Abstract
The excessive production of inflammatory cytokines during invasive infection primarily mediates the pathophysiology of sepsis. To improve the survival of septic patients, many selective or mediator-specific anti-inflammatory agents have been developed. Saikosaponin A (SsA), a triterpenoid saponin isolated from Radix Bupleuri, inhibits the production of proinflammatory mediators in several cell types and protects against CCl4-induced liver injury in rats. However, whether SsA treatment provides protective effects against sepsis remains unknown. The aim of the present study was to investigate the anti-inflammatory role of SsA in septic rats and the possible involvement of the nucleotide-binding oligomerization domain 2 (NOD2)/NF-κB signaling pathway in the regulation of inflammatory cytokine expression. Sixty male Wistar rats were randomly divided into six groups (10 rats per group): Sham surgery, cecal ligation and puncture (CLP), CLP plus SsA (1.0 mg/kg), CLP plus SsA (2.5 mg/kg), CLP plus SsA (5.0 mg/kg) and sham surgery plus SsA (2.5 mg/kg) groups. Rats in the SsA groups were intraperitoneally (i.p.) injected with different doses of SsA following the CLP surgery. Tissues from the ileum were harvested 8 h after CLP or sham surgery and the levels of inflammatory cytokines and NOD2 mRNA, and the activation of NF-κB were measured. The concentrations of the cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6, as well as the NOD2 mRNA expression levels and NF-κB activation in the intestinal tissues were significantly increased in the septic rats of the CLP group compared with those in the sham group. SsA administration effectively suppressed the increase in the levels of TNF-α and IL-6. Moreover, the upregulation of NOD2 mRNA expression and phospho-NF-κB p65 levels was significantly inhibited following the administration of SsA. SsA may exert a protective role in the septic process by suppressing TNF-α and IL-6 concentrations in the intestines of septic rats and these effects appear to be mediated, at least partly, via inhibition of the NOD2/NF-κB signaling pathway.
Keywords: sepsis, saikosaponin A, tumor necrosis factor-α, interleukin-6, cecal ligation and puncture, NOD2/NF-κB signaling pathway
Introduction
Sepsis, which is characterized by a systemic inflammatory response to invasive microbial pathogens, is one of the major causes of mortality in intensive care units globally (1). During the years 1979–2000, the overall mortality rate of sepsis rose from 22 to 44 per 100,000 population (2), accounting for ~9% of the total annual mortality in the United States (3). Despite advances in understanding the pathogenesis of sepsis, efforts to use new treatment methods in clinical settings have not been proved successful and the mortality rate for sepsis remains high. Thus, it is necessary to develop new therapies in order to improve clinical outcomes in the future.
The proinflammatory cytokine tumor necrosis factor (TNF)-α, together with secondary proinflammatory mediators such as interleukin (IL)-6 and IL-8, appear to be generated to modulate the human immune response to severe infections. TNF-α and IL-6 are considered to be important regulatory factors in the cytokine network during sepsis (4,5). The excessive production of cytokines may increase vascular permeability, cause coagulopathy and change the metabolism of cells, which frequently contributes to the vulnerability of multiple organ dysfunction syndrome (6).
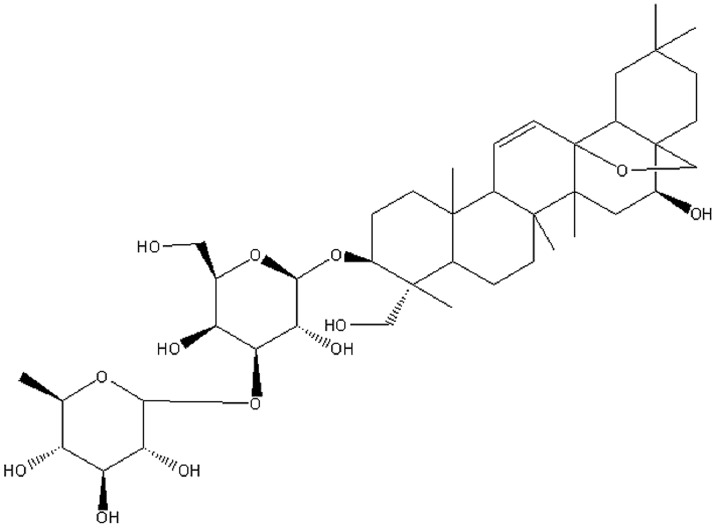
Radix Bupleuri (RB), the dried roots of Bupleurum falcatum L., is frequently included in traditional Chinese herbal formulas designed to provide anti-inflammatory, antipyretic and antihepatotoxic effects in the treatment of common cold, fever and hepatitis (7). Saikosaponin A (SsA) is a major bioactive triterpenoid saponin isolated from RB and has been identified as (3β,4α,16β)-13,28-epoxy-16,23-dihydroxyolean-11-en-3-yl-6-deoxy-3-O-β-D-glucopyranosyl-β-D-galactopyranoside (Fig. 1) (8). Previous studies have demonstrated that SsA exhibits anti-inflammatory activity in vitro and in vivo (9,10). However, little is known concerning the protective effects of SsA against sepsis.
Figure 1.
Chemical structure of saikosaponin A.
In the present study, the inhibitory effects of SsA on the important proinflammatory cytokines, TNF-α and IL-6 in the intestinal tissues of septic rats were investigated. Furthermore, nucleotide-binding oligomerization domain 2 (NOD2) mRNA expression levels and the activation of the NF-κB were examined in order to explore the mechanism underlying the effects of SsA on the inflammatory response.
Materials and methods
Animals
Sixty male Wistar rats weighing 180–200 g were provided by the Laboratory Animal Center of Henan Province [Zhengzhou, China; Certificate No. SYXK (Yu2011-0001)]. During the experiments, all rats were kept in wire-bottomed cages at 25±2°C, given tap water and standard pellet diet and exposed to a 12-h light/dark cycle at 50–60% humidity. Animal use was in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (4th edition, 2008). The study was approved by the Ethics Committees of Zhengzhou University (Zhengzhou, China).
Experimental protocol
The 60 rats were assigned equally to six groups: Sham surgery, cecal ligation and puncture (CLP), CLP plus SsA (1.0 mg/kg), CLP plus SsA (2.5 mg/kg), CLP plus SsA (5.0 mg/kg) and sham surgery plus SsA (2.5 mg/kg). Immediately following CLP surgery, rats were intraperitoneally (i.p.) treated with SsA (purity > 98%; Shanghai Institute of Pharmaceutical Industry, Shanghai, China) at the specified dose or phosphate-buffered saline (PBS; 5 ml). In each group, 2 surviving rats were sacrificed under ether anesthesia at 1, 2, 4, 6 or 8 h after surgery respectively. The ileal tissues were collected and stored in liquid nitrogen for later use.
CLP procedure
CLP was performed according to the procedure described previously (11). The rats were anesthetized i.p. with 2.5% pentobarbital sodium (40 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). Following a 3-cm midline incision, the cecum was exposed and ligated with a 3-0 silk suture below the ileocecal valve. The cecum was then punctured between the ligation and the tip of the cecum with a 20-gauge needle. After extruding a small amount of feces from the punctured site, the cecum was replaced into the peritoneum and the incision was closed using a sterile 6-0 silk suture. Rats in the sham group underwent the same laparotomy without the CLP.
Enzyme-linked immunosorbent assay (ELISA)
The ileal tissues were thawed, washed in PBS, blotted on filter paper, and weighed. Thereafter, homogenization was performed in ice-cold homogenate buffer, containing 10 mM HEPES (pH 7.9), 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 1.0 mM dithiothreitol and 0.5 mM phenylmethanesulfonyl fluoride. The homogenates were centrifuged at 3,000 × g for 15 min at 4°C. The supernatants were collected and were stored at −80°C until assayed. TNF-α and IL-6 levels in the tissue homogenates were determined by ELISA using commercial kits (TNF-α ELISA kit; Diaclone, Besançon, France; IL-6 Rat ELISA kit, cat. no. KRC0061; BioSource Europe SA, Nivelles, Belgium). The concentrations of TNF-α and IL-6 were expressed in units of pg of cytokine per mg total protein.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Tissues from the ileum were homogenized and total RNA was isolated using TRIzol reagent according to the manufacturer's instructions (Invitrogen Corp., Carlsbad, CA, USA). The RNA was converted into cDNA using M-MLV Reverse Transcriptase (Promega Corporation, Madison, WI, USA) and oligo(dT) primers. qPCR analysis with SYBR Green was performed using the Rotor-Gene™ 3000 real-time DNA analysis system (Corbett Research, Sydney, Australia). Amplifications were performed in triplicate using a standard shuttle PCR protocol (30 sec at 94°C, 30 sec at 55°C and 30 sec at 72°C) for 40 cycles. Primer sequences were as follows (all 5′ to 3′): NOD2 forward, ATC CCT CGG TTA CTA TGT TG; reverse, GCT TCC TGA ATA CTC CTC CT; β-actin forward, CCC ATC TAT GAG GGT TAC GC; reverse, TTA ATG TCA CGC ACG ATT TC. Primers were designed with Primer Premier 6.0 software (Premier Biosoft, Palo Alto, CA, USA). The cDNA results for NOD2 were normalized to β-actin measured on the same plate.
Western blot analysis
Following the determination of protein concentrations using a bicinchoninic acid (BCA) kit (Sigma-Aldrich), proteins were separated by SDS-PAGE on a 10% Tris-glycine gel, transferred to polyvinylidene membranes, and incubated with phospho-NF-κB p65 rabbit polyclonal antibody (1:1,000 dilution; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight, then washed twice with Tris-buffered saline and Tween 20 (TBST), followed by horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 1:1,000 dilution for 1 h at room temperature. After exensive washing with TBST, protein bands were visualized by the enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Tokyo, Japan). The relative expression ratio was determined with the density of the band of the protein of interest to that of a β-actin reference band using the software UN-SCAN-IT gel version 6.1 (Silk Scientific, Inc., Orem, UT, USA). Experiments were repeated at least three times.
Statistical analysis
Statistical analyses were performed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA). Measurements were expressed as the mean ± standard deviation (SD). Differences among multiple groups were examined by one-way analysis of variance (ANOVA). P<0.05 was considered to indicate a statistically significant result.
Results
Proinflammatory cytokine concentrations
Following CLP surgery, the concentrations of TNF-α and IL-6 in the intestines of the rats increased in a time-dependent manner, and the maximum levels were recorded at 8 h post-surgery (P<0.01; Fig. 2). The effect of SsA on the CLP-induced increase in cytokine levels was also studied at this time point. At doses of ≥1.0 mg/kg, SsA significantly repressed the elevation of TNF-α (P<0.05). The inhibitory effects on TNF-α were accompanied by a statistically significant inhibition of IL-6 elevation at doses of ≥2.5 mg/kg following CLP (P<0.05). Additionally, SsA did not affect the levels of proinflammatory cytokines in the rats that did not undergo CLP treatment (P>0.05; Fig. 3).
Figure 2.

Intestinal concentrations of TNF-α and IL-6 measured by ELISA. Intestinal concentrations of (A) TNF-α in sham (control) and (B) CLP group rats at 1, 2, 4, 6 and 8 h following CLP surgery. Results are presented as the means ± standard deviation of three independent experiments. TNF, tumor necrosis factor; IL, interleukin; CLP, cecal ligation and puncture.
Figure 3.
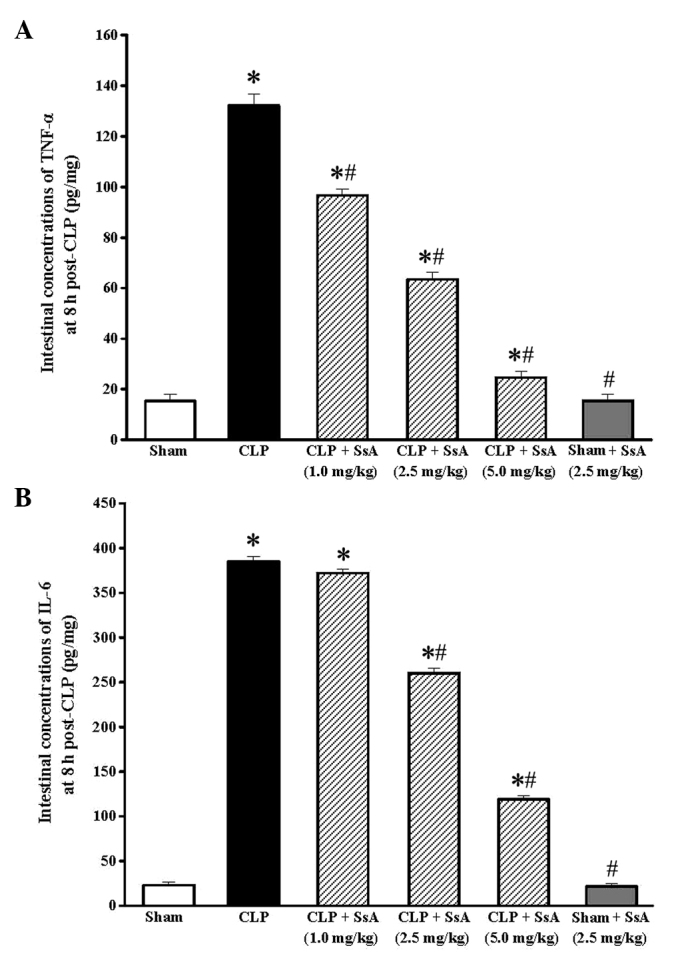
Effects of SsA on the levels of TNF-α and IL-6. Effects of SsA at different doses on the concentrations of (A) TNF-α and (B) IL-6 in rat intestines at 8 h after CLP or sham surgery. Results are presented as the means ± standard deviation of three independent experiments. *P<0.05 compared with the sham group; #P<0.05 compared with the CLP group. SsA, saikosaponin A; TNF, tumor necrosis factor; IL, interleukin; CLP, cecal ligation and puncture.
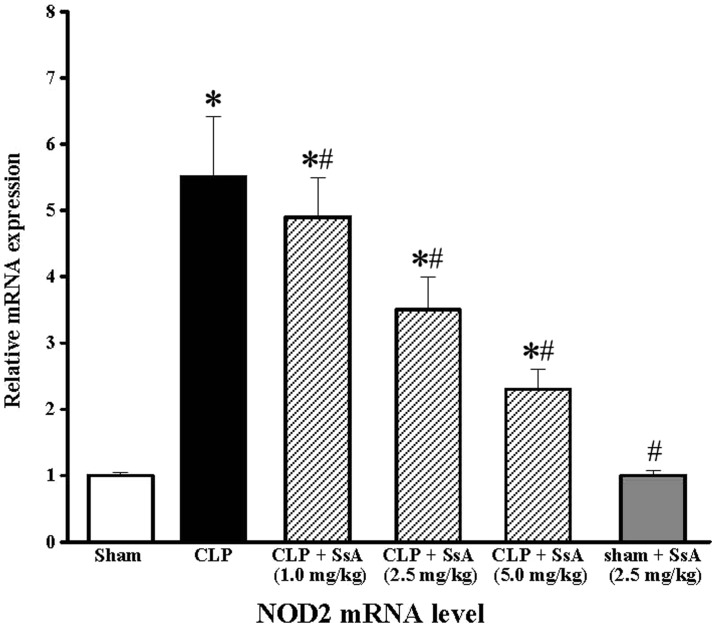
NOD2 mRNA expression
As shown in Fig. 4, in the CLP group, NOD2 mRNA expression in the intestines was significantly increased at 8 h post-surgery compared with that in the sham surgery group (P<0.05), and SsA markedly suppressed the upregulation of NOD2 mRNA expression in a dose-dependent manner (P<0.05). In addition, the expression of NOD2 mRNA in the sham surgery group was found to be similar to that of the sham surgery plus SsA group.
Figure 4.
Effect of SsA at different doses on NOD2 mRNA expression. NOD2 mRNA expression levels in rat intestines at 8 h after CLP or sham surgery were analyzed by reverse transcription-quantitative polymerase chain reaction. Results are presented as the means ± standard deviation of three independent experiments. *P<0.05 compared with the sham group; #P<0.05 compared with the CLP group. SsA, saikosaponin A; NOD2, nucleotide-binding oligomerization domain 2.
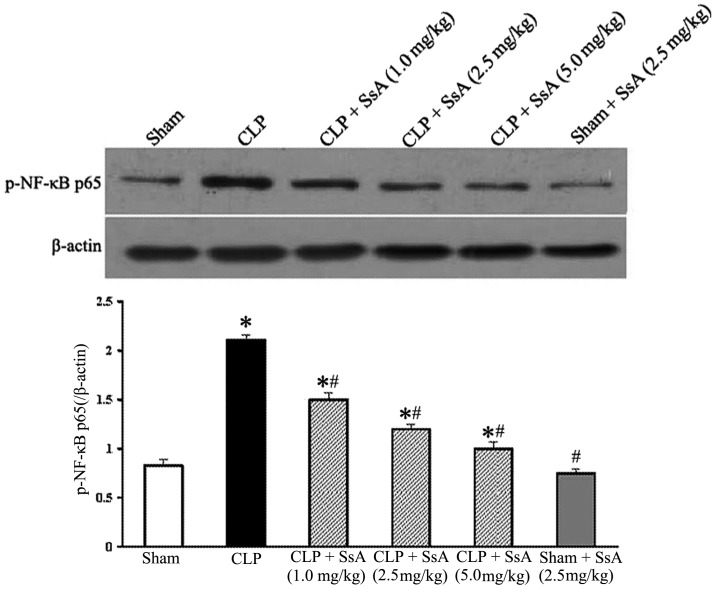
Expression of phospho-NF-κB p65
To investigate the effect of SsA on the activation of NF-κB in the ileum, the phosphorylation of the NF-κB p65 subunit was examined by western blotting 8 h after CLP or sham surgery. As demonstrated in Fig. 5, the p65 level increased significantly at 8 h after CLP, while SsA treatment markedly inhibited the activation of p65 in a dose-dependent manner (P<0.05). Moreover, the p65 level in the sham surgery plus SsA group was similar to that of the sham surgery group. These results suggest that SsA blocked the elevation of NF-κB activation induced by CLP.
Figure 5.
Effect of SsA on the activation of NF-κB in intestines 8 h after CLP or sham surgery. Expression of phospho-NF-κB p65 was analyzed by western blotting and the relative ratio was calculated from the density of the band relative to that of β-actin. *P<0.05 compared with the sham group; #P<0.05 compared with the CLP group. SsA, saikosaponin A; CLP, cecal ligation and puncture.
Discussion
Sepsis is a complex clinical syndrome comprising a systemic inflammatory response to invasive infection, which can cause cell injury and progress to multi-organ dysfunction (1,12). It is believed that the intestine is not only a major ‘victim’ that is passively damaged, but also a driving force due to the systemic release of inflammatory cytokines affecting the function and integrity of other remote organs during the sepsis process (13). To mimic clinical polymicrobial sepsis derived from the intestinal tract, a CLP model was established in the present study and the concentrations of proinflammatory cytokines in the rat intestine were measured. The results showed that the intestinal levels of TNF-α and IL-6 markedly increased following CLP in a time-dependent manner.
Several authors have reported that SsA is able to reduce the secretion of proinflammatory mediators, such as TNF-α, IL-1β, IL-6 and prostaglandin E2 in a number of cell types (10,14–16). Furthermore, SsA has been shown to suppress the contents of TNF-α, IL-1β and IL-6, and increase the IL-10 level in rats with CCl4-induced liver inflammation and fibrogenesis (17,18). SsA has also been found to decrease the serum TNF-α level in a murine model of allergic rhinitis (15). In agreement with these previous studies, the results of the present study demonstrated that SsA at different doses inhibited the increases in the levels of TNF-α and IL-6 in the ileal tissues of septic rats.
NF-κB is an inducible nuclear transcription factor, which plays a key role in regulating the transcription of several genes, including those encoding proinflammatory cytokines such as TNF-α, IL-1β and IL-6 involved in severe sepsis and septic shock (1,19). It is now well established that the persistent activation of NF-κB is associated with a higher mortality rate in septic patients (20). Clinical evidence has demonstrated that the suppression of NF-κB activity may exert an beneficial effect on sepsis (21,22). NOD2 is known to be an important innate cytosolic receptor involved in protective immunity against infectious agents (23). NOD2 in the host cells senses the peptidoglycan component of gram-positive and -negative bacteria, transmits signals to receptor-interacting protein 2, and then triggers a NF-κB-mediated proinflammatory and antibacterial response (24,25), which leads to a positive feedback loop during the infection process (26). Polymorphisms in the gene encoding NOD2 in humans have been associated with early mortality in septic patients as they affect the ability of NOD2 to recognize bacteria and activate NF-κB (27). From the studies described above, a potential role of the NOD2/NF-κB pathway in sepsis is suggested. In the present study, it was shown that the activation of NF-κB was greatly enhanced following CLP and that SsA suppressed NF-κB activation. Moreover, a noteworthy observation is that NOD2 expression was markedly upregulated in the intestines of rats following CLP and SsA inhibited its expression. Collectively, the present findings indicate that SsA may exert a protective role against sepsis, possibly via the downregulation of the expression of NOD2 mRNA, which is necessary for NF-κB activation and TNF-α and IL-6 expression.
There are certain shortcomings in the present study. First, although NOD2 is necessary for initiating the proinflammatory function via NF-κB activation, it is not possible to rule out the possibility that SsA inhibits the activation of NF-κB via other signaling pathways. Further studies are required to explore the function of the NOD2-mediated NF-κB pathway independently. Secondly, data in the literature indicates that the delicate balance between proinflammatory and anti-inflammatory mediators determines the severity of infection (28,29). In the present study, cytokines with notably anti-inflammatory properties, such as IL-10 and IL-13, which reduce inflammation by suppressing NF-κB activation (30,31) were not investigated. Further studies to demonstrate the effect of SsA on anti-inflammatory cytokine production and the potential mechanism involved are required.
In conclusion, the present study reveals the protective effect of SsA during the CLP-induced septic process, which may be achieved, at least in part, through the inhibition of the NOD2-mediated NF-κB signaling pathway. Thus, the potent anti-inflammatory actions of SsA indicate that it may be useful as a new therapeutic agent for sepsis.
Acknowledgements
This study was supported by a grant from the Science Foundation of Henan Province (no. 2013A310660) and the Research Foundation for Talent Recruitment of Zhengzhou University.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell TS, Christman JW. Sepsis and cytokines: Current status. Br J Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 7.Ashour ML, Wink M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. 2011;63:305–321. doi: 10.1111/j.2042-7158.2010.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KH, Park J, Koh D, Lim Y. Effect of saikosaponin-A, a triterpenoid glycoside, isolated from Bupleurum falcatum on experimental allergic asthma. Phytother Res. 2002;16:359–363. doi: 10.1002/ptr.903. [DOI] [PubMed] [Google Scholar]
- 9.Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ, Liao M, Chen JX. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int Immunopharmacol. 2012;14:121–126. doi: 10.1016/j.intimp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Kim SO, Park JY, Jeon SY, Yang CH, Kim MR. Saikosaponin a, an active compound of Radix Bupleuri, attenuates inflammation in hypertrophied 3T3-L1 adipocytes via ERK/NF-κB signaling pathways. Int J Mol Med. 2015;35:1126–1132. doi: 10.3892/ijmm.2015.2093. [DOI] [PubMed] [Google Scholar]
- 11.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: The mystery continues. Am Surg. 2012;78:1–8. [PubMed] [Google Scholar]
- 13.Yu M, Shao D, Liu J, Zhu J, Zhang Z, Xu J. Effects of ketamine on levels of cytokines, NF-kappaB and TLRs in rat intestine during CLP-induced sepsis. Int Immunopharmacol. 2007;7:1076–1082. doi: 10.1016/j.intimp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Luo C, Wang P, He Q, Zhou J, Peng H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp Ther Med. 2013;5:1345–1350. doi: 10.3892/etm.2013.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han NR, Kim HM, Jeong HJ. Inactivation of cystein-aspartic acid protease (caspase)-1 by saikosaponin A. Biol Pharm Bull. 2011;34:817–823. doi: 10.1248/bpb.34.817. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Cai TT, Zhou XB, Xu Q. Saikosaponin A inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int Immunopharmacol. 2009;9:978–983. doi: 10.1016/j.intimp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Wu SJ, Lin YH, Chu CC, Tsai YH, Chao JC. Curcumin or saikosaponin A improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J Med Food. 2008;11:224–229. doi: 10.1089/jmf.2007.555. [DOI] [PubMed] [Google Scholar]
- 18.Wu SJ, Tam KW, Tsai YH, Chang CC, Chao JC. Curcumin and saikosaponin A inhibit chemical-induced liver inflammation and fibrosis in rats. Am J Chin Med. 2010;38:99–111. doi: 10.1142/S0192415X10007695. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Arnalich F, Garcia-Palomero E, López J, Jiménez M, Madero R, Renart J, Vázquez JJ, Montiel C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun. 2000;68:1942–1945. doi: 10.1128/IAI.68.4.1942-1945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X, Ren B, Xie W, Huang Z, Liu J, Guan R, Duan M, Xu J. Influence of hydroxyethyl starch 130/0.4 in pulmonary neutrophil recruitment and acute lung injury during polymicrobial sepsis in rats. Acta Anaesthesiol Scand. 2006;50:1081–1088. doi: 10.1111/j.1399-6576.2006.01113.x. [DOI] [PubMed] [Google Scholar]
- 22.Feng X, Yan W, Liu X, Duan M, Zhang X, Xu J. Effects of hydroxyethyl starch 130/0.4 on pulmonary capillary leakage and cytokines production and NF-kappaB activation in CLP-induced sepsis in rats. J Surg Res. 2006;135:129–136. doi: 10.1016/j.jss.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Frutuoso MS, Hori JI, Pereira MS, Junior DS, Sônego F, Kobayashi KS, Flavell RA, Cunha FQ, Zamboni DS. The pattern recognition receptors Nod1 and Nod2 account for neutrophil recruitment to the lungs of mice infected with Legionella pneumophila. Microbes Infect. 2010;12:819–827. doi: 10.1016/j.micinf.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Núñez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magalhaes JG, Lee J, Geddes K, Rubino S, Philpott DJ, Girardin SE. Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. Eur J Immunol. 2011;41:1445–1455. doi: 10.1002/eji.201040827. [DOI] [PubMed] [Google Scholar]
- 26.Hu C, Sun L, Hu Y, Lu D, Wang H, Tang S. Functional characterization of the NF-kappaB binding site in the human NOD2 promoter. Cell Mol Immunol. 2010;7:288–295. doi: 10.1038/cmi.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenmoehl J, Herfarth H, Glück T, Audebert F, Barlage S, Schmitz G, Froehlich D, Schreiber S, Hampe J, Schölmerich J, et al. Genetic variants in the NOD2/CARD15 gene are associated with early mortality in sepsis patients. Intensive Care Med. 2007;33:1541–1548. doi: 10.1007/s00134-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 28.Girardin E, Grau GE, Dayer JM, Roux-Lombard P, Lambert PH. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 29.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappaB (NF kappaB) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 31.Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappaB and preservation of I kappaB alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]