Abstract
Nesidioblastosis is a major cause of persistent hyperinsulinemic hypoglycemia of infancy and is caused by hypertrophy of the pancreatic endocrine islands. The disease can be categorized histologically into diffuse and focal forms. The condition rarely occurs in adults and only one adult case of suspected, but not histologically confirmed, focal nesidioblastosis has been reported. The present study describes the case of a 62-year-old man suffering from symptomatic hypoglycemia for 3 years and exhibiting a nodule in the pancreatic tail. Pathological evaluation following surgical enucleation of the pancreatic body and tail revealed focal nesidioblastosis. The hypoglycemic symptoms of the patient disappeared postoperatively. To the best of our knowledge, this is the first histologically-confirmed case of focal adult nesidioblastosis, suggesting that the possibility of nesidioblastosis should be taken into account in adult patients with persistent hypoglycemia.
Keywords: nesidioblastosis, hypoglycemia, adult
Introduction
Hypoglycemia in nondiabetic patients is not a common clinical problem and can be a diagnostic and therapeutic challenge (1). Persistent hyperinsulinemic hypoglycemia (PHH) is a functional disorder caused by aberrant insulin release by pancreatic β cells (2). Nesidioblastosis is the major cause of PHH in infants and children, but in adults it is usually a consequence of a solitary insulinoma. Nesidioblastosis has been reported infrequently in adults (1–3). It should be noted that in pediatric patients nesidioblastosis may be classified histologically as either diffuse or focal, but only diffuse lesions have been reported in adult patients with histologically-confirmed nesidioblastosis. A single case of suspected focal nesidioblastosis in an adult was reported by McElroy et al in 2010 (4), but was not confirmed histologically. Due to the lack of evidence, most physicians do not take a possibility of focal nesidioblastotosis into account when confronted with an adult patient with PHH‥
The present study focused on the case of a 62-year-old man with a 3-year history of intermittent episodes of symptomatic hypoglycemia. A 72-h fasting test, elevated levels of insulin and C-peptide, concomitant with decreased blood glucose levels and imaging, led to the discovery of a nodule in the pancreatic tail. The pancreatic corpus and tail were enucleated laparoscopically and the presence of focal nesidioblastosis was confirmed histologically. We propose that focal nesidioblastosis should be taken into consideration when confronted with PHH, even in middle-aged patients.
Case report
Case presentation
A 62-year-old man with a body mass index of 26.99 presented with a 3-year history of intermittent episodes of dizziness, weakness and sweating, which were apparently associated with work load and subsided upon food intake. Previous clinical evaluations had not included blood glucose measurements. The patient had no history of diabetes, pancreatic diseases, von Hippel-Lindau or multiple endocrine neoplasia syndromes and denied using insulin or medications associated with hypoglycemia.
Based on the clinical presentation of the patient, a 72-h fasting test was performed to address the possibility of endogenous hyperinsulinemic hypoglycemia, but was discontinued after 61 h due to complaints of fatigue and dizziness. During the hypoglycemic episodes, laboratory tests revealed that the glucose, insulin and C-peptide levels were 48 mg/dl (normal range, 70–100), 162.20 pmol/l (normal range, 17.0–173.0) and 1.394 nmol/l (normal range, 0.37–1.47), respectively. A nodule in the pancreatic tail was observed on abdominal computed tomography (CT) (Fig. 1A), which prompted further examination using other imaging modalities. Magnetic resonance imaging (MRI) revealed the presence of an unevenly enhanced lesion in the pancreatic tail, measuring 2.2 cm in diameter (Fig. 1B and C). The patient underwent laparoscopic enucleation of the pancreatic body and tail following an initial diagnosis of a distal pancreatic tumor.
Figure 1.
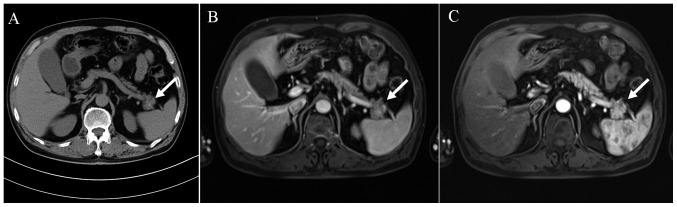
Preoperative imaging showing an exophytic mass (2.2 cm diameter) arising from the pancreatic tail. (A) Abdominal computed tomography of the lesion. (B) T1-weighted MRI of the pancreas. (C) Enhanced T1-weighted MRI showing uneven enhancement of the lesion. White arrows indicate the position of the lesion. MRI, magnetic resonance imaging.
Investigations
The collected pancreatic specimen measured ~3.5×2.5×1.5 cm. This specimen was fixed in formalin, and hematoxylin and eosin-stained serial sections were prepared for microscopic examination by an expert pathologist. Immunohistochemical staining was also performed, using rabbit monoclonal antibodies against synaptophysin (1:1; #RMA-0537) and chromogranin A (1:1; #MAB-0202), and mouse monoclonal antibodies against neuron-specific enolase (1:1; #MAB-0584) and Ki-67 (1:1; #Kit-0005-2) purchased from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou, China). In addition, rabbit polyclonal antibody against insulin Rβ (1:100; #sc-711) was purchased from Santa Cruz Biotechnology Inc., Dallas, TX, USA).
The pancreatic tissue had a normal appearance; the cut surface was light brown and soft. The microscopic architecture of the exocrine pancreas and the histology of the pancreatic ducts and epithelium were normal. A significant abnormality was noted in the endocrine pancreas, which contained substantially more than the normal number of islets (Fig. 2A). The endocrine islets also contained multiple enlarged cells with enlarged nucleoli and abundant clear cytoplasm that was granulated and slightly eosinophilic (Fig. 2B). The hyperplastic nature of the islets decreased substantially from the center of the lesion toward the perimeter (Fig. 2A and C), and therefore it was concluded that the lesion was characterized by the presence of hyperplastic islets.
Figure 2.
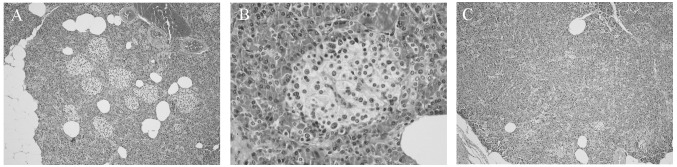
Hematoxylin and eosin-stained sections of pancreatic tissue. (A) Multiple hypertrophic islets are shown in the center of the pancreatic lesion (magnification, x100). (B) Islets including multiple, enlarged cells with enlarged nucleoli and abundant clear, granulated, slightly eosinophilic cytoplasm (magnification, x400). (C) In contrast to the center of the lesion, the number of islets at the margin of the lesion is nearly normal (x100).
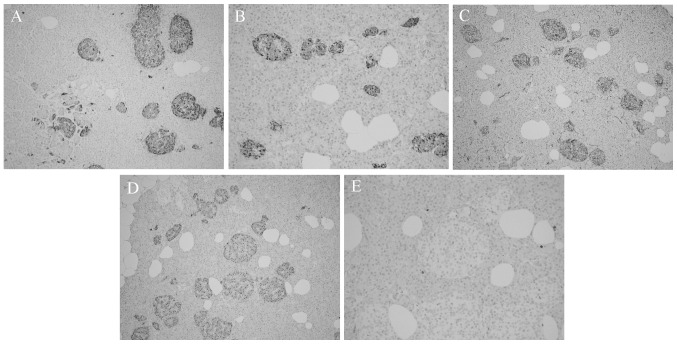
Immunohistochemical staining showed islet cells that were positive for synaptophysin (Fig. 3A), chromogranin A (Fig. 3B) and neuron-specific enolase (Fig. 3C). Insulin staining revealed enlarged, insulin-secreting β cells (Fig. 3D). Ki-67 staining was nearly absent in the islets (Fig. 3E). The pathological findings were consistent with a diagnosis of adult nesidioblastosis (3) and had the histological characteristics of a focal lesion. Postoperatively, the hypoglycemic symptoms of the patient disappeared, with fasting glucose ranging from 120 to 144 mg/dl.
Figure 3.
(A–C) Immunohistochemical staining showing islet cells positive for (A) synaptophysin (magnification, x100) (B) chromogranin A (magnification, x100) and (C) neuron-specific enolase (magnification, x100). (D) Insulin staining showing enlarged, insulin-secreting β cells (magnification, x100). (E) Islets showing almost no Ki-67 staining (magnification, x200).
Discussion
Nesidioblastosis is the most common cause of PHH of infancy, but is rarely encountered in adults. The term nesidioblastosis was coined by Laidlaw (5) in 1938 to describe the neoformation of endocrine islets from pancreatic duct epithelium. The first adult case was reported in 1975 (6), and nesidioblastosis is currently estimated to be the cause of PHH in 0.5–7% of adult patients (4,7).
The cause of adult nesidioblastosis is unknown, but it may be genetically induced, as in congenital hyperinsulinism in neonates, or be a response to metabolic and hormonal changes, such as those following gastric bypass surgery and weight loss (3). A paradoxical elevation of glucagon-like peptide 1 reported following gastric bypass surgery (8) may influence islet cell neogenesis and apoptosis, thus producing islet hyperplasia and PHH (9).
Preoperative diagnosis of adult nesidioblastosis is challenging, as there are no defining clinical symptoms or history and no highly specific functional tests (10). The occurrence of postprandial hypoglycemia may indicate adult nesidioblastosis (3,5) if insulinoma or factitious hypoglycemia can be excluded. In addition, a selective arterial calcium stimulation test may indicate hyperactive β-cell activity and provide guidance for the localization of the disease and direct resection of the appropriate pancreatic regions (11). A final diagnosis of adult nesidioblastosis is best established postoperatively by histological examination (3,7,10,11). Diagnostic criteria of adult nesidioblastosis proposed by Klöppel et al (3) involve exclusion of an insulinoma and various histological characteristics.
The definitive treatment of adult nesidioblastosis is surgical, but the resection volumes remain undefined (10). Subtotal (75 to 90%) pancreatectomy is generally considered to be the more suitable choice, but recurrent hypoglycemia and diabetes mellitus are frequent postoperative complications (11).
In the present case, the histological findings met the diagnostic criteria for adult nesidioblastosis (3) and the gradient observed in islet hyperplasia, progressing from the center to the periphery of the lesion, was consistent with the diagnosis of focal nesidioblastosis.
Despite the fact that postprandial hypoglycemia has been reported in adult nesidioblastosis, this was not evident in the present case, in which the disease episodes appeared to be due to increased physical activity or physical work stress. Insulin and C-peptide were in the upper half of the normal range but laboratory testing did not provide a definitive indication of nesidioblastosis. Imaging techniques are not generally helpful for indicating preoperative localization of adult nesidioblastosis (10), but in this patient both CT and MRI revealed the location of an exophytic lesion in the pancreas. The intraoperative localization of the lesion was consistent with the imaging and with the previous case report of suspected (but histologically unconfirmed) focal nesidioblastosis (4). Since positive imaging was also apparent in the previous case, we propose that imaging may assist in the localization of focal nesidioblastosis and its distinction from diffuse nesidioblastosis.
The patient of the present study developed diabetes mellitus following the resection of the pancreatic tissue. In the aforementioned case of suspected focal nesidioblastosis, euglycemia was restored following simple enucleation of the lesion, which comprised <5% of the total pancreatic mass (4). In patients with focal nesidioblastosis, partial pancreatectomy would be expected to control the hypoglycemia and result in a good long-term outcome.
In conclusion, focal nesidioblastosis is rare in adults and can be difficult to diagnose preoperatively. The present case report, however, suggests that physicians should consider focal nesidioblastosis when confronted with patients with PHH, since early detection may prevent unnecessary full resection of the pancreatic corpus and tail. Imaging can be useful, particularly when combined with moderately high levels of insulin following fasting; patients exhibiting these characteristics should be considered for partial pancreatectomy.
References
- 1.Ng CL. Hypoglycaemia in nondiabetic patients - an evidence. Aust Fam Physician. 2010;39:399–404. [PubMed] [Google Scholar]
- 2.Anlauf M, Wieben D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: Diagnostic criteria, incidence and characterization of beta-cell changes. Am J Surg Pathol. 2005;29:524–533. doi: 10.1097/01.pas.0000151617.14598.ae. [DOI] [PubMed] [Google Scholar]
- 3.Klöppel G, Anlauf M, Raffel A, et al. Adult diffuse nesidioblastosis: Genetically or environmentally induced. Hum Pathol. 2008;39:3–8. doi: 10.1016/j.humpath.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 4.McElroy MK, Lowy AM, Weidner N. Case report: Focal nesidioblastosis (‘nesidioblastoma’) in an adult. Hum Pathol. 2010;41:447–451. doi: 10.1016/j.humpath.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Laidlaw GF. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol. 1938;14:125–134. [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler R, Horwitz DL, Rubenstein AH, Kuzuya H. Hypoglycemia and endogenous hyperinsulinism complicating diabetes mellitus. Application of the C-peptide assay to diagnosis and therapy. Am J Med. 1975;59:730–736. doi: 10.1016/0002-9343(75)90234-X. [DOI] [PubMed] [Google Scholar]
- 7.Kaczirek K, Niederle B. Nesidioblastosis: An old term and a new understanding. World J Surg. 2004;28:1227–1230. doi: 10.1007/s00268-004-7598-7. [DOI] [PubMed] [Google Scholar]
- 8.Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: Evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–2940. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 10.Raffel A, Krausch MM, Anlauf M, et al. Diffuse nesidioblastosis as a cause of hyperinsulinemic hypoglycemia in adults: A diagnostic and therapeutic challenge. Surgery. 2007;141:179–186. doi: 10.1016/j.surg.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Kenney B, Tormey CA, Qin L, et al. Adult nesidioblastosis. Clinicopathologic correlation between pre-operative selective arterial calcium stimulation studies and post-operative pathologic findings. JOP. 2008;9:504–511. [PubMed] [Google Scholar]