Abstract
Multiple signal transduction pathways can affect each other considerably through crosstalk. However, the presence and extent of this phenomenon have not been rigorously studied. The aim of the present study was to identify strong and normal interactions between pathways in breast cancer and determine the main pathway. Five sets of breast cancer data were downloaded from the high-throughput Gene Expression Omnibus (GEO) and analyzed to identify differentially expressed (DE) genes using the Rank Product (RankProd) method. A list of pathways with differential expression was obtained by gene set enrichment analysis (GSEA) of the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The DE genes that overlapped between pathways were identified and a crosstalk network diagram based on the overlap of DE genes was constructed. A total of 1,464 DE genes and 26 pathways were identified. In addition, the number of DE genes that overlapped between specific pathways were determined, and the greatest degree of overlap was between the extracellular matrix (ECM)-receptor interaction and Focal adhesion pathways, which had 22 overlapping DE genes. Weighted pathway analysis of the crosstalk between pathways identified that Pathways in cancer was the main pathway in breast cancer.
Keywords: crosstalk, RankProd, gene set enrichment analysis, breast cancer, pathway
Introduction
Breast cancer is a malignant type of tumor with the highest incidence in women, and it seriously affects the quality of life of patients (1). The occurrence of breast cancer is considered to be the result of the abnormal expression of numerous genes. The overexpression of a variety of oncogenes and tumor suppressor gene deletion, mutation or low expression accompany carcinogenesis (2,3). However, the mechanism of the occurrence and development of breast cancer remains incompletely understood, and numerous genes and their functions remain to be discovered and understood. A certain theoretical basis for further study of the pathogenesis of breast cancer is provided by the screening of differentially expressed (DE) genes in breast cancer tissue and normal breast tissue and analyzing the pathways in which these genes are enriched using biological information (4,5).
Experimental high-throughput genomics has identified a number of notable genes, including DE genes having significant changes in expression level. A main task in bioinformatics is to elucidate the biological significance of these genes. Based on a variety of biological knowledge databases such as the Gene Ontology (GO) database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database, numerous research groups are systemically analyzing the biological processes and single pathways associated with the enriched genes using a variety of statistical analysis strategies. At present, studies have clearly shown that UBE2C (6,7), EGFR (8) and interferon regulatory factor binding protein 2 (IRF2BP2) (9) are DE genes associated with the development of breast cancer. Further study of these genes may be helpful in preventing the occurrence of this disease.
Pathways can affect each other through a phenomenon known as crosstalk, rather than acting independently (10). Although it is evident that different pathways could influence each other, particularly when there is an overlap of DE genes having significant changes in expression level, the presence and extent of this phenomenon have not been rigorously studied. Identification of the interaction of pathways has important implications for the understanding of numerous diseases; it may contribute to their prevention and treatment by enabling inhibition of the interaction among pathways, which may play a key role in the invasion and proliferation of cancer cells (11–13). Wang et al (14) showed that EGFR activity was increased in a PTGS2 (COX-2) transgenic mouse and that forced expression of PTGS2 (COX-2) in human colorectal cancer (CRC) cells stimulated cellular proliferation. In addition, it was demonstrated that a crosstalk of PTGS2 (COX-2) and EGFR pathways synergistically promoted CRC progression and metastasis. The study conducted by Krysan et al (15) indicated that PGE2 was able to induce the proliferation of colon and lung cancer cells through the activation of MAPK in an EGFR-independent manner in vitro. Wang et al (16) generated a mouse model characterized by the co-expression of activated forms of AKT and Ras in the liver. The results indicated that concomitant suppression of AKT/mTOR and Ras/MAPK pathways was highly detrimental for the growth of AKT/Ras-expressing cells in vitro. This finding has important implications for the understanding of HCC pathogenesis and its prevention.
The identification of DE genes and the pathways involved in the development of disease has been the subject of numerous studies. However, in many cases the crosstalk between pathways was not investigated, and the enriched DE genes and the most important pathway were not identified. In the present study, 5 sets of breast cancer data were downloaded from the Gene Expression Omnibus (GEO) platform and analyzed with the RankProd package (17) to detect DE genes. The pathways in which the DE genes were enriched were identified by the gene set enrichment analysis (GSEA) (18,19) method. The DE genes that overlapped between pathways were identified for further analysis. A network diagram of crosstalk among these pathways was constructed based on the overlapping DE genes with the aim of identifying the main pathway on the basis of the connections with other pathways.
Materials and methods
Detection of differentially expressed genes
The study design was to obtain experimental data for breast cancer from a genomic database, and to identify DE genes and their pathways from the data using analytical software. The aim was to obtain an improved understanding of the interaction among pathways by analyzing the crosstalk of pathways.
Five biological data sets for breast cancer (E-GEOD-29431, E-GEOD-3744, E-GEOD-42568, E-GEOD-50567 and E-GEOD-7904) from different experimental origins were downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). There were 281 breast cancer samples and 69 normal samples in total. After pretreating these data by RAM, quantiles and median polish summarization methods, unqualified chips were eliminated leaving only qualified data to enter the next step. The gene expression values of all data were transformed to a comparable level, which provided a digital expression profile for subsequent analysis. As the 5 sets of data were from different experiments, DE genes were detected using RankProd (http://www.bioconductor.org/packages/release/bioc/html/RankProd.html), which is a powerful meta-analysis tool for integrating multiple array datasets from various experimental platforms. In this analysis, T and C represent two experimental conditions (treatment versus control), and there are nT and nC replicates in the first dataset, mT and mC, sT and sC, wT and wC, and fT and fC replicates in the second, third, fourth and fifth data sets, respectively. The rank product for each gene (RPg) was calculated using the following formula:
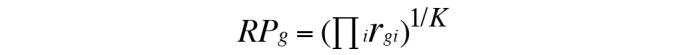
rgi is the rank of the gth gene under ith comparison. i=1, …, K, where K = (nT × nC) + (mT × mC) + (sT × sC) + (wT × wC) + (fT × fC). The genes with |logFC|>2 and percentage of false predictions (pfp)<0.01 were considered differentially expressed.
GSEA
Following the detection of DE genes from the 5 breast cancer data sets, the next step was to find pathways in which DE genes were enriched using gene enrichment analysis. There are three types of common gene enrichment analysis: singular enrichment analysis (SEA), GSEA and modular enrichment analysis (MEA). In this study, a broader application of the GSEA (20) analysis method was used. The prior knowledge of biology such as that available from KEGG (http://www.genome.jp/kegg/) was used to identify a set of genomes. Each genome was given an enrichment score (ES) by statistical calculation, the difference in significance level of the ES for different groups was detected (i.e., breast cancer and normal), then the significance level was adjusted to P<0.05 to obtain a list of enriched gene pathways.
The ES was calculated as follows:
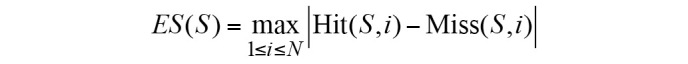
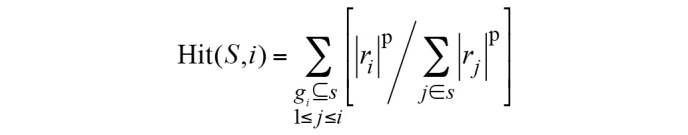
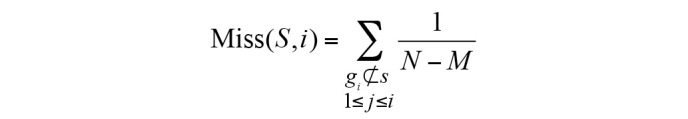
S indicates the biological pathway, rj is the correlation of gene and phenotype, M is the number of genes in S and N is the total number of DE genes in the KEGG database. P is used to correct the ES; it avoids erroneous inferences when the gene is located in the middle of gene set, as they would otherwise yield high ES values. Hit(S,i) indicates the total increase in the ES for cases when gene i belongs to S; Miss(S,i) indicates the total reduction in ES for cases when gene i does not belong to S.
Pathway crosstalk network analysis
The interactions between pathways that were obtained by the GSEA method were analyzed. It was assumed that if DE genes overlapped between pathways, an interaction existed between the pathways. If the number of DE genes that overlapped between pathways was >5, the two pathways were considered to have a strong interaction. Under these conditions, a network diagram was designed, programmed and constructed in order to represent the crosstalk between pathways visually.
The formula used was as follows:
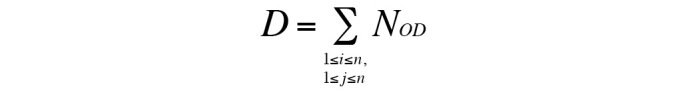
D indicates the degree of interaction between pathways. NOD indicates the number of DE genes that are overlapped, i and J represent DEGs in two different pathways. When i=j, NOD increases one; when i≠j, NOD is unchanged. ΣNOD ≥5 indicates a strong connection, whereas ΣNOD <5 indicates a normal connection.
The total number of DE genes in a pathway overlapping with another pathway is Psum; the pathway with the maximum number (MaxPsum) was considered to be the central pathway.
Results
Detection of DE genes between breast cancer and normal breast tissue
DE genes were identified between breast cancer and normal breast tissue. Genes that were upregulated and downrregulated, respectively, in breast cancer compared with normal tissue were identified. Analysis of the DE genes revealed that there were 1,464 DE genes in total, including 1,038 upregulated genes and 426 downregulated genes that had an estimated pfp<0.01 and |logFC|>2.
Pathway enrichment analysis
The majority of the pathways that were identified by the GSEA method to be significant (P<0.05) were cancer-related signaling pathways (Table I), for example, Pathways in cancer, Prostate cancer, Small cell lung cancer, peroxisome proliferator-activated receptor (PPAR) signaling pathway and p53 signaling pathway. As can be seen in Table I, a total of 26 pathways were included following enrichment analysis and the majority of the DE genes, with a count of 55, were contained in Pathways in cancer. The significance level (P<0.001) of the top five pathways in the list suggests the high reliability of the enrichment analysis.
Table I.
Significant pathways.
| KEGG ID | Term | Count | P-value |
|---|---|---|---|
| 04512 | ECM-receptor interaction | 25 | 1.99E-07 |
| 04510 | Focal adhesion | 43 | 3.87E-07 |
| 05200 | Pathways in cancer | 55 | 2.95E-05 |
| 05144 | Malaria | 13 | 5.66E-04 |
| 03320 | PPAR signaling pathway | 16 | 7.59E-04 |
| 04110 | Cell cycle | 23 | 1.69E-03 |
| 04115 | p53 signaling pathway | 14 | 5.14E-03 |
| 05222 | Small cell lung cancer | 16 | 8.33E-03 |
| 05218 | Melanoma | 14 | 8.75E-03 |
| 00565 | Ether lipid metabolism | 8 | 8.89E-03 |
| 04530 | Tight junction | 22 | 8.95E-03 |
| 04270 | Vascular smooth muscle contraction | 19 | 9.89E-03 |
| 04114 | Oocyte meiosis | 19 | 9.89E-03 |
| 04520 | Adherens junction | 14 | 9.91E-03 |
| 04350 | TGF-β signaling pathway | 15 | 1.21E-02 |
| 05215 | Prostate cancer | 16 | 1.29E-02 |
| 04610 | Complement and coagulation cascades | 13 | 1.29E-02 |
| 04710 | Circadian rhythm - mammal | 6 | 1.37E-02 |
| 04914 | Progesterone-mediated oocyte maturation | 15 | 1.67E-02 |
| 04614 | Renin-angiotensin system | 5 | 2.12E-02 |
| 00982 | Drug metabolism - cytochrome P450 | 11 | 2.81E-02 |
| 00564 | Glycerophospholipid metabolism | 13 | 3.12E-02 |
| 04916 | Melanogenesis | 16 | 3.29E-02 |
| 05219 | Bladder cancer | 8 | 4.49E-02 |
| 05221 | Acute myeloid leukemia | 10 | 4.60E-02 |
| 04360 | Axon guidance | 19 | 4.65E-02 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; ECM, extracellular matrix; PPAR, peroxisome proliferator-activated receptor; TGF, transforming growth factor.
Pathway crosstalk network analysis
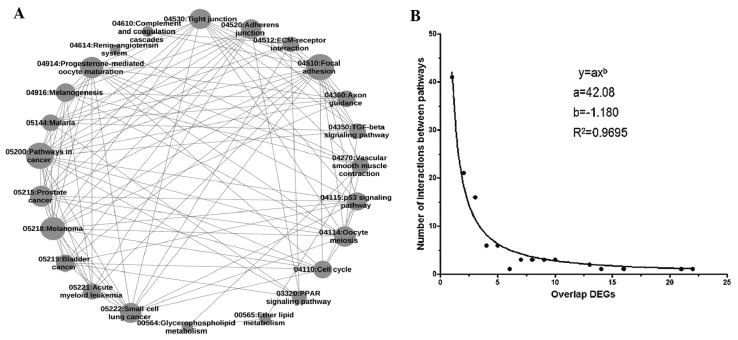
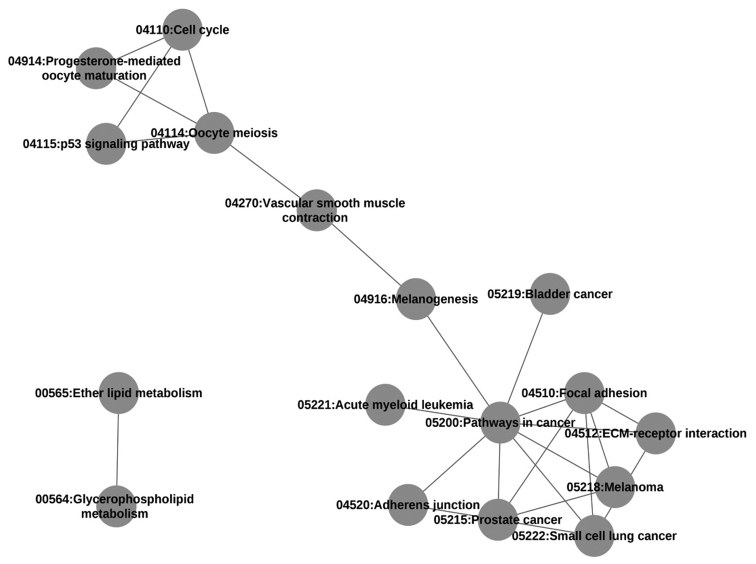
According to the overlap of DE genes between pathways, a program was designed to generate an overall network diagram that could describe the crosstalk of all 26 pathways (Fig. 1A). In addition, a scattergram of overlapping DE genes in the pathway crosstalk network was drawn (Fig. 1B). It showed that the distribution of overlapping DE genes followed a power law (y=axb, where a=42.08 and b=-1.18). In addition, a network diagram for pathways with a high degree of crosstalk, that is, those which contained >5 overlapping DE genes was generated (Fig. 2) and the main pathway was identified. It was found that the network of pathways with strong crosstalk conformed to a scale-free network whose pathway degree distribution followed a power law (y=axb, where a=43.48 and b=-0.5288).
Figure 1.
Interaction beween pathways based on the overlap of DE genes. (A) Network diagram of crosstalk among pathways; each circle represents a pathway, the size of the circles indicates how many DE genes are in the pathway; and the line between any two pathways indicates that there were DE genes that overlapped between them. (B) Scattergram of overlapping DE genes in the pathway crosstalk network. The network was a scale-free network whose distribution of overlapping DE genes followed a power law. DE, differentially expressed; DEGs, DE genes.
Figure 2.
Network diagram of strong crosstalk among pathways. Each circle represents a pathway, and a line between circles indicates that there were >5 overlapping differentially expressed genes between the two pathways.
The pathways with the greatest number of DE genes overlapping between them were the extracellular matrix (ECM)-receptor interaction and Focal adhesion pathways, which had 22 overlapping DE genes. From the analytical results, it was found that Pathways in cancer, the most important pathway with a MaxPsum of 9, had DE genes that overlapped with the Focal adhesion, Small cell lung cancer, Prostate cancer, Melanoma, ECM-receptor interaction, Acute myeloid leukemia, Melanogenesis, Adherens junction and Bladder cancer pathways (Table II). Furthermore, the DE genes that overlapped between the Pathways in cancer pathway and the other 9 pathways are listed in Table III.
Table II.
Differentially expressed genes that overlap between the main pathway (Pathways in cancer) and 9 other pathways.
| KEGG ID | Term | Overlap DEGs | Count |
|---|---|---|---|
| 04510 | Focal adhesion | IGF1, EGFR, LAMA2, PTEN, MAPK10, LAMA4, LAMC1, MET, LAMA3, LAMB2, BCL2, PDGFA, COL4A6, FN1, PIK3R1, PDGFRA, JUN, COL4A2, LAMB3, VEGFC, ITGA6 | 21 |
| 05222 | Small cell lung cancer | LAMA2, LAMA4, LAMC1, PTGS2, LAMB3, LAMA3, LAMB2, BCL2, ITGA6, COL4A6, CCNE2, FN1, E2F3, PTEN, COL4A2, PIK3R1 | 16 |
| 05215 | Prostate cancer | IGF1, FOXO1, TCF7L2, BCL2, FGFR1, EGFR, PDGFRA, PDGFA, PTEN, TCF7L1, CCNE2, LEF1, E2F3, PIK3R1 | 14 |
| 05218 | Melanoma | IGF1, EGFR, FGF2, PTEN, FGF1, MET, PDGFRA, FGFR1, PIK3R1, MITF, PDGFA, E2F3, CDKN2A | 13 |
| 04512 | ECM-receptor interaction | LAMA2, COL4A2, LAMA4, LAMC1, LAMB3, LAMA3, LAMB2, ITGA6, COL4A6, FN1 | 10 |
| 05221 | Acute myeloid leukemia | ZBTB16, CEBPA, RUNX1T1, TCF7L2, STAT5A, STAT5B, TCF7L1, KIT, PIK3R1, LEF1 | 10 |
| 04916 | Melanogenesis | FZD4, TCF7L2, FZD5, TCF7L1, KIT, MITF, FZD7, LEF1 | 8 |
| 04520 | Adherens junction | EGFR, TCF7L2, TGFBR, MET, TCF7L1, FGFR1, LEF1 | 7 |
| 05219 | Bladder cancer | EGFR, VEGFC, MMP1, MMP9, FGFR3, E2F3, CDKN2A | 7 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; ECM, extracellular matrix.
Table III.
Degree of connection among pathways.
| KEGG ID | Pathway name | Psum |
|---|---|---|
| 04270 | Vascular smooth muscle contraction | 2 |
| 00564 | Glycerophospholipid metabolism | 1 |
| 00565 | Ether lipid metabolism | 1 |
| 04115 | p53 signaling pathway | 2 |
| 05219 | Bladder cancer | 1 |
| 04520 | Adherens junction | 2 |
| 04916 | Melanogenesis | 2 |
| 04914 | Progesterone-mediated oocyte maturation | 2 |
| 04114 | Oocyte meiosis | 4 |
| 04110 | Cell cycle | 3 |
| 05221 | Acute myeloid leukemia | 1 |
| 05218 | Melanoma | 3 |
| 05215 | Prostate cancer | 5 |
| 05222 | Small cell lung cancer | 4 |
| 05200 | Pathways in cancer | 9 |
| 04510 | Focal adhesion | 5 |
| 04512 | ECM-receptor interaction | 3 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; ECM, extracellular matrix.
Discussion
Overall, 1,464 DE genes were detected from 5 breast cancer data sets and 26 pathways were identified by the GSEA method. Network diagrams of normal and strong crosstalk between pathways were constructed and Pathways in cancer was identified as the main pathway.
The hypothesis that the occurrence of breast cancer is the result of the abnormal expression of numerous genes (2) was confirmed. It is urgently necessary to identify genes that undergo changes in expression level in the process of breast cancer development in order to prevent the occurrence and development of tumors. Although numerous studies of DE genes in breast cancer have been conducted, the results have not been uniform (21,22). The methods for detecting DE genes from microarray data are the significance analysis of microarrays (SAM) method (23), two sample t-test (24), Bonferroni correction and the Benjamini and Hochberg method (25). In the present study, the RankProd method was applied, which can analyze data sets of multiple origins simultaneously, and also offers several advantages including the biologically intuitive fold-change (FC) criterion, fewer assumptions under the model, and low numbers of replicates (26). A total of 1,464 DE genes were identified from the 5 experimental data sets through analysis. Among them, certain DE genes were consistent with those identified in previous studies, for instance, EGFR (8), BCL2 (27) and FN1 (28).
A broader application of the GSEA analysis method was used to conduct pathway enrichment analysis. The enrichment analysis strategy has two advantages: i) It reduces the impact of DE gene selection on the enrichment analysis; and ii) all the information from the chip experiments is used. The GSEA analysis identified 26 pathways enriched in DE genes, and the top ranked DE gene-enriched pathways were Pathways in cancer, Focal adhesion and ECM-receptor interaction. This finding was consistent a previous study in which certain pathways, such as Pathways in cancer and Cell cycle, were identified (29). Huan et al (29) determined the changes in metabolic pathways at different time points after the treatment of breast cancer samples with estradiol, using KEGG pathway enrichment analysis for the DE genes. They concluded that the changes were mainly focused on the Pathways in cancer, Focal adhesion, and Chemokine signaling pathways.
Due to the fast-growing human interactome knowledge base, network-based approaches have become increasingly powerful and informative for the study of disease mechanisms (30). Computational methods have been proposed for the detection of disease-related networks, including co-expression (31), protein-protein interaction (PPI) (32), protein phosphorylation (33) and DNA methylation (34) networks. To the best of our knowledge, no previous study has constructed a network based on overlapping DE genes and identified the most significant pathway in breast cancer using RankProd and GSEA methods. Furthermore, the present study showed overlapping genes in all pathways. Among them, EGFR (35), IGF-1 (36), E2F3 (37) are associated with lung cancer, prostate cancer and other diseases, which may be helpful for the study of diseases by understanding of these pathways. Although the crosstalk between pathways was analyzed and the main pathway was identified, further evaluation of how the pathways effect each other would be worthwhile. In addition, as high-throughput genomic technologies become more affordable and accurate, their use is likely to become more prevalent in the identification of candidate genes in the future.
References
- 1.Igene H. Global health inequalities and breast cancer: An impending public health problem for developing countries. Breast J. 2008;14:428–434. doi: 10.1111/j.1524-4741.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 2.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–5704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 3.Planas-Silva MD, Bruggeman RD, Grenko RT, Smith JS. Overexpression of c-Myc and Bcl-2 during progression and distant metastasis of hormone-treated breast cancer. Exp Mol Pathol. 2007;82:85–90. doi: 10.1016/j.yexmp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Ota D, Mimori K, Yokobori T, et al. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int J Oncol. 2011;38:955–962. doi: 10.3892/ijo.2011.926. [DOI] [PubMed] [Google Scholar]
- 5.Ohira M, Nakagawara A. Global genomic and RNA profiles for novel risk stratification of neuroblastoma. Cancer Sci. 2010;101:2295–2301. doi: 10.1111/j.1349-7006.2010.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita T, Ikeda H, Kawasaki K, Taira N, Ogasawara Y, Nakagawara A, Doihara H. Clinicopathological relevance of UbcH10 in breast cancer. Cancer Sci. 2009;100:238–248. doi: 10.1111/j.1349-7006.2008.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berlingieri MT, Pallante P, Sboner A, et al. UbcH10 is overexpressed in malignant breast carcinomas. Eur J Cancer. 2007;43:2729–2735. doi: 10.1016/j.ejca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Chen C, Meng Y, et al. Effective suppression of breast tumor growth by an anti-EGFR/ErbB2 bispecific antibody. Cancer Lett. 2012;325:214–219. doi: 10.1016/j.canlet.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Tinnikov AA, Yeung KT, Das S, Samuels HH. Identification of a novel pathway that selectively modulates apoptosis of breast cancer cells. Cancer Res. 2009;69:1375–1382. doi: 10.1158/0008-5472.CAN-08-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donato M, Xu Z, Tomoiaga A, et al. Analysis and correction of crosstalk effects in pathway analysis. Genome Res. 2013;23:1885–1893. doi: 10.1101/gr.153551.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley EW, Ruan MM, Vrable A, Oursler MJ. Pathway crosstalk between Ras/Raf and PI3K in promotion of M-CSF-induced MEK/ERK-mediated osteoclast survival. J Cell Biochem. 2008;104:1439–1451. doi: 10.1002/jcb.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: Comradeship and hostility. Cell Signal. 2008;20:1592–1607. doi: 10.1016/j.cellsig.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Pham H, Chong B, Vincenti R, Slice LW. Ang II and EGF synergistically induce COX-2 expression via CREB in intestinal epithelial cells. J Cell Physiol. 2008;214:96–109. doi: 10.1002/jcp.21167. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Xia D, Dubois RN. The crosstalk of PTGS2 and EGF signaling pathways in colorectal cancer. Cancers (Basel) 2011;3:3894–3908. doi: 10.3390/cancers3043894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, Dubinett SM. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–6281. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Cigliano A, Delogu S, Armbruster J, et al. Functional crosstalk between AKT/mTOR and Ras/MAPK pathways in hepatocarcinogenesis: Implications for the treatment of human liver cancer. Cell Cycle. 2013;12:1999–2010. doi: 10.4161/cc.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong FX, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J. A bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics. 2006;22:2825–2827. doi: 10.1093/bioinformatics/btl476. [DOI] [PubMed] [Google Scholar]
- 18.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 19.Suárez-Fariñas M, Lowes MA, Zaba LC, Krueger JG. Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA) PLoS ONE. 2010;5:e10247. doi: 10.1371/journal.pone.0010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisowska KM, Dudaladava V, Jarzab M, et al. BRCA1-related gene signature in breast cancer: The role of ER status and molecular type. Front Biosci (Elite Ed) 2011;3:125–136. doi: 10.2741/e227. [DOI] [PubMed] [Google Scholar]
- 22.Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani R. Empirical Bayes methods and false discovery rates for microarrays. Genet Epidemiol. 2002;23:70–86. doi: 10.1002/gepi.1124. [DOI] [PubMed] [Google Scholar]
- 24.Cui X, Churchill GA. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 2003;4:210. doi: 10.1186/gb-2003-4-4-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yekutieli D, Benjamini Y. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J Stat Plan Inference. 1999;82:171–196. doi: 10.1016/S0378-3758(99)00041-5. [DOI] [Google Scholar]
- 26.Breitling R, Herzyk P. Rank-based methods as a non-parametric alternative of the T-statistic for the analysis of biological microarray data. J Bioinform Comput Biol. 2005;3:1171–1189. doi: 10.1142/S0219720005001442. [DOI] [PubMed] [Google Scholar]
- 27.Long JM, Bell CW, Fagg WS, IV, et al. Microarray and pathway analysis reveals decreased CDC25A and increased CDC42 associated with slow growth of BCL2 overexpressing immortalized breast cell line. Cell Cycle. 2008;7:3062–3073. doi: 10.4161/cc.7.19.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: Microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huan J, Wang L, Xing L, et al. Insights into significant pathways and gene interaction networks underlying breast cancer cell line MCF-7 treated with 17β-estradiol (E2) Gene. 2014;533:346–355. doi: 10.1016/j.gene.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 30.del Sol A, Balling R, Hood L, Galas D. Diseases as network perturbations. Curr Opin Biotechnol. 2010;21:566–571. doi: 10.1016/j.copbio.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Miller JA, Oldham MC, Geschwind DH. A systems level analysis of transcriptional changes in Alzheimer's disease and normal aging. J Neurosci. 2008;28:1410–1420. doi: 10.1523/JNEUROSCI.4098-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao L, Wang L, Wei Z, et al. Dynamic network of transcription and pathway crosstalk to reveal molecular mechanism of MGd-treated human lung cancer cells. PLoS ONE. 2012;7:e31984. doi: 10.1371/journal.pone.0031984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian M, Chen X, Xiong Q, et al. Phosphoproteomic analysis of protein phosphorylation networks in Tetrahymena thermophila, a model single-celled organism. Mol Cell Proteomics. 2014;13:503–519. doi: 10.1074/mcp.M112.026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartlett TE, Olhede SC, Zaikin A. A DNA methylation network interaction measure, and detection of network oncomarkers. PLoS ONE. 2014;9:e84573. doi: 10.1371/journal.pone.0084573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remon J, Morán T, Reguart N, Majem M, Carcereny E, Lianes P. Beyond EGFR TKI in EGFR-mutant non-small cell lung cancer patients: Main challenges still to be overcome. Cancer Treat Rev. 2014;40:723–729. doi: 10.1016/j.ctrv.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Wang Z, Liang Z, et al. Expression and clinical significance of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues from patients with non-small cell lung cancer. Onco Targets Ther. 2013;6:1437–1444. doi: 10.2147/OTT.S51997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilke S, Schwentner R, Yang F, et al. Oncogenic ETS fusions deregulate E2F3 target genes in Ewing sarcoma and prostate cancer. Genome Res. 2013;23:1797–1809. doi: 10.1101/gr.151340.112. [DOI] [PMC free article] [PubMed] [Google Scholar]