Abstract
According to the cancer stem cell theory, a small subpopulation of cancer cells, known as cancer stem cells (CSCs), exist that are self-renewing and are involved in tumor invasion, metastasis and recurrence. A number of studies have reported that certain cancer cells are able to efflux the Hoechst 33342 dye. These cells are termed side population (SP) cells and share characteristic features of CSCs. The results of the present study revealed that 2.7% of primary head and neck squamous cell carcinoma (HNSCC) cells were SP cells. This was reduced to 0.7% following treatment with verapamil. The immunofluorescence and reverse transcription polymerase chain reaction analysis revealed that SP cells have an enhanced expression of the ATP-binding cassette (ABC) transporter protein ABC subfamily G, member 2 (ABCG2), which has been identified to be actively involved in drug exclusion. Similarly, the mRNA level of the oncogene B lymphoma Mo-MLV insertion region-1 and the stem cell surface proteins nestin and octamer-binding transcription factor-4 were highly expressed in the SP cells compared with the non-SP cells. In addition, it was demonstrated that HNSCC SP cells exhibited increased proliferation and were highly resistant to multiple drugs. These findings suggest that the presence of CSCs, such as SP cells, may be responsible for chemotherapy failure and tumor relapse in patients with HNSCC. Therefore, the identification of a novel therapeutic drug that could effectively target CSCs may help to eradicate refractory tumors.
Keywords: cancer stem cells, chemotherapy, tumor recurrence, side population cells, ATP-binding cassette transporters
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the tenth most common malignancy worldwide, and confers a low overall survival rate following diagnosis (1). Despite recent advances in treatment strategies, patients often develop secondary tumors within 2–5 years of receiving chemotherapy or radiotherapy (1). Previous studies have suggested that treatment failure and tumor recurrence are the result of a small population of tumor-initiating cancer stem cells (CSCS) (2–4). CSCs are a small subpopulation of cancer cells that are self-renewing, exhibit a high differentiation potential, are highly tumorigenic and express stem cell surface markers, including cluster of differentiation (CD)44, CD133 and octamer-binding transcription factor (Oct-4). Furthermore, CSCs are highly resistant to chemotherapeutic drugs, and therefore prevent the complete elimination of a tumor. Previous data has revealed the ability of CSCs that are CD44- and CD133-positive are able to initiate tumor growth in NOD/SCID mice (5–7). Therefore, it is important to understand the molecular mechanisms underlying CSC-induced tumorigenesis in order to develop effective anti-cancer therapies. Studies concerning solid tumors have identified tumorigenic subpopulations of cancer cells by their expression of cell surface markers, such as CD44 and CD133, or by using the Hoechst 33342 dye exclusion technique. Cancer cells with the ability to efflux the DNA binding dye Hoechst 33342 during fluorescence-activated cell sorting (FACS) analysis are termed side population (SP) cells (8). These SP cells possess the characteristic features of CSCs. The ability of SP cells to exclude dye or exhibit chemotherapeutic drug resistance is believed to be associated with the overexpression of the ATP-binding cassette (ABC) transporter protein ABC subfamily G, member 2 (ABCG2) (9,10). Therefore, the isolation and characterization of SP cells may help to identify the tumor cell of origin and enable the complete eradication of tumors. The isolation and characterization of SP cells has been documented in several solid tumors, including oral, breast, lung, prostate and gastric cancers (11–13). Therefore, the present study aimed to isolate and characterize cancer stem-like SP cells from HNSCCs using the Hoechst 33342 dye exclusion assay.
Materials and methods
Sphere-forming cell culture from primary HNSCC specimens
The present study was approved by the Medical Ethics Committee of The Second Hospital of Jilin University (Changchun, China). Human HNSCC samples were obtained from patients at the time of surgery according to the approved method, at the Department of Otolaryngology, Head and Neck Surgery, Tumor Hospital of Jilin (Changchun, China). The primary tumor samples were cut into small sections using blades, enzymatically digested with collagenase, hyaluronidase and DNase (Takara Bio, Inc., Otsu, Japan), and then incubated for 2 h at 37°C with 5% CO2. Subsequent to careful pipetting up and down 10 times with a 1-ml pipette every 15 min, the cell disaggregates were washed twice with phosphate-buffered saline (PBS), and then subjected to centrifugation in order to remove the cell debris and red blood cells. The cell suspensions were then filtered and the resulting single cells were incubated under stem cell suspension culture conditions, consisting of serum-free Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Gibco Life Technologies, Carlsbad, CA, USA) supplemented with N2, B27, 20 ng/ml human recombinant epidermal growth factor (EGF) and 20 ng/ml human basic fibroblast growth factor (bFGF)(Prospec-Tany TechnoGene, Ltd., East Brunswick, NJ, USA).
FACS analysis
First, the cells were cultured in DMEM containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) supplemented with antibiotics, and then maintained in T-75 flasks at 37°C in a humidified 5% CO2 and 95% air atmosphere. Upon reaching 90% confluency, the cells were removed using Trypsin-EDTA (0.25%-53 mM EDTA; Sigma-Aldrich) from the culture flask, washed, and then suspended in 10% DMEM. The cell count was obtained using a C-Chip Disposable hemocytometer (Polysciences Inc., Warrington, PA, USA).
Study groups
The control cells received Hoechst 33342 dye alone (n=3), whilst the drug-treated cells received Hoechst 33342 dye in combination with verapamil (n=3). The cells were counted using a hemocytometer. In total, ~106 cells/ml in 10% DMEM were labeled with 5 µl/ml Hoechst 33342-bis-benzimide stock (Sigma-Aldrich) either alone or in combination with 0.8 µl/ml of verapamil, an ABC transporter inhibitor. The cells were then counterstained using 2 µg/ml propidium iodide (PI; Sigma-Aldrich), and an Attune® NxT Acoustic Focusing Flow Cytometer (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) was used for sorting. Next, the sorted cells were cultured and maintained in DMEM/F-12 supplemented with 10% FBS. The Hoechst 33342 emission was first split using a 610 nm dichroic short-pass filter, and then the red and blue emissions were collected through 670/30 and 450/65 nm bandpass filters, respectively.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted and the complementary DNA was prepared using a Reverse Transcriptase kit (Thermo Fisher Scientific, Inc.). The RT-qPCR analysis was conducted on an iCycler IQ real-time detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using IQ Supermix with SYBR-Green (Bio-Rad Laboratories, Inc.). The sequences of the human-specific primers used in the present study are as follows: Oct-4 forward, 5′-GCA ATT TGC CAA GCT CCT GAA-3′ and reverse, 5′-GCA GATGGTCGTTTGGCTGA-3′; Nestin forward, 5′-AGAGGG AGGACA AAGTCCCT-3′ and reverse, 5′-CACTTCCTCAGACTG CTCCA-3′; ABCG2 forward, 5′-TCAATCAAAGTGCTT CTTTTTTATG-3′ and reverse, 5′-TTGTGGAAGAATCAC GTGGC-3′; GAPDH forward, 5′-ATG TCG TGG AGT CTA CTG GC-3′ and reverse, 5′-TGACCTTGCCCACAGCCTTG-3′; B lymphoma Mo-MLV insertion region-1 (Bmi-1) forward, 5′-CTCCCAACTGGTTCGACCTT-3′ and reverse, 5′-CGG TTTCCATATTTCTCAGT-3′. The amplified products were separated by electrophoresis on ethidium bromide-stained 1.2% agarose gels. The band intensities from three independent experiments were measured using ImageJ software (National Institutes of Health, Bethesda, MA, USA).
Immunofluorescent staining
The immunostaining of the squamous spheres was performed as previously described (14). First, the spheres were fixed onto glass slides in ice-cold 4% paraformaldehyde at 4°C for 10 min, and then blocked with normal serum for 30 min. The slides were then incubated overnight with mouse monoclonal anti-human Oct-4 (#SAB3300035) and CD44 antibodies (#SAB1405590) (dilution, 1:200; Sigma-Aldrich). Subsequent to washing with PBS, the slides were incubated with fluorescein isothiocyanate (FITC)-conjugated chicken anti-rat immunoglobulin G (IgG) (#ab46969; Abcam, Cambridge, UK) overnight in a dark room. The nuclei were then counterstained with DAPI (Molecular Probes Life Technologies, Carlsbad, CA, USA) and viewed under an AX80TR fluorescence microscope (Olympus Corporation, Tokyo, Japan).
In order to investigate the expression of ABCG2, the sorted SP and non-SP cells were incubated overnight at 4̊C with a mouse monoclonal anti-human ABCG2 antibody (dilution, 1:500; #ab95692; Abcam, Cambridge, MA, USA). Subsequent to washing with PBS, goat anti-mouse horseradish peroxidase (HRP)-conjugated IgG (#10004302; Cayman Europe, Tallinn, Estonia) was added, and the cells were incubated for a further 30 min at room temperature. Next, the cells were counterstained with hematoxylin and eosin and then mounted using glycerol vinyl alcohol aqueous mounting solution (15). A BX50 optical microscope (Olympus Corporation) was used to observe the red color of the ABCG2+ cells. All images were processed using Adobe Photoshop CS4 (Adobe Systems, Inc., San Jose, CA, USA).
Sphere formation assay
The sphere formation assay was conducted as previously described (16). First, the sorted SP and non-SP cells were placed at a density of 1000 cells/ml resuspended in a tumor sphere medium consisting of a serum-free 1:1 mixture of Ham's F-12/DMEM and N2 supplement, 10 ng/ml human recombinant bFGF and 10 ng/ml EGF. The cells were then cultured in ultra-low attachment plates for ~2 weeks.
Cell resistance assay
In total, ~1×103 cells/plate were cultured in 96-well plates and treated with 10 µg/ml 5-fluorouracil (5-FU), 20 µmol/l cisplatin, 2 µmol/l paclitaxel or 2 µg/ml docetaxel (all Sigma-Aldrich). The mean optical densities at 450 nm (OD450) are shown in Fig. 1B. The cell resistance of the control and verapamil-treated groups was calculated using the following formula, as previously described (17):
Figure 1.
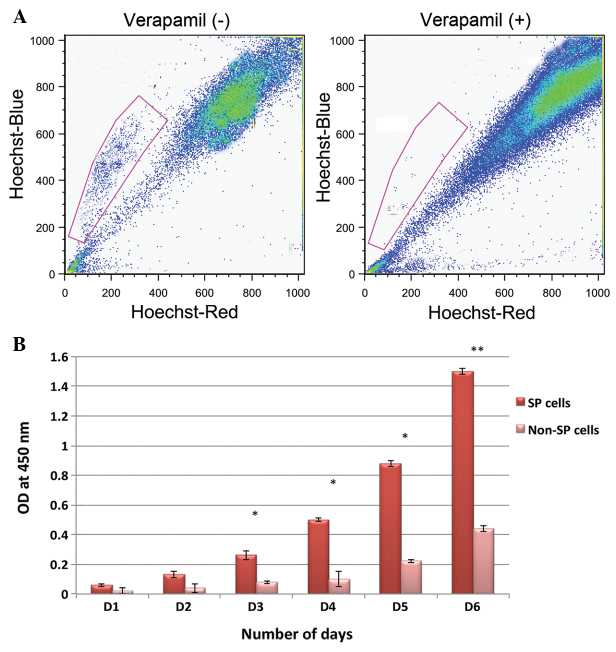
Representative images of the fluorescence-activated cell sorting dot plot analysis. Live cells were selected by propidium iodide staining. (A) Graph (left panel) revealing that 2.7% of the HNSCC cells were a population of SP cells. Following treatment with the ATP-binding cassette transporter inhibitor, verapamil, the percentage of SP cells was reduced to 0.7% (right panel). (B) Cell proliferation rates of the SP and non-SP cells. *P<0.05; **P<0.01. HNSCC, head and neck squamous cell carcinoma; SP, side population; OD, optical density; D, day; -, absent; +, present.
In vitro proliferation activity assay
In total, 2×106 cells/well of the sorted SP and non-SP cells were seeded into a 96-well plate and cultured in a CO2 incubator. Each group was set up in triplicate. The rate of cell proliferation was measured each day for 7 days. The wells were supplemented with 10 µl cell counting kit 8 solution and incubated for 2–3 h in a CO2 incubator. The OD450 was then assessed. The mean OD450 values and the standard deviation values for each well were then used to construct cell growth graphs.
Statistical analysis
A one-way analysis of variance and Student's t-test was used to identify any significant differences between the experimental and control groups. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Isolation and phenotypic characterization of cancer stem-like SP cells in HNSCCs
The present study investigated the presence of cancer stem-like SP cells in HNSCCs using a FACS-based Hoechst 33342 dye exclusion method. As shown in Fig. 1A, ~2.7% of the HNSCC cells were SP cells. These small populations of cells exhibit a distinct pattern and fall to the side of the FACS dot plot analysis. It has been established that the Hoechst 33342 dye efflux of SP cells actively involves ABC transporter proteins. Therefore, the cells were treated with the APC transporter inhibitor, verapamil. Following treatment with verapamil, the SP cells constituted 0.7% of the overall HNSCC cell population (Fig. 1A). This indicates that the expression of ABC transporter proteins has an important role in Hoechst 3342 dye and drug exclusion. Subsequent to careful sorting of SP and non-SP cells, an in vitro cell proliferation assay was performed in order to determine the growth rate. Starting from day 3, the FACS-sorted SP cells underwent rapid cell proliferation and became more confluent on day 8 (data not shown). However, the growth rate of non-SP cells was significantly lower compared with the SP cells (Fig. 1B). The growth rate of the SP cells at 450 nm was significantly higher.
Following the in vitro proliferation assay, the SP cells were further subjected to immunocytochemistry to assess the expression of the ABC transporter protein ABCG2, which has been established to be involved in multi-drug resistance. Almost all FACS-sorted SP cells were positive for ABCG2 expression, which indicated an enhanced expression of ABCG2 compared with the non-SP cells (Fig. 2A). The fact that the sorted SP cells were highly resistant to drug uptake may result from the overexpression of ABC transporters. Therefore, these cells were further analyzed for the expression of the ABC transporter gene and stem cell surface markers.
Figure 2.
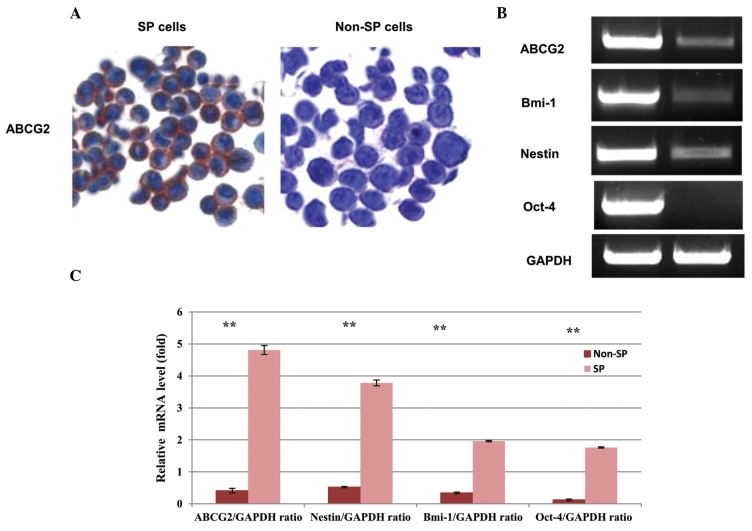
(A) Immunocytochemistry analysis of the sorted HNSCC SP and non-SP cells. SP cells exhibiting an enhanced expression of ABCG2 (red color) compared with the non-SP cells. The cell nuclei were counterstained with Hoechst 33342 (blue color). (B) Expression of stem cell markers in SP and non-SP cells. The elevated expression of ABCG2, Oct-4, nestin and Bmi-1 in SP cells was detected by reverse transcription quantitative polymerase chain reaction. GAPDH was used as a house keeping gene. (C) Quantification graph presenting the data of three separate independent experiments. Bar represents the standard deviation. **P<0.01 compared with the SP and non-SP cells, respectively. HNSCC, head and neck squamous cell carcinoma; SP, side population; ABC, ATP-binding cassette; Oct-4, octamer-binding transcription factor-4; Bmi-1, B lymphoma Mo-MLV insertion region-1; ABCG2, adenosine triphosphate-binding cassette subfamily G, member 2.
RT-PCR for the expression of stem cell surface markers and ABC transporter genes
In order to further investigate the stem cell phenotype of isolated HNSCC SP cells, the present study used RT-PCR analysis to examine the expression of the stem cell surface genes, Bmi-1, Oct-4, Nestin, and the ABC transporter gene, ABCG2. It has been reported that ABCG2 is overexpressed in breast cancer stem cells, and also that ABCG2 expression increases with higher tumor grade (18,19). The data from the present study revealed that the expression of ABCB2 mRNA was significantly higher in SP cells. Similarly, the expression of the stem cell surface genes, Bmi-1, Nestin and Oct-4, was higher in SP than non-SP cells (Fig. 2B). The quantification graph illustrates that elevated levels of Bmi-1, Nestin and Oct-4 were present in SP cells (Fig. 2C). GAPDH was used as a housekeeping gene. The data clearly indicates that a higher expression of ABCG2 and stem cell surface marker genes in SP cells may contribute to drug resistance and the rapid malignancy of HNSCCs.
Self-renewal and chemoresistance in HNSCC SP cells
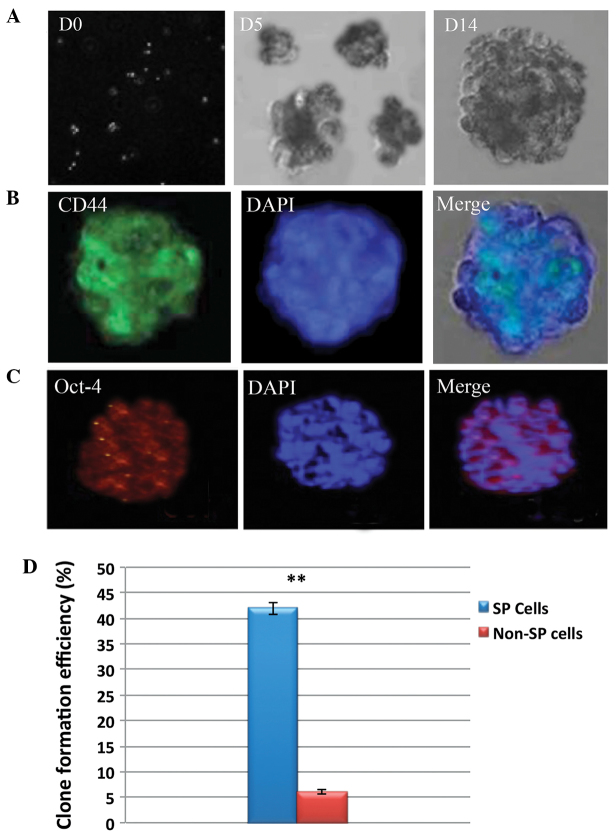
In order to determine the self-renewal potential and drug resistance abilities of SP cells, the present study performed a sphere formation and drug resistance assay. The sphere formation assay revealed that SP cells were able to rapidly generate tumor spheres. In addition, the size of the spheres increased over time (Fig. 3A). The total number of squamospheres generated by HNSCC SP cells was significantly higher than that of non-SP cells (Fig. 3D). Interestingly, the fluorescence microscopic analysis revealed that the squamospheres generated by SP cells were positive for CD44 and Oct-4 (Fig. 3B and C). Furthermore, the SP cells exhibited high resistance to docetaxel, 5-FU, cisplatin and paclitaxel. Upon treatment with these drugs, the SP cells demonstrated increased resistance and had a significantly higher survival rate than non-SP cells (Fig. 4). Taken together, these data clearly indicate that SP cells have a higher tumor initiation ability, which may contribute to rapid tumor invasion. In addition, it can be hypothesized that the drug resistance and increased survival rate of SP cells results from the overexpression of ABCG2, and possibly the overexpression of anti-apoptotic factors.
Figure 3.
(A) Bright field micrographs of the tumor spheres generated by SP cells on days 0, 5 and 14. Representative immunostaining images of (B) CD44 and (C) Oct-4 in the tumor spheres generated by HNSCC SP cells. The nuclei were stained with DAPI. (D) Graph revealing the clone formation efficiency of the SP cells. The total number of tumor spheres generated by the HNSCC SP cells was significantly higher than that of the non-SP cells. The bar represents the standard deviation. **P<0.01. SP, side population; CD44, cluster of differentiation 44; HNSCC, head and neck squamous cell carcinoma; octamer-binding transcription factor-4, Oct-4; D, day.
Figure 4.
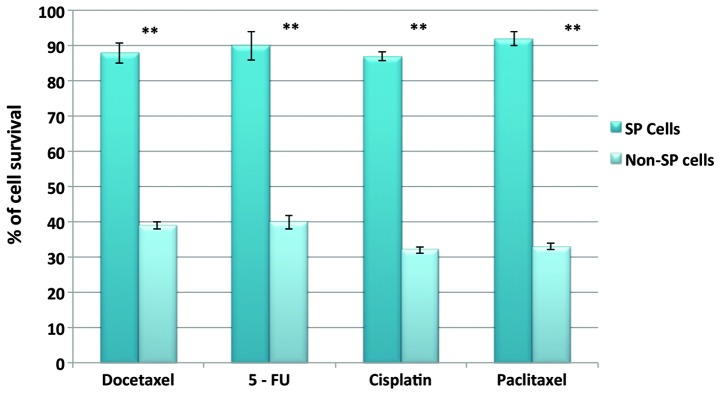
Comparison of the cell survival rates of SP and non-SP cells following treatment with docetaxel, 5-FU, cisplatin and paclitaxel. The SP cells were highly resistant to drug treatment and exhibited increased survival rates compared with the non-SP cells. SP, side population; 5-FU, 5-fluorouracil.
Discussion
A subset of cells within tumors, known as CSCs, have the ability to undergo self-renewal, exhibit high differentiation potentials, and demonstrate resistance to anticancer drugs. These properties of CSCs are a major limitation in the treatment of cancer, and ultimately result in treatment failure and tumor relapse (20). Studies concerning several solid tumors identified the presence of CSCs, whose functions were heterogeneous within the tumor population (21). The two primary methods for isolating CSCs are based on the expression of the stem cell surface markers CD133 and CD44, and the Hoechst 33342 dye exclusion method. In the dye exclusion method, the small subset of cancer cells that exhibit a low staining pattern or Hoechst 33342 dye efflux are termed SP cells. These cells possess the characteristic features of CSCs (22). Therefore, the isolation and characterization of SP cells can aid in designing novel anticancer drugs that could target and kill CSCs, and therefore achieve complete eradication of tumors.
SP cells have been identified in several solid tumors (23). Previous studies have revealed that the HNSCC cell lines, M3a2 and M4e, contain SP cells that are highly tumorigenic and are involved in chemoresistance and tumor invasion (24). In accordance with these findings, the present study identified that 2.7% of the metastatic HNSCC cells were SP cells. Furthermore, these isolated SP cells exhibited an increased cell proliferation rate and demonstrated chemoresistance, which suggests that SP cells have a major role in tumor progression and metastasis. This may be due to the overexpression of stem cell genes, ABC transporters and a reduction in apoptosis. Studies have established that the ABCG2 transporter protein expressed by SP cells is involved in chemotherapy resistance (25,26). In the present study, the percentage of SP cells was reduced to 0.7% following treatment with verapamil. The presence and overexpression of ABCG2 in HNSCC SP cells was further confirmed by RT-PCR and immunofluorescence analysis, whereby the SP cells demonstrated elevated ABCG2 mRNA expression and increased ABCG2 immunoreactivity, respectively. Similarly, the expression of stem cell genes was significantly higher in SP cells, which indicates that SP cells share characteristic features of CSCs. However, there may be additional factors involved in the chemoresistance and tumorigenesis of SP cells that are yet to be elucidated.
In vitro and in vivo studies using the HNSCC M3a2 and M4e cell lines have demonstrated that a significantly small number of SP cells are able to initiate large tumor growth at high frequencies; SP cells from the HNSCC M3a2 and M4e cell lines were found to be highly invasive in vitro and tumorigenic in vivo, when compared with non-SP cells (24). This suggests that SP cells are highly self-renewing and tumorigenic. Similarly, the present study revealed that isolated SP cells are able to rapidly generate a larger number of tumor spheres that are positive for the stem cell surface proteins CD44 and Oct-4. Therefore, it can be hypothesized that the presence of cancer stem-like SP cells within a tumor is the primary factor underlying the initiation of tumor growth and metastasis (9,27,28). From these findings, it is clear that SP cells are enriched CSCs that lead to treatment failure and tumor recurrence. Therefore, understanding the molecular mechanisms and signaling pathways involved in CSC-induced tumor recurrence and invasion is important in order to design novel treatment strategies.
References
- 1.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2015;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 2.Rehman AO, Wang CY. CXC12/SDF-1 alpha activates NF-kappaB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 2009;1:105–118. doi: 10.4248/IJOS.09059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkert J, Wright NA, Alison MR. Stem cells and cancer: an intimate relationship. J Pathol. 2006;209:287–297. doi: 10.1002/path.2016. [DOI] [PubMed] [Google Scholar]
- 4.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Goodell MA. Current Protocols in Cytometry. Wiley; New York, NY, USA: 2002. Stem cell identification and sorting using the Hoechst 33342 side population (SP) pp. 9.18.1–9.18.11. [DOI] [PubMed] [Google Scholar]
- 9.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct ‘side population’ of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 12.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Jackson JD, Das AV, et al. Direct identification and enrichment of retinal stem cells/progenitors by Hoechst dye efflux assay. Invest Ophthalmol Vis Sci. 2003;44:2764–2773. doi: 10.1167/iovs.02-0899. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Lai D, Liu T, et al. Cancer stem-like cells can be isolated with drug selection in human ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin (Shanghai) 2010;42:593–602. doi: 10.1093/abbs/gmq067. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Liu W, Feng X, Wang L, et al. Identification of ABCG2+ cells in nasopharyngeal carcinoma cells. Oncol Rep. 2012;27:1177–1187. doi: 10.3892/or.2011.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanamoto S, Kawasaki G, Yamada S, et al. Isolation and characterization of cancer stem-like side population cells in human oral cancer cells. Oral Oncol. 2011;47:855–860. doi: 10.1016/j.oraloncology.2011.06.501. [DOI] [PubMed] [Google Scholar]
- 17.He QZ, Luo XZ, Wang K, et al. Isolation and characterization of cancer stem cells from high-grade serous ovarian carcinomas. Cell Physiol Biochem. 2014;33:173–184. doi: 10.1159/000356660. [DOI] [PubMed] [Google Scholar]
- 18.Britton KM, Eyre R, Harvey IJ, et al. Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012;323:97–105. doi: 10.1016/j.canlet.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faneyte IF, Kristel PM, Maliepaard M, et al. Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res. 2002;8:1068–1074. [PubMed] [Google Scholar]
- 20.Dean M, Fojo T, Bates S. Tumor stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 21.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 23.Hill RP. Identifying cancer stem cells in solid tumors: Case not proven. Cancer Res. 2006;66:1890–1895. doi: 10.1158/0008-5472.CAN-05-3450. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5:e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 26.Kopper L, Hajdú M. Tumor stem cells. Pathol Oncol Res. 2004;10:69–73. doi: 10.1007/BF02893458. [DOI] [PubMed] [Google Scholar]
- 27.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 28.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]