Abstract
Inflammatory myofibroblastic tumors (IMTs) of the inguinal region are exceptionally rare. The current study reported the case of a 49 year-old male patient with IMT, who presented with a fever, night sweats, anorexia, loss of weight and frequent urination. Computed tomography (CT) revealed a lesion occupying the soft tissue of the right inguinal region and surgery was performed to resect the lesion. Histopathological analysis of the lesion revealed a composition of spindle and inflammatory cells, including plasma cells and lymphocytes. In addition, immunohistochemical analysis demonstrated that the tumor cells were positive for CD34, vimentin, actin, Ki-67, B cell lymphoma-2, CD99, epithelial membrane antigen and CD38; however, tumor cells were negative for CD117, desmin, anaplastic lymphoma kinase and creatine kinase. Thus, the patient was diagnosed with IMT and was advised to return for regular follow-up appointments. Subsequently, the patient developed a local recurrence 12 months following the initial surgery. Of note, the histopathological characteristics of the recurrent lesions were consistent with those of the initial specimen. Thus, a second surgery was performed, followed by fractionated radiotherapy (FRT). At 3 and 6 months following the FRT, magnetic resonance imaging scans did not indicate tumor recurrence or metastasis. In conclusion, surgical excision is the current recommended treatment for IMT; however, for cases similar to that of the current study, which are not successfully controlled by surgical excision, radiotherapy should be considered and long-term follow-up is essential.
Keywords: inguinal region, inflammatory myofibroblastic tumor, recurrence, radiotherapy
Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare and primarily occur in patients <16 years of age (1). An IMT may also be defined as a plasma cell granuloma, inflammatory myofibrohistiocytic proliferation, fibroxanthoma, histiocytoma, fibrous histiocytoma, xanthomatous pseudotumor, inflammatory pseudotumor, mast cell tumor or plasma cell-histiocytoma (2). The most common sites for IMTs include the lungs, mesenteries and omentum (3), although they are also observed in the head and neck region (4), liver (5), spleen (6), thyroid (7), gastrointestinal tract (8) and genitourinary tract (9), among other systems (10). Although these types of tumors are primarily benign, up to 25% of patients suffer from recurrences (11). Recurrence rates have been associated with body site, multifocality and whether the initial tumor was completely resected. Rare malignant transformations have been reported (12). To the best of our knowledge, the current study is the first to report the occurrence of IMT in the inguinal region. Furthermore, recurrence and metastasis of IMT are comparatively rare. The present study describes a 49-year-old male patient with IMT of the inguinal region, which recurred 12 months following initial surgery. The aims of this report were to describe a novel case of IMT in this uncommon region and to emphasize that IMT may occasionally demonstrate malignant biological behaviors, despite its intermediate biological potential as a neoplasm that frequently recurs, but rarely metastasizes.
Case report
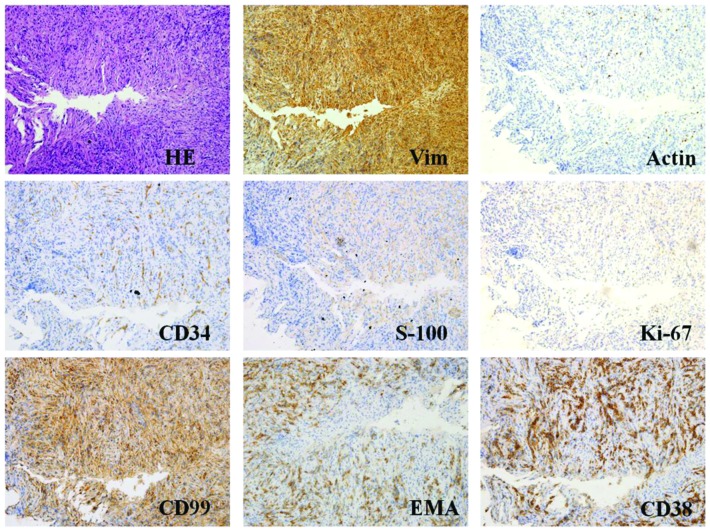
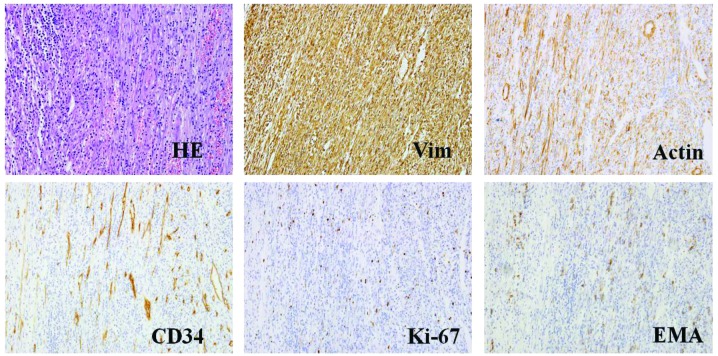
A 49-year-old male patient with an unremarkable medical history was admitted to Nanfang Hospital, Southern Medical University (Guangdong, China) and presented with a low fever, night sweats, anorexia, weight loss of 5 kg and 1 month of frequent urination. A visible mass, measuring ~6×5 cm, was located in the right inguinal region. The oval mass presented no adherence with the surrounding tissue, and the position had no influence on the size of the mass. The laboratory examination of the patients blood revealed anemia (hemoglobin, 81 g/l), thrombocythemia (platelet count, 517 g/l), hypoproteinemia (albumin, 29 g/l) and increased inflammatory markers with an erythrocyte sedimentation rate and C-reactive protein values of 130 mm/h and 113.6 mg/l, respectively. All other laboratory results were within the normal range. Computed tomography (CT) of the abdomen revealed an undefined lesion, 6.5×5.2 cm, occupying the soft tissue of the right inguinal region (Fig. 1A). The mass was inhomogeneous in density and was lower in density in the middle compared with the periphery. A CT enhancement scan demonstrated moderate enhancement of the solid portion of the lesion. The rectus abdominis muscles and the bladder were compacted and difficult to differentiate from the mass (Fig. 1B). The patient then underwent a fine needle aspiration procedure. Histopathological analysis of the lesion revealed a composition of spindle and inflammatory cells, including plasma cells and lymphocytes. In addition, immunohistochemical analysis established that the tumor cells were positive for vimentin, actin, Ki-67, B cell lymphoma-2, CD99, epithelial membrane antigen, CD34, S-100 and CD38; however, tumor cells were negative for CD117, desmin, anaplastic lymphoma kinase (ALK) and creatine kinase (Fig. 2). Following the initial surgery, the histopathological characteristics of the mass were determined again and the results were comparable to those of the initial specimen from the fine needle aspiration (Fig. 3). Thus, the patient was diagnosed with IMT and was advised to return for regular follow-up appointments, at 3, 6, 12 and 24 months after surgery.
Figure 1.
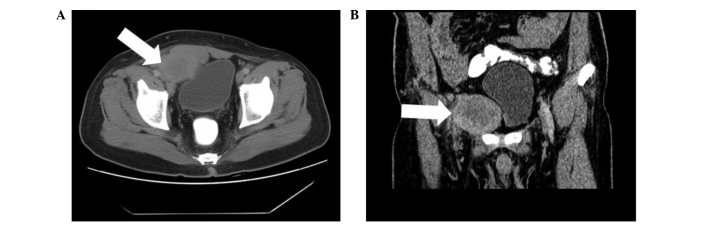
CT and enhanced CT scans revealed a lesion in the inguinal region. (A) CT scan of the abdomen revealed an undefined lesion, 6.5×5.2 cm, occupying the soft tissue of the right inguinal region. The mass was inhomogeneous in density, with a decreased density at the center. (B) CT enhancement scan demonstrated moderate enhancement of the solid portion of the lesion. The rectus abdominis muscles and the bladder were compacted and difficult to differentiate from the mass. CT, computed tomography.
Figure 2.
HE staining and immunohistochemical findings of the fine needle aspiration samples (magnification, x400; microscope, Olympus IX71). Representative histological cross-sections from the original tumor, obtained via fine needle aspiration, demonstrated variable myofibroblasts, myxoid stroma and mixed inflammation with lymphocytes, plasma cells and eosinophils. Tumor tissue stained positive for Vim, actin, CD34, S-100, Ki-67, CD99, EMA, CD38 and B cell lymphoma-2, but negative for CD117, desmin, anaplastic lymphoma kinase and creatine kinase. HE, hematoxylin and eosin; Vim, vimentin; EMA, epithelial membrane antigen.
Figure 3.
HE staining and representative histological cross-sections from the original tumor following surgery (magnification, x400; microscope, Olympus IX71). The tumor stained positive for Vim, actin, Ki-67, EMA, CD34, S-100, but negative for CD117, desmin, anaplastic lymphoma kinase, β-catenin, myogenin, creatine kinase and p53. HE, hematoxylin and eosin; Vim, vimentin; EMA, epithelial membrane antigen.
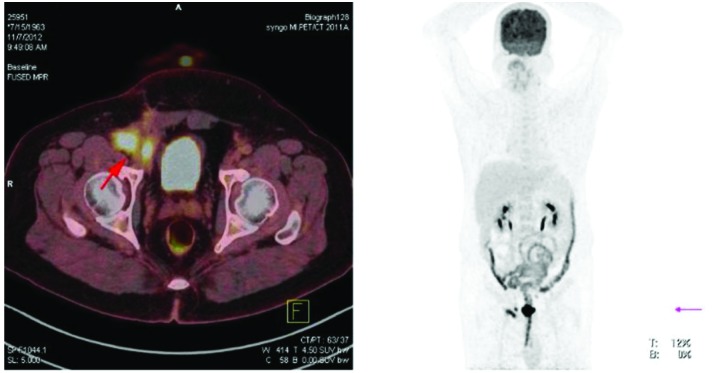
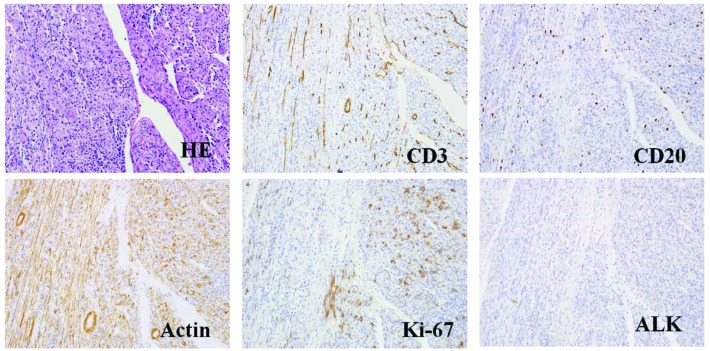
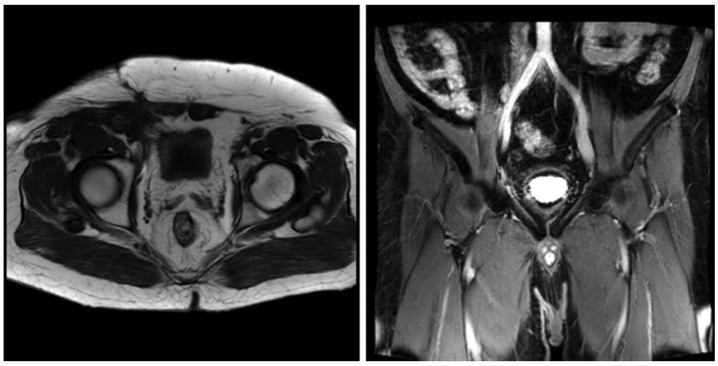
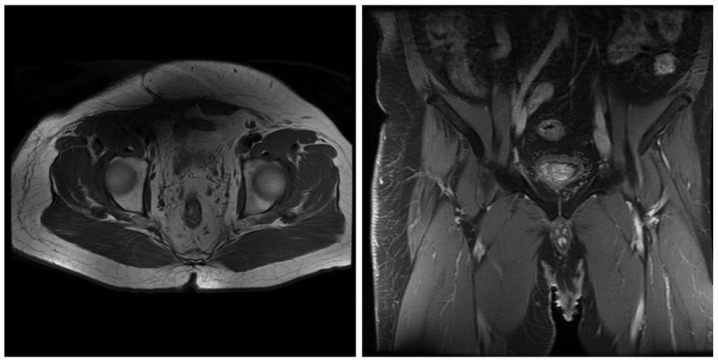
The patient developed a local recurrence 12 months following the initial surgery with no clinical symptoms and negative laboratory results. Positron emission tomography-CT revealed multiple integration lesions in the right inguinal region and iliac fossa; the largest measured ~3.3×2.0×2.0 cm and was invading into the right abdominal wall (Fig. 4). Thus, a second surgery was performed. Of note, the histopathological characteristics of the recurrent lesions were comparable to those of the initial specimen (Fig. 5). Following the second surgery, the patient received fractionated radiotherapy (FRT; 46 Gy/23 fractions/30 days; the patient received radiotherapy, 5 days/week, at 2 Gy/fraction, for a total of 30 days). At 3 and 6 months following radiotherapy, magnetic resonance imaging was performed and the scans did not indicate tumor recurrence or metastasis (Figs. 6 and 7, respectively).
Figure 4.
Positron emission tomography-computed tomography scan revealing local recurrence. At 12 months following initial surgery, multiple integration lesions were identified in the right inguinal region and iliac fossa that were invading into the right abdominal wall.
Figure 5.
HE staining and representative histological cross-sections from the recurrent tumor (magnification, x400; microscope, Olympus IX71). Representative histological cross-sections from the recurrent tumors revealed variable myofibroblasts, myxoid stroma and mixed inflammation with lymphocytes, plasma cells and eosinophils. The tumor stained positive for actin, Ki-67, desmin, CD3 and CD20, but negative for ALK and p53. HE, hematoxylin and eosin; ALK, anaplastic lymphoma kinase.
Figure 6.
Magnetic resonance imaging at 3 months post radiotherapy demonstrated no evidence of tumor recurrence or metastasis.
Figure 7.
Magnetic resonance imaging at 6 months post radiotherapy demonstrated no evidence of tumor recurrence or metastasis.
Written informed consent was obtained from the patient prior to publication of the study and the study was approved by the ethics committee of Nanfang Hospital.
Discussion
The first case of IMT was described in the lungs in 1939 (13). IMT, also referred to as plasma cell granulomas, plasma cell pseudotumors, inflammatory myofibrohistiocytic proliferations, omental mesenteric myxoid hamartomas and inflammatory pseudotumors, is a rare type of low-grade malignant mesenchymal tumor. In 2002, the World Health Organization defined IMT as a distinctive lesion consisting of myofibroblastic spindle cells with an inflammatory infiltrate of plasma cells, lymphocytes and eosinophils (14).
As reported in the literature, IMT predominantly occurs in the soft tissue and viscera of children and young adults (15); in addition, IMT is most commonly localized to the lung (16), although it may also occur in other regions of the body, including the lymph nodes, soft tissue, viscera, gastrointestinal tract, omentum majus and central nervous system. Among these extra-pulmonary IMTs, 43% arise in the mesenteries (17) or omentum (18). IMTs are exceptionally rare in the inguinal region and to the best of our knowledge no similar cases have been previously reported.
The etiology of IMT remains to be defined; however, trauma to the affected region secondary to inflammation has been proposed as a cause of IMT. A previous study demonstrated the occurrence of ectopic chromosomal rearrangements in chromosomes 2 (long arm) and 9 (short arm); in addition, this previous study confirmed the monoclonal identity of IMT through genetic and molecular techniques (19). Furthermore, it has been reported that ~50% of IMTs exhibit clonal cytogenetic aberrations, which results in the genetic activation ALK-receptor tyrosine kinase at 2p23 (20). This therefore suggested that IMT is a true neoplasm, as opposed to an inflammatory pseudotumor, as it was previously considered. In the present case report, the aggressive features of IMT, including local recurrence, metastasis and malignant transformation, indicated that IMT development may be a neoplastic process.
Due to the inconsistency of the pathological diagnoses of IMTs and the limited number of patients typically diagnosed with IMTs, the treatment of choice for IMT patients remains controversial. IMTs are commonly located in the peritoneum, liver, spleen, breast, spinal cord, brain or respiratory system (6,21–25). They are more frequently observed in the lower lobe of the right lung and form a solitary, oval-shaped and well-defined mass that is located peripherally (26–28).
At present, due to the occurrence of rare IMT cases with a more aggressive clinical picture, including local recurrence, malignant transformation or metastasis, IMTs are classed as low-grade mesenchymal malignancies. Surgical resection should be considered as the first therapeutic option when feasible; however, radiotherapy (29), anticancer chemotherapy (30), steroids (31) or non-steroidal anti-inflammatory drugs (32) have previously been used for the treatment of anatomically and functionally inoperable patients as well as in patients with disease recurrence. The results of these treatments have been variable, ranging from ineffective to complete regression.
A limited number of studies have investigated the use of radiation therapy for IMT. Seider et al (33) reported a case in which progression of IMT was observed at a 1 month following initial resection of the tumor. Further surgery to eradicate the tumor completely would have been extensive and disfiguring; therefore, the patient was administered 40 Gy FRT in 20 fractions and a 27 months follow-up demonstrated local control of the IMT (33).
Certain studies have reported 66–100% complete remission rates in orbital inflammatory pseudotumor patients following radiotherapy (29,34–36). Sasagawa et al (37) observed local control following 20 Gy FRT treatment and other studies have also demonstrated clinical responses following FRT (38,39).
Ong et al (40) classified head and neck IMT patients into categories according to risk of relapse (high, moderate or low), which were dependent on the diameter of their tumors, the composition of the pseudocapsule and immunohistochemistry, among other prognostic factors. This previous study suggested that high and moderate-risk groups required post-operative radiotherapy. Adjunct radiation therapy of 60–64 Gy was performed for the moderate-risk group and 66–70 Gy was used for the high-risk group. In the low-risk group, post-operative radiotherapy of 50–54 Gy was recommended if the lesion had a diameter of >5 cm with conditioned ALK and Ki-67 overexpression. For other cases of the low-risk group, post-operative radiotherapy was not required.
The prognosis of IMT is usually good; however, in rare cases, this type of tumor may exhibit local invasion. Recurrence has been associated with the tumors location, resection ability and multinodularity. The metastatic rate of IMTs has been reported as 5% (41) and metastasis is predominantly observed in children with intra-abdominal tumors.
The malignant potential of IMT is incompletely characterized. IMT has been previously confused with malignant conditions based on commonalities in the pathological examination, radiological appearance and clinical presentation. However, an increasing number of studies have reported the malignant potential of IMTs. For instance, Anderson et al (42) reported the case of a 15-year-old boy that was diagnosed with IMT of the heart and experienced recurrence 6 months after the initial surgery. In addition, Navinan et al (43) reported the case of a 33-year-old South Asian male who was diagnosed with inoperable IMT of the paranasal sinuses and orbit. As curative excision of the tumour was not feasible, medical management was offered. Despite early features of remission to glucocorticoids, tapering resulted in recurrence.
In conclusion, IMTs, in particular those with inguinal region involvement, are rare in adults. The most relevant therapy for this type of tumor is open surgical resection. Regular follow-up is recommended to monitor patients for recurrence. In addition, radiotherapy should be considered in patients whose surgical resection was incomplete, in those with postoperative recurrences and in those whose tumors are non-resectable due to associated medical conditions.
Glossary
Abbreviations
- IMT
inflammatory myofibroblastic tumors
- FRT
fractionated radiotherapy
References
- 1.Cassivi SD, Wylam ME. Pulmonary inflammatory myofibroblastic tumor associated with histoplasmosis. Interact Cardiovasc Thorac Surg. 2006;5:514–516. doi: 10.1510/icvts.2006.129809. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SH, Kim KJ, Chung SK, et al. Inflammatory myofibroblastic tumor in the intradural extramedullary space of the lumbar spine with spondylolisthesis: Case report and review of the literature. Eur Spine J. 2010;19(Suppl 2):S153–S157. doi: 10.1007/s00586-009-1212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour. In: Fletcher CDM, Unni KK, Mertens F, editors. WHO Pathology & Genetics of Tumours of Soft Tissue and Bone. IARC Press; Lyon: 2002. pp. 90–93. [Google Scholar]
- 4.Gao F, Zhong R, Li GH, Zhang WD. Computed tomography and magnetic resonance imaging findings of inflammatory myofibroblastic tumors of the head and neck. Acta Radiol. 2014;55:434–440. doi: 10.1177/0284185113500165. [DOI] [PubMed] [Google Scholar]
- 5.Nagarajan S, Jayabose S, McBride W, et al. Inflammatory myofibroblastic tumor of the liver in children. J Pediatr Gastroenterol Nutr. 2013;57:277–280. doi: 10.1097/MPG.0b013e31829e0b3b. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi T, Mochizuki K, Kizu T, et al. Inflammatory pseudotumor of the liver and spleen diagnosed by percutaneous needle biopsy. World J Gastroenterol. 2012;18:90–95. doi: 10.3748/wjg.v18.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima M, Suzuki M, Shimizu K, Masawa N. Inflammatory pseudotumor of the thyroid gland showing prominent fibrohistiocytic proliferation. A case report. Endocr Pathol. 2009;20:186–190. doi: 10.1007/s12022-009-9080-4. [DOI] [PubMed] [Google Scholar]
- 8.Arslan D, Gündüz S, Tural D, et al. Inflammatory myofibroblastic tumor: a rarely seen submucosal lesion of the stomach. Case Rep Oncol Med. 2013;2013:328108. doi: 10.1155/2013/328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HW, Choi YH, Kang SM, et al. Malignant inflammatory myofibroblastic tumor of the bladder with rapid progression. Korean J Urol. 2012;53:657–661. doi: 10.4111/kju.2012.53.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan W, Xu Y, Dong Y, Cao L, Tong J, Zhou X. Ectopic expression of miR-34a enhances radiosensitivity of non-small cell lung cancer cells, partly by suppressing the LyGDI signaling pathway. J Radiat Res. 2013;54:611–619. doi: 10.1093/jrr/rrs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oztuna F, Pehlivanlar M, Abul Y, Tekinbas C, Ozoran Y, Ozlu T. Adult inflammatory myofibroblastic tumor of the trachea: Case report and literature review. Respir Care. 2013;58:e72–e76. doi: 10.4187/respcare.02198. [DOI] [PubMed] [Google Scholar]
- 13.Brunn H. Two interesting benign lung tumors of contradictory histopathology: remarks on the necessity for maintaining the chest tumor registry. J Thorac Cardiovasc Surg. 1939;9:119–131. [Google Scholar]
- 14.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 15.El-Desoky T, Nasef N, Osman E, Osman A, Zaki A, Zalata K. Endobronchial inflammatory pseudotumor: A rare cause of a pneumothorax in children. J Bronchol Interv Pulmonol. 2013;20:256–260. doi: 10.1097/LBR.0b013e31829bccba. [DOI] [PubMed] [Google Scholar]
- 16.Toma CL, Belaconi IN, Dumitrache-Rujinski S, et al. A rare case of lung tumor - pulmonary inflammatory pseudotumor. Pneumologia. 2013;62:30–32. [PubMed] [Google Scholar]
- 17.Shatzel J, Wooten K, Ankola A, Cheney RT, Morrison CD, Skitzki JJ. Inflammatory myofibroblastic tumor of the mesentery: A clinical dilemma. Int J Clin Oncol. 2012;17:380–384. doi: 10.1007/s10147-011-0297-0. [DOI] [PubMed] [Google Scholar]
- 18.Gupta CR, Mohta A, Khurana N, Paik S. Inflammatory pseudotumor of the omentum: An uncommon pediatric tumor. Ind J Pathol Microbiol. 2009;52:219–221. doi: 10.4103/0377-4929.48924. [DOI] [PubMed] [Google Scholar]
- 19.Cole B, Zhou H, McAllister N, Afify Z, Coffin CM. Inflammatory myofibroblastic tumor with thrombocytosis and a unique chromosomal translocation with ALK rearrangement. Arch Pathol Lab Med. 2006;130:1042–1045. doi: 10.5858/2006-130-1042-IMTWTA. [DOI] [PubMed] [Google Scholar]
- 20.O'Malley DP, Poulos C, Czader M, Sanger WG, Orazi A. Intraocular inflammatory myofibroblastic tumor with ALK overexpression. Arch Pathol Lab Med. 2004;128:e5–e7. doi: 10.5858/2004-128-e5-IIMTWA. [DOI] [PubMed] [Google Scholar]
- 21.al-Sarraj S, Wasserberg J, Bartlett R, Bridges LR. Inflammatory pseudotumour of the central nervous system: Clinicopathological study of one case and review of the literature. Br J Neurosurg. 1995;9:57–66. doi: 10.1080/02688699550041764. [DOI] [PubMed] [Google Scholar]
- 22.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: A clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 23.Neuhauser TS, Derringer GA, Thompson LD, et al. Splenic inflammatory myofibroblastic tumor (inflammatory pseudotumor): A clinicopathologic and immunophenotypic study of 12 cases. Arch Pathol Lab Med. 2001;125:379–385. doi: 10.5858/2001-125-0379-SIMTIP. [DOI] [PubMed] [Google Scholar]
- 24.Pettinato G, Manivel JC, Insabato L, De Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol. 1988;90:627–632. doi: 10.1093/ajcp/90.5.627. [DOI] [PubMed] [Google Scholar]
- 25.Zemmoura I, Hamlat A, Morandi X. Intradural extramedullary spinal inflammatory myofibroblastic tumor: Case report and literature review. Eur Spine J. 2011;20(Suppl 2):S330–S335. doi: 10.1007/s00586-011-1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedlund GL, Navoy JF, Galliani CA, Johnson WH., Jr Aggressive manifestations of inflammatory pulmonary pseudotumor in children. Pediatr Radiol. 1999;29:112–116. doi: 10.1007/s002470050553. [DOI] [PubMed] [Google Scholar]
- 27.Kobashi Y, Fukuda M, Nakata M, Irei T, Oka M. Inflammatory pseudotumor of the lung: Clinicopathological analysis in seven adult patients. Int J Clin Oncol. 2006;11:461–466. doi: 10.1007/s10147-006-0611-4. [DOI] [PubMed] [Google Scholar]
- 28.Laufer L, Cohen Z, Mares AJ, Maor E, Hirsch M. Pulmonary plasma-cell granuloma. Pediatr Radiol. 1990;20:289–290. doi: 10.1007/BF02019671. [DOI] [PubMed] [Google Scholar]
- 29.Maire JP, Eimer S, San Galli F, et al. Inflammatory myofibroblastic tumour of the skull base. Case Rep Otolaryngol. 2013;2013:103646. doi: 10.1155/2013/103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian H, Liu T, Wang C, Tang L, Chen Z, Xing G. Inflammatory pseudotumor of the temporal bone: Three cases and a review of the literature. Case Rep Med. 2013;2013:480476. doi: 10.1155/2013/480476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DK, Cho YS, Hong SH, Chung WH, Ahn YC. Inflammatory pseudotumor involving the skull base: Response to steroid and radiation therapy. Otolaryngol Head Neck Surg. 2006;135:144–148. doi: 10.1016/j.otohns.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Moon CH, Yoon JH, Kang GW, et al. A case of recurrent pulmonary inflammatory myofibroblastic tumor with aggressive metastasis after complete resection. Tuberc Respir Dis. 2013;75:165–169. doi: 10.4046/trd.2013.75.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seider MJ, Cleary KR, van Tassel P, et al. Plasma cell granuloma of the nasal cavity treated by radiation therapy. Cancer. 1991;67:929–932. doi: 10.1002/1097-0142(19910215)67:4<929::AID-CNCR2820670412>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 34.Ampil FL, Bahrassa FS. Primary orbital lymphoma-pseudotumor (pseudolymphoma): Case reports and review of radiotherapy literature. J Surg Oncol. 1985;30:91–95. doi: 10.1002/jso.2930300206. [DOI] [PubMed] [Google Scholar]
- 35.de Jesús O, Inserni JA, Gonzalez A, Colón LE. Idiopathic orbital inflammation with intracranial extension. Case report. J Neurosurg. 1996;85:510–513. doi: 10.3171/jns.1996.85.3.0510. [DOI] [PubMed] [Google Scholar]
- 36.Noble SC, Chandler WF, Lloyd RV. Intracranial extension of orbital pseudotumor: A case report. Neurosurgery. 1986;18:798–801. doi: 10.1097/00006123-198606000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Sasagawa Y, Akai T, Itou S, Iizuka H. Multiple intraosseous inflammatory myofibroblastic tumors presenting with an aggressive clinical course: Case report. Neurosurgery. 2011;69:E1010–E1015. doi: 10.1227/NEU.0b013e318223b651. [DOI] [PubMed] [Google Scholar]
- 38.Frohman LP, Kupersmith MJ, Lang J, et al. Intracranial extension and bone destruction in orbital pseudotumor. Arch Ophthalmol. 1986;104:380–384. doi: 10.1001/archopht.1986.01050150080032. [DOI] [PubMed] [Google Scholar]
- 39.Kaye AH, Hahn JF, Craciun A, Hanson M, Berlin AJ, Tubbs RR. Intracranial extension of inflammatory pseudotumor of the orbit. Case report. J Neurosurg. 1984;60:625–629. doi: 10.3171/jns.1984.60.3.0625. [DOI] [PubMed] [Google Scholar]
- 40.Ong HS, Ji T, Zhang CP, et al. Head and neck inflammatory myofibroblastic tumor (IMT): Evaluation of clinicopathologic and prognostic features. Oral Oncol. 2012;48:141–148. doi: 10.1016/j.oraloncology.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhao HD, Wu T, Wang JQ, et al. Primary inflammatory myofibroblastic tumor of the breast with rapid recurrence and metastasis: A case report. Oncol Lett. 2013;5:97–100. doi: 10.3892/ol.2012.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen ND, DiBernardo LR, Linardic CM, Camitta MG, Lodge AJ. Recurrent inflammatory myofibroblastic tumor of the heart. Circulation. 2012;125:2379–2381. doi: 10.1161/CIRCULATIONAHA.111.066191. [DOI] [PubMed] [Google Scholar]
- 43.Navinan MR, Liyanage I, Herath S, et al. Inoperable inflammatory myofibroblastic tumour of the para-nasal sinuses and orbit with recurrence responding to methotrexate and prednisolone: A case report. BMC Res Notes. 2015;8:27. doi: 10.1186/s13104-015-0993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]