjuvant chemotherapy; RT, radiotherapy.
Table IV.
Acute toxicities following NACT and RT.
| NACT | RT | |||
|---|---|---|---|---|
| Toxicity | Grade 1–2 (%) | Grade 3–4 (%) | Grade 1–2 (%) | Grade 3–4 (%) |
| Hematological | ||||
| Leukopenia | 61 (70.9) | 10 (11.6) | 49 (57.0) | 6 (7.0) |
| Neutropenia | 42 (48.8) | 25 (29.1) | 25 (29.1) | 6 (7.0) |
| Neutropenia fever | 2 (2.3) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Thrombocytopenia | 20 (23.3) | 8 (9.3) | 29 (33.7) | 6 (7.0) |
| Anemia | 59 (68.6) | 3 (3.5) | 54 (56.3) | 2 (2.3) |
| Non-hematological | ||||
| Liver dysfunction | 50 (58.1) | 8 (9.3) | 15 (22.7) | 2 (2.3) |
| Kidney dysfunction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 2 (2.3) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Rash | 12 (14.0) | 0 (0.0) | 5 (5.8) | 0 (0.0) |
| Nausea/vomiting | 35 (40.7) | 7 (8.1) | 41 (47.7) | 10 (11.6) |
| Neurotoxicity | 8 (9.3) | 0 (0.0) | 10 (11.6) | 0 (0.0) |
| Oropharyngeal | 6 (7.0) | 0 (0.0) | 76 (83.7) | 14 (16.3) |
| Mucositis | ||||
| Hearing loss | 3 (3.5) | 0 (0.0) | 2 (2.3) | 0 (0.0) |
| Radiodermatitis | N/A | N/A | 15 (22.7) | 4 (4.7) |
NACT, neoadjuvant chemotherapy; RT, radiotherapy; N/A, not applicable.
The combined response rate for the primary tumor and metastatic nodes following NACT was 89.0% (73/82). The CR and PR rates were 8.5% (7/82) and 80.5% (66/82), respectively. Eight (9.8%) patients had SD and one patient (1.2%) developed PD. The combined response rate for the primary tumor and metastatic nodes three months after the completion of radiotherapy was 94.2% (81/86). The CR and PR rates were 61.6% (53/86) and 32.6% (28/86), respectively. Five (5.8%) patients had SD and none developed PD.
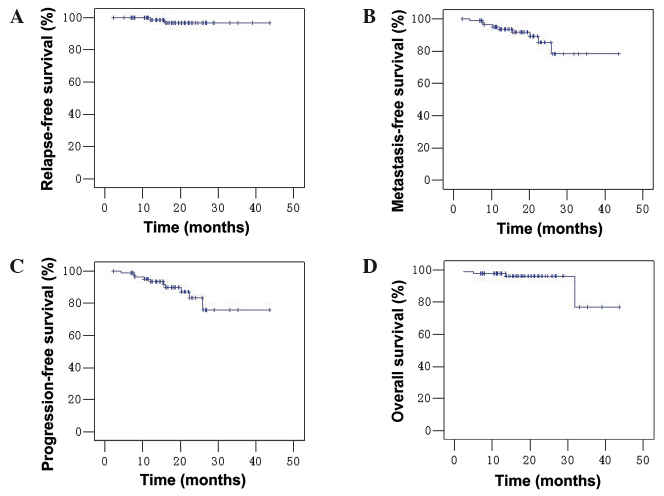
During the median follow-up period of 18 months (range, 5–44 months), 1 patient (1/86; 1.2%) developed local recurrence, while 9 (10.5%) developed distant metastasis, including 1 patient with nodal and distant metastasis (Table V). Four patients did not survive the follow-up period; three due to their cancer and one due to unknown causes. The 2-year RFS, DMFS, PFS and OS rates for the whole group were 96.6, 85.4, 83.3 and 96.1%, respectively (Fig. 1).
Table V.
Incidence and sites of recurrent disease.
| Site of recurrence | Number of patients (%) (n=86) |
|---|---|
| Local only | 1 |
| Distant only | 9 |
| Lung | 4 |
| Bone | 3 |
| Stomach | 1 |
| Nodal and liver | 1 |
Figure 1.
Survival curves for 86 patients with locoregionally-advanced nasopharyngeal carcinoma following neoadjuvant gemcitabine + nedaplatin and radiotherapy or concurrent chemoradiatherapy. (A) Relapse-free survival; (B) distant metastasis-free survival; (C) progression-free survival; (D) overall survival.
Treatment-related toxicities
Overall, the toxicity of neoadjuvant GEM + NDP was acceptable. A total of 73/86 (84.9%) patients completed two courses of NACT, 12 (14.0%) completed three courses and 1 (1.2%) completed four courses. Four patients (4.9%) received a 25% dose reduction due to treatment-related toxicity. As shown in Table IV, the most commonly observed grade 3–4 hematological and non-hematological adverse events following NACT were neutropenia (25 patients; 29.1%) and liver dysfunction (8 patients; 9.3%), respectively. Two (2.3%) of the patients with neutropenia had fever. Other common severe (grade 3–4) adverse events included leukopenia (10 patients, 11.6%), thrombocytopenia (8 patients, 9.3%), nausea/vomiting (7 patients; 8.1%) and anemia (3 patients, 3.5%). No grade 5 toxicities were observed.
All 86 patients completed radiotherapy. Additionally, 50 patients (58.1%) completed one course of CCRT (NDP, d1-3, every three weeks), 19 (22.1%) completed two courses of CCRT and 17 (19.8%) did not receive CCRT. Of the 17 patients who did not receive CCRT, 6 patients experienced grade 3–4 neutropenia and one patient experienced grade 3–4 thrombocytopenia during radiotherapy. Two patients refused CCRT due to poor appetite and fatigue during radiotherapy, and 5 patients refused CCRT as they experienced grade 3–4 neutropenia (2 patients), grade 3–4 thrombocytopenia (1 patient) or grade 2–3 liver dysfunction (2 patients) during NACT, although these patients did not experience grade 3–4 toxicities during radiotherapy. The remaining 3 patients refused CCRT for economic reasons.
Of the 50 patients who received only one course of CCRT, 13 patients experienced grade 2–4 thrombocytopenia, 4 patients experienced grade 3–4 neutropenia or leukopenia, and 3 patients experienced grade 2–3 liver dysfunction during radiotherapy. Of these 50 patients, 3 refused additional cycles of CCRT due to oropharyngeal mucositis, 2 patients due to poor appetite and fatigue, and 1 due to unexplained hyperpyrexia. Thirteen patients refused additional cycles of CCRT as they experienced grade 3–4 neutropenia or leukopenia (11 patients), grade 3–4 thrombocytopenia (3 patients), or grade 2–3 liver dysfunction (2 patients) during NACT, although these patients did not develop grade 3–4 toxicity during radiotherapy. Eleven patients refused a second course of CCRT as they were concerned about the side effects associated with additional chemotherapy. The treatment-related toxicities associated with CCRT are summarized in Table IV.
Discussion
Clinical response and outcome
Compared with the CBP + GEM or CDDP + GEM regimens, neoadjuvant GEM + NDP exhibited a similar efficacy in the treatment of locoregionally advanced NPC. As shown in Table VI, neoadjuvant GEM + NDP resulted in a higher primary site response rate (RR; 95.1%) compared with CBP + GEM (77.8%) (30), and similar a RR to CDDP + GEM (95%) (25). Additionally, neoadjuvant GEM + NDP achieved a similar nodal RR (90.3%) to CDDP + GEM (92%) (32), and markedly higher nodal RR than CBP + GEM (59.2%) (30). The primary reason for the large differences between the present results and those of Lim et al (30) is that 5/27 patients in the latter study exhibited SD, resulting in a low RR. In addition, each of these studies employed different chemotherapy regimens and drug-delivery methods, and the evaluation methods also varied. For example, the current study defined small nodes with no blood flow signals or unenhanced nodes on MRI as CR, while Lim et al (30) may have classified these features as PR, using the modified RECIST 1.0 criteria. Additionally, Yau et al (32) reported a higher CR rate for the primary site compared with the present study (49% vs. 13.4%). However, Yau et al evaluated tumor response using endoscopy and clinical examinations, which have a low accuracy for this purpose, whereas the current study used CT or MRI.
Table VI.
Comparison of tumor response following neoadjuvant chemotherapy in different studies.
| CR rate (%) | PR rate (%) | RR rate (%) | ||||
|---|---|---|---|---|---|---|
| Study | Primary | Nodes | Primary | Nodes | Primary | Nodes |
| Present study (NDP + GEM) | 13.4 | 35.4 | 81.7 | 54.9 | 95.1 | 90.3 |
| Lim et al (30) (CBP + GEM) | 22.2 | 11.1 | 55.6 | 48.1 | 77.8 | 59.2 |
| He et al (33) (CDDP + GEM) | 34 | 55 | 89 | |||
| Yau et al (32) (CDDP + GEM) | 49 | 46 | 46 | 46 | 95 | 92 |
NDP, nedaplatin; GEM, gemcitabine; CBP, carboplatin; CDDP, cisplatin; CR, complete remission; PR, partial remission; RR, response rate.
Due to the short median follow-up period, only 2-year survival rates were calculated in the present study. As shown in Table VII, the 2-year survival rates achieved using neoadjuvant GEM + NDP were similar to the 3-year survival rates reported for CDDP + GEM by He et al (33) and superior to those reported by Yau et al (32). However, all of the patients in the latter study had stage IV (A or B) disease, which may indicate that in terms of survival outcomes, GEM + NDP is inferior to CDDP + GEM. However, it should be noted that all of the studies described used relatively small sample sizes. One possible reason to explain why the GEM + NDP regimen resulted in poorer survival outcomes is that only 19 patients (22.1%) in the present study completed two courses of CCRT (NDP 25 mg/m2 d1-3, every 3 weeks), while 34 patients (92%) patients completed two or more courses of CCRT (CDDP 100 mg/m2 on d1, every 3 weeks) in the study conducted by Yau et al (32). Secondly, only 8.5% of patients achieved CR (in both the primary tumor and nodes) in the current study, while 34 to 46% of patients achieved a CR in the other studies (32–33). The GEM + NDP regimen was also inferior to the CBP + GEM, docetaxel + CDDP + 5-FU, and docetaxel + CDDP regimens (30,34–38), perhaps for similar reasons. Neoadjuvant GEM + NDP did not appear to provide any additional benefit compared with the outcomes of CCRT with CDDP-containing regimens, which were reported by Al-Sarraf et al (3-year PFS and OS rates of ~69 and 76%, respectively) (14) and Wee et al (3-year PFS and OS rates of ~70 and 80%, respectively) (11). Therefore, the present study indicates that the neoadjuvant GEM + NDP regime does not significantly improve any survival outcome compared with radiotherapy or CCRT with alternative CDDP-containing neoadjuvant regimens in locoregionally advanced NPC.
Table VII.
Comparison of survival rates following neoadjuvant chemotherapy in different studies.
| Study | RFS (%) | MFS (%) | PFS (%) | OS (%) |
|---|---|---|---|---|
| Present study (NDP + GEM) | 96.6 (2-year) | 85.5 (2-year) | 83.3 (2-year) | 96.1 (2-year) |
| Du et al (37) (Docetaxel + CDDP + 5-FU) | 96.6 (2-year) | 93.3 (2-year) | 89.9 (2-year) | 98.3 (2-year) |
| Zheng et al (28) (5-FU + NDP) | – | – | 75.0 (2-year) | 88.5 (2-year) |
| Yau et al (32) (CDDP + GEM) | 78.0 (3-year) | 76.0 (3-year) | 63.0 (3-year) | 76.0 (3-year) |
| He et al (33) (CDDP + GEM) | 94.9 (3-year) | 86.2 (3-year) | – | 87.7 (3-year) |
| Kong et al (38) (Docetaxel + CDDP + 5-FU) | 100 (3-year) | 88.0 (3-year) | 85.1 (3-year) | 90.2 (3-year) |
| Ekenel et al (36) (Docetaxel + CDDP) | 84.7 (3-year) | 94.9 (3-year) | ||
| Zhong et al (35) (Docetaxel + CDDP) | 72.7 (3-year) | 94.1 (3-year) | ||
| Lim et al (30) (CBP + GEM) | 92.9 (3-year) | 89.1 (3-year) | 82.1 (3-year) | 89.3 (3-year) |
NDP, nedaplatin; GEM, gemcitabine; CBP, carboplatin; CDDP, cisplatin; 5-FU, 5-fluorouracil; RFS, recurrence-free survival; MFS, metastasis-free survival; PFS, progression-free survival; OS, overall survival.
Of the 86 patients with locoregionally advanced NPC who received NACT in the present study, 4 patients (4.9%) received a 25% dose reduction due to treatment-related toxicities. In terms of hematological toxicities following NACT, GEM + NDP resulted in a higher frequency of grade 3–4 neutropenia (29.1 vs. 14%) and a similar frequency of grade 3–4 anemia (3.5 vs. 4%), compared with CBP+GEM (30). In terms of non-hematological toxicities following NACT, GEM + NDP resulted in a higher frequency of liver dysfunction (67.4 vs. 4%) and nausea/vomiting (48.8 vs. 7%), compared with CBP+GEM (30).
In terms of hematological toxicities following NACT, GEM + NDP resulted in a higher frequency of grade 3–4 leukopenia (11.6 vs. 6%) and a similar frequency of grade 3–4 thrombocytopenia (9.3 vs. 9%) compared with CDDP + GEM (33). However, Yau et al (32) reported that 19/37 (52%) patients developed grade 3–4 neutropenia following neoadjuvant CDDP + GEM, which may have been due to the fact that 1,250 mg/m2 GEM (rather than 1,000 mg/m2) was administered on d1 and d8 of chemotherapy. With respect to non-hematological toxicities following administration of NACT, GEM + NDP resulted in a higher frequency of liver dysfunction (67.4 vs. 9%) and lower frequency of nausea/vomiting (48.8 vs. 68%) compared with the CDDP + GEM regimen (33). In the same study, there was a significantly higher incidence of grade 3–4 oropharyngeal mucositis (16.3%, 14/86) in the CCRT arm. However, this did not affect the administration of radiotherapy, as a result of the administration of appropriate antibiotic and hormone therapy; grade 3–4 hematological toxicity was treated using thrombopoietin, colony-stimulating factor or blood transfusion, and nausea and vomiting were treated with antiemetics.
The results of the present study should be interpreted with caution, as it was a retrospective study involving a relatively small number of patients. There are also certain limitations to the design of the current study. One notable feature is that 13 of the 99 patients were excluded from the analysis as they refused additional cycles following the first course of NACT. Additionally, only 19 patients (22.1%) completed two courses of CCRT, although all of the patients completed radiotherapy. Finally, only 2 year survival rates were measured due to the relatively short median follow-up period, while the majority of the other studies referred to here, reported 3 year survival rates. Thus, it is difficult to accurately compare the current results with the results of these other studies. Therefore, the conclusions of this study require validation in the future.
Compared with other NACT regimens in combination with radiotherapy or CCRT, neoadjuvant GEM + NDP did not provide significant survival benefits and led to a higher frequency of liver dysfunction. Therefore, neoadjuvant GEM + NDP chemotherapy should be used with caution in patients with locoregionally advanced NPC.
Table I.
Chemotherapy delivery.
| Chemotherapy | Number of patients (%) |
|---|---|
| Neoadjuvant chemotherapy (GEM + NDP) | |
| Two courses | 73 (84.9) |
| Three courses | 12 (14.0) |
| Four courses | 1 (1.2) |
| 25% dose reduction | 4 (4.7) |
| Concurrent chemotherapy (NDP d1-3) | |
| None | 17 (19.8) |
| One course | 50 (58.1) |
| Two courses | 19 (22.1) |
| Adjuvant chemotherapy | |
| None | 26 (30.2) |
| One course | 40 (46.5) |
| Two courses | 20 (23.3) |
GEM, gemcitabine; NDP, nedaplatin; d, day.
Acknowledgements
The authors would like to thank the Elixigen Company for editing this article prior to submission. This study was supported by the National Natural Science Foundation of China (grant no. 81372437), the China International Medical Foundation (grant no. CIMF-F-H001-043) and the Excellent Talents Project of Zhejiang Cancer Hospital.
References
- 1.Fandi A, Altun M, Azli N, Armand JP, Cvitkovic E. Nasopharyngeal cancer: Epidemiology, staging, and treatment. Semin Oncol. 1994;21:382–397. [PubMed] [Google Scholar]
- 2.Henle G, Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976;17:1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 3.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. New Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Hsu MM, Tu SM. Nasopharyngeal carcinoma in Taiwan. Clinical manifestations and results of therapy. Cancer. 1983;52:362–368. doi: 10.1002/1097-0142(19830715)52:2<362::AID-CNCR2820520230>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Vokes EE, Liebowitz DN, Weichselbaum RR. Nasopharyngeal carcinoma. Lancet. 1997;350:1087–1091. doi: 10.1016/S0140-6736(97)07269-3. [DOI] [PubMed] [Google Scholar]
- 7.Geara FB, Sanguineti G, Tucker SL, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: Determinants of distant metastasis and survival. Radiother Oncol. 1997;43:53–61. doi: 10.1016/S0167-8140(97)01914-2. [DOI] [PubMed] [Google Scholar]
- 8.Baujat B, Audry H, Bourhis J, et al. MAC-NPC Collaborative Group: Chemotherapy in locally advanced nasopharyngeal carcinoma: An individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: Positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 10.Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: Progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038–2044. doi: 10.1200/JCO.2002.08.149. [DOI] [PubMed] [Google Scholar]
- 11.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapyin patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. 2008;71:1356–1364. doi: 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Pfister DG, Ang KK, Brizel DM, et al. National Comprehensive Cancer Network Head and Neck Cancers, Version 2.2013 Featured updates on the NCCN guidelines. J Natl Compr Cancer Netw. 2013;11:917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 14.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 15.Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: A relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101:300–306. doi: 10.1002/cncr.20358. [DOI] [PubMed] [Google Scholar]
- 16.Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int J Radiat Oncol. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 17.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/S0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 18.Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: A meta-analysis of the published literature. J Clin Oncol. 2004;22:4604–4612. doi: 10.1200/JCO.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 19.Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: A pooled data analysis of two phase III trials. J Clin Oncol. 2005;23:1118–1124. doi: 10.1200/JCO.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 20.Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatinradiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242–249. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 21.OuYang PY, Xie C, Mao YP, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol. 2013;24:2136–2146. doi: 10.1093/annonc/mdt146. [DOI] [PubMed] [Google Scholar]
- 22.Barton-Burke M. Gemcitabine: A pharmacologic and clinical overview. Cancer Nurs. 1999;22:176–183. doi: 10.1097/00002820-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Leong SS, Wee J, Tay MH, et al. Paclitaxel, carboplatin and gemcitabine in metastatic nasopharyngeal carcinoma: A phase II trial using a triplet combination. Cancer. 2005;103:569–575. doi: 10.1002/cncr.20804. [DOI] [PubMed] [Google Scholar]
- 24.Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol. 2002;13:150–156. doi: 10.1093/annonc/mdf002. [DOI] [PubMed] [Google Scholar]
- 25.Ngan RK, Yiu HH, Lau WH, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: Report of a phase II study. Ann Oncol. 2002;13:1252–1258. doi: 10.1093/annonc/mdf200. [DOI] [PubMed] [Google Scholar]
- 26.Wang CC, Chang JY, Liu TW, et al. Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck. 2006;28:74–80. doi: 10.1002/hed.20310. [DOI] [PubMed] [Google Scholar]
- 27.Kurita H, Yamamoto E, Nozaki S, et al. Multicenter phase I trial of induction chemotherapy with docetaxel and nedaplatin for oral squamous cell carcinoma. Oral Oncol. 2004;40:1000–1006. doi: 10.1016/j.oraloncology.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J, Wang G, Yang GY, et al. Induction chemotherapy with nedaplatin with 5-FU followed by intensity-modulated radiotherapy concurrent with chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Jpn J Clin Oncol. 2010;40:425–431. doi: 10.1093/jjco/hyp183. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto M, Takeda Y, Maki H, et al. Preclinical in vivo antitumor efficacy of nedaplatin with gemcitabine against human lung cancer. Jpn J Cancer Res. 2001;92:51–58. doi: 10.1111/j.1349-7006.2001.tb01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim AM, Corry J, Collins M, et al. A phase II study of induction carboplatin and gemcitabine followed by chemoradiotherapy for the treatment of locally advanced nasopharyngeal carcinoma. Oral Oncol. 2013;49:468–474. doi: 10.1016/j.oraloncology.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Gu MF, Liu LZ, He LJ, et al. Sequential chemoradiotherapy with gemcitabine and cisplatin for locoregionally advanced nasopharyngeal carcinoma. Int J Cancer. 2012;132:215–223. doi: 10.1002/ijc.27638. [DOI] [PubMed] [Google Scholar]
- 32.Yau TK, Lee AW, Wong DH, et al. Induction chemotherapy with cisplatin and gemcitabine followed by accelerated radiotherapy and concurrentcisplatin in patients with stage IV (A-B) nasopharyngeal carcinoma. Head Neck. 2006;28:880–887. doi: 10.1002/hed.20421. [DOI] [PubMed] [Google Scholar]
- 33.He X, Ou D, Ying H, et al. Experience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2012;269:1027–1033. doi: 10.1007/s00405-011-1669-9. [DOI] [PubMed] [Google Scholar]
- 34.Han SH, Yu L, Zhang Z, et al. Evaluation of induction chemotherapy with vinorelbine plus cisplatin (NP) or docetaxel plus cisplatin (TP) combined with concurrent chemoradiotherapy for patients with locally advanced nasopharyngeal carcinoma. Zhonghua Zhong Liu Za Zhi. 2013;35:623–626. (In Chinese) [PubMed] [Google Scholar]
- 35.Zhong YH, Dai J, Wang XY, et al. Phase II trial of neoadjuvant docetaxel and cisplatin followed by intensity-modulated radiotherapy with concurrent cisplatin in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71:1577–1583. doi: 10.1007/s00280-013-2157-2. [DOI] [PubMed] [Google Scholar]
- 36.Ekenel M, Keskin S, Basaran M, et al. Induction chemotherapy with docetaxel and cisplatin is highly effective for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2011;47:660–664. doi: 10.1016/j.oraloncology.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Du C, Ying H, Zhou J, Hu C, Zhang Y. Experience with combination of docetaxel, cisplatin plus 5-fluorouracil chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Int J Clin Oncol. 2013;18:464–471. doi: 10.1007/s10147-012-0403-y. [DOI] [PubMed] [Google Scholar]
- 38.Kong L, Hu C, Niu X, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: Interim results from 2 prospective phase 2 clinical trials. Cancer. 2013;119:4111–4118. doi: 10.1002/cncr.28324. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Mao YP, Xie FY, et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol. 2012;104:331–337. doi: 10.1016/j.radonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 41.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Liu YJ, Zhu GP, Guan XY. Comparison of the NCI-CTCAE version 4.0 and version 3.0 in assessing chemoradiation-induced oral mucositis for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48:554–559. doi: 10.1016/j.oraloncology.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Thompson L. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. [PubMed] [Google Scholar]