Abstract
The aim of the present study was to investigate the effect of lymphokine-activated killer (LAK) cells, which received low-dose ionizing radiation, on the treatment of osteosarcoma in rats. The cultured UMR-106 cells were inoculated under the anterior chest skin of 24 rats to establish an osteosarcoma model. In addition, the LAK cells from 24 mice were exposed to doses of 0 (control group), 0.65 or 3.25 mGy X-ray radiation. The tritiated thymidine (3H-TdR) release method and Winn assay were performed to determine the antitumor effects of the LAK cells. The proliferation of the mouse LAK cells treated with 3.25 mGy radiation was significantly higher than that for those treated with 0 or 0.65 mGy radiation, which suggested that low-dose ionizing radiation stimulates the proliferation of LAK cells. The tumor-bearing rats were divided into three groups and injected with LAK cells that had already received 0, 0.65 or 3.25 mGy radiation. The mean survival time of the 3.25-mGy group was longer than that of the 0- and 0.65-mGy groups. After 30 days, tumors with weights of ~6.25 and 2.0 g were identified in the rats of the 0- and 0.65-mGy groups, respectively. However, tumor proliferation was not detectable in the rats of the 3.25-mGy radiation group. Therefore, low-dose ionizing radiation effectively kills osteosarcoma cells in rats by stimulating the proliferation and enhancing the cytotoxicity of LAK cells.
Keywords: low-dose ionizing, lymphokine-activated killer cells, osteosarcoma, proliferation, antitumor
Introduction
Osteosarcoma is one of the most common highly malignant forms of bone tumors, occurring most frequently in children and adolescents, with a poor clinical prognosis (1–3). Amputation surgery is the primary treatment for patients with osteosarcoma, but confers a five-year survival rate of just 10–20% (4). However, with the application of neoadjuvant chemotherapy, the survival rate of patients with osteosarcoma has increased to 60–70% in the last 20 years (5,6). Despite this, resistance to chemotherapy remains a major challenge for patients, particularly in cases of recurrence or distant metastasis (5). At present, immunotherapy, gene therapy and cell therapy are implemented for the treatment of osteosarcoma and osteosarcoma-induced lung metastases. The application of lymphokine-activated killer (LAK) cells is a recent advance in cell therapy for the treatment of osteosarcoma (7–11). The purpose of the present study was to investigate the effects of low doses of ionizing radiation on LAK cell therapy for animal osteosarcoma.
Materials and methods
Reagents and equipment
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA, USA). An industrial portable X'Pert-Pro MPD X-ray unit was purchased from Philips Medical Systems, Inc., (Bothell, WA, USA).
Animals
For the present study, 24 Sprague-Dawley (SD) rats were provided by the Experimental Animal Center of the Fourth Military Medical University (Xi'an, China; license number, SCXK; Army 2002–005). A total of 24, 6–8-week-old, male C57BL/6 mice, weighing between 18 and 22 g, were provided by the Chinese Academy of Medical Sciences Animal Breeding Company (Xi'an, China; animal license number, SCXK; Beijing 2004-0001). The osteosarcoma UMR-106 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were cultured in DMEM containing 10% FBS. The LAK cells were derived from the spleens of the tumor-bearing mice. All animal experiments were conducted in accordance with the ethical guidelines of the Provincial Hospital Affiliated to Shandong University (Jinan, China) and the study was approved by the ethics committee of the Provincial Hospital Affiliated to Shandong University.
Cell inoculation
The cultured UMR-106 cells were counted, and 1×107 cells were seeded subcutaneously into the anterior chest wall of the SD rats. Rats were randomly divided into the following groups: The control group (0 mGy radiation); the 0.65-mGy group; and the 3.25-mGy group, with 8 SD rats in each group. In each group, the rats were examined for tumor growth following inoculation with the cells. The tumor size of each rat was measured weekly with a caliper, and the average values of the tumor diameters were calculated.
Pathological staining and determination of alkaline phosphatase activity
The rats were euthanized by cervical dislocation after anesthesia with 1% pentobarbital (Beyotime Institute of Biotechnology, Haimen, China), and the tumor tissues were collected. Hematoxylin and eosin (HE) staining was performed to examine the extent of tumor metastasis. Serum from the tail vein of eight rats in each group was collected each week to determine the alkaline phosphatase activity using an Alkaline Phosphatase Assay kit (ab83369) purchased from Abcam (Burlingame, CA, USA) according to the manufacturer's instructions.
LAK cells
The LAK cells were derived from the spleens of the tumor-bearing mice. The isolated bioactive cells were diluted to 2×105/ml. In total, 1,000 units interleukin 2 (IL-2) were added per ml of the culture system. The separated LAK cells were divided into three groups and treated with 0 (control), 0.65 or 3.25 mGy ionizing radiation. Subsequent to treatment with the different doses of radiation, the LAK cells were cultured at 37°C in 5% CO2.
Winn assay
In the present study, 24 healthy C57BL/6 mice were divided into three groups. In total, 2×106 LAK cells exposed to different radiation doses, in combination with 2×105 osteosarcoma cells, were subcutaneously injected into the hind limbs of the rats. The rats were then raised under the same conditions for 30 days to observe tumor growth. The Winn assay was performed as described by Tjota et al (12).
Tritiated thymidine (3H-TdR) release assay
The 3H-TdR release assay was performed to determine LAK cell killing activity as in a previous study (13). In total, 1×106 osteosarcoma cells/ml were treated with 3.7×105 Bq/ml 3H-TdR. The solution was incubated at 37°C for 2h, with agitation every 30 min. The cells were then washed with phosphate-buffered saline (PBS), and the density of the cells was diluted to 2×105/ml. Next, 50 µl cells/well were added to a 96-well plate. Subsequent to washing with PBS, the obtained LAK and tumor cells were mixed at a ratio of 40:1. Each experimental sample had three replicate wells. The radioactivity of each sample was measured using a Tri-Carb 2810TR Low Activity Liquid Scintillation Analyzer (PerkinElmer, Inc., Boston, MA, USA). The LAK cell killing activity was calculated with the following formula: LAK cell killing activity (%) = [spontaneous release group (min-1) - experimental group (min-1)]/[spontaneous release group (min-1) - maximum release group (min-1)] × 100.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for analysis of the data. The experimental data are presented as the mean ± standard error. An analysis of variance was applied for the comparison of mean values of the samples. P<0.05 was considered to indicate a statistically significant difference.
Results
Establishment of an osteosarcoma model
The cultured UMR-106 cells were inoculated under the anterior chest skin of 18 of the 24 rats (the experimental groups) to establish an osteosarcoma model. In total, six of the 24 rats were inoculated with phosphate-buffered saline to serve as the control group. The tumor growth curves were plotted from the average weekly tumor diameter of the rats in the experimental groups. There were no tumors detected in the rats of the control group, which suggested that the tumor model was successfully established. HE staining was used to determine the extent of tumor metastasis. As revealed in Fig. 1, pale-blue Mallory's trichrome staining of the tumor tissues indicated evident osteoid growth. These results suggested that the tumor tissues exhibited distinctive osteogenesis characteristics.
Figure 1.
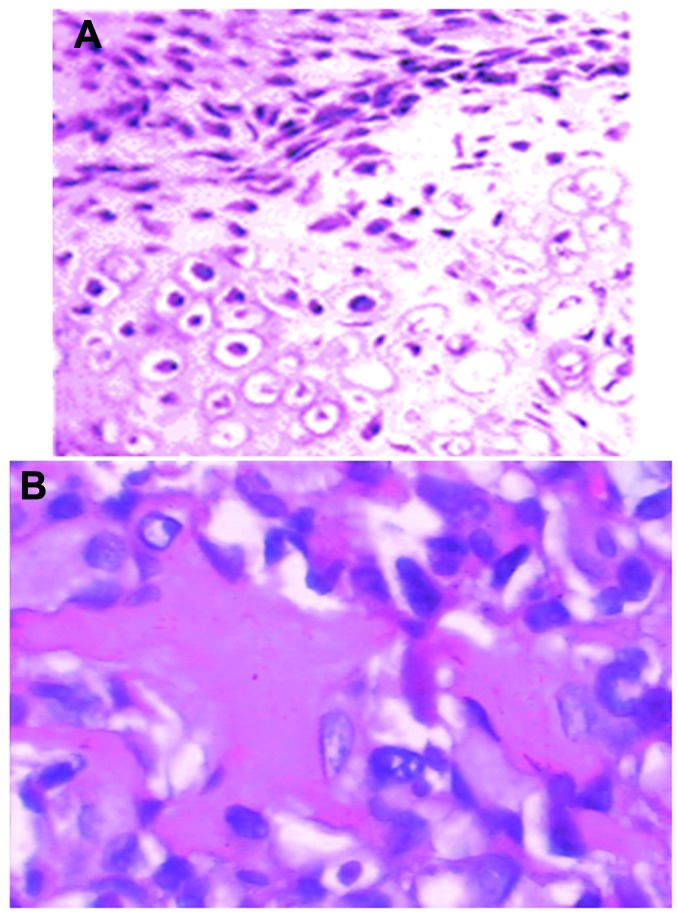
Immunohistochemical staining of tissue from rats inoculated with either phosphate-buffered saline or cultured UMR-106 cells (magnification, x400). (A) Normal tissues without tumors. (B) Tumor tissues.
Effects of different doses of X-ray radiation on the proliferation of cells
The LAK cells from the 24 mice were exposed to doses of 0 (control), 0.65 or 3.25 mGy X-ray radiation. The number and survival rates of the LAK cells cultured in a CO2 incubator were determined every three days. The results demonstrated that the LAK cells began to grow from day three post-radiation, and subsequent to attaining a higher level on day six, the cell proliferation rate was maintained at a stable level. As revealed in Fig. 2, the cells that received 3.25 mGy X-ray radiation demonstrated a higher proliferation rate than those receiving 0.65 mGy. Compared with the control group that received 0 mGy X-ray radiation, the proliferation rates in the 0.65- and 3.25-mGy groups were significantly higher (P<0.01).
Figure 2.
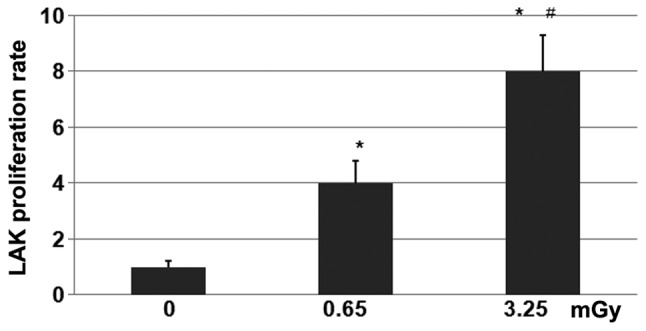
Proliferation rates of lymphokine-activated killer (LAK) cells treated with 0, 0.65 or 3.25 mGy X-ray radiation. LAK cells were derived from the spleens of tumor-bearing mice. The isolated bioactive cells were diluted to 2×105/ml. In total, 1,000 units interleukin-2 was added per ml of the culture system. The separated LAK cells were divided into three groups, and treated with 0, 0.65 or 3.25 mGy ionizing radiation. Subsequent to treatment with the different doses of radiation, the LAK cells were cultured at 37°C with 5% CO2 for future use. * P<0.01 vs. control; #P<0.01 vs. 0.65-mGy group.
Therapeutic effect of LAK cells treated with low-dose ionizing radiation on osteosarcoma
Subsequent to exposure to 0, 0.65 or 3.25 mGy X-ray radiation, the LAK cells were inoculated into the tumor-bearing rats. Following tumor cell inoculation, alkaline phosphatase levels in the blood serum of the tumor-bearing rats were elevated compared with those of rats without tumors. After 30 days, the rats were euthanized and the tumors were collected and weighed (Fig. 3A). The mean weight of the tumors in the control and 0.65-mGy groups was 6.25 and 2.0 g, respectively. However, the weights of the tumors in the rats inoculated with 3.25 mGy radiation-treated LAK cells were undetectable after 30 days. Furthermore, the rats in the group treated with 3.25 mGy radiation demonstrated the longest survival rate (Fig. 3B), followed by those in the 0.65-mGy group. The differences between these two groups were revealed to be statistically significant (P<0.01). In addition, the differences between the 0.65- and 3.25-mGy groups and the control 0-mGy group were statistically significant (P<0.01).
Figure 3.
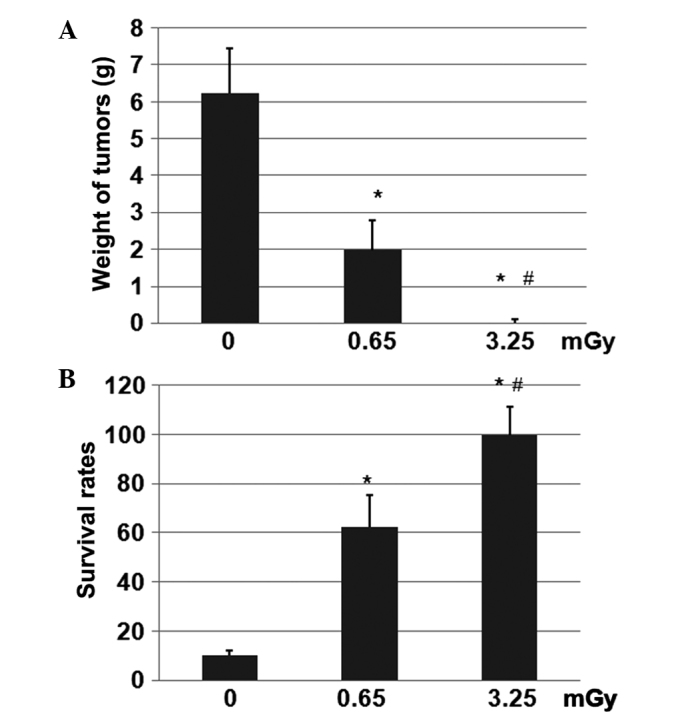
Weight of tumors and survival rates of the rats. The 24 healthy C57BL/6 mice were divided into three groups. In total, 2×106 LAK cells exposed to different radiation doses, in combination with 2×105 osteosarcoma cells, were subcutaneously injected into the hind limbs of the rats. The rats were then raised under the same conditions for 30 days to observe tumor growth and survival rates. (A) Weight of tumors. (B) Survival rates. *P<0.01 vs. control; #P<0.01 vs. 0.65-mGy group.
Discussion
The results of the present study demonstrated that the optimal dose of X-ray radiation for the stimulation of LAK cell activity was 3.25 mGy. Tomuleasa et al (14) reported that low doses of ionizing radiation were able to enhance the biological activity of stem and bone cells. The results of the present study demonstrated that the viability and proliferation of the LAK cells that received low doses of X-ray radiation were higher than those of the cells that received no radiation (P<0.01). This indicates that low doses of ionizing radiation may promote the proliferation of certain cells. This may be due to low doses of ionizing radiation simultaneously accelerating cellular mitosis and increasing IL-2-mediated proliferation. The LAK cell proliferation rate reached a peak on days one and two, prior to gradually decreasing until day 21, where the rate returned to the initial level. This indicated that the proliferative ability of the cells was progressively weakened over time.
In the present study, the viability of the freshly-isolated cells was 98%, which gradually decreased over the incubation period. However, the viability of the cells treated with 0.65 and 3.25 mGy X-ray radiation was 20% higher than that of the cells in the control group. The differences were statistically significant (P<0.01). The results of the present study indicated that low doses of radiation may not only increase the proliferation rate, but also decrease the mortality of LAK cells. Therefore, radiation provides a method to shorten the culture time and increase the proliferation of cells. The reduced mortality may be due to the ability of low doses of radiation to specifically stimulate certain dormant cells, but not affect the internal or external environment of the cells.
The effect of low doses of radiation may provide a novel approach in increasing the proliferation of cells for clinical applications. A previous study (15) revealed that osteosarcoma cells had the ability to produce cancer stem cells (CSCs), which are recognized to lead to tumor recurrence. Therefore, osteosarcoma CSCs were considered to be a major target for cancer therapy, as they were believed to contribute to chemotherapy resistance (13,16–18). Another previous study identified that low doses of radiation were able to enhance immunity, stimulate the differentiation and proliferation of T lymphocytes and natural killer (NK) cells, and promote IL-2 secretion of spleen cells and peripheral lymphocytes (19). In addition, previous studies have demonstrated that low doses of ionizing radiation can stimulate LAK cell killing activity (20). This may be due to the ability of radiation to promote the secretion of IL-2 and stimulate NK cell activity.
LAK cells are derived from a group of heterogeneous large particles of lymphocytes that are cytotoxic to either autologous or allogeneic tumor cells. IL-2 is necessary for LAK cell activation, and activated LAK cells can directly or indirectly kill tumor cells by the release of cytotoxic granules and the secretion of cytokines (21). As a biological form of tumor treatment, the clinical application of LAK cells attracts a large amount of attention.
LAK cell therapy is an adoptive immunotherapy (22), and is administered to patients by the injection of IL-2-activated LAK cells. This leads to immunity and an improvement in the body's anti-tumor ability.
Subsequent to a certain amount of IL-2-mediated proliferation, LAK cells demonstrate antitumor activity (23). However, the clinical culture and proliferation of LAK cells is difficult to achieve. Therefore, to enhance the clinical antitumor effects of LAK cells, a dose of IL-2 is usually co-administered (24). However, high doses of IL-2 have certain side-effects that may affect treatment. Tumor necrosis factor (TNF) can significantly enhance LAK cell activity in vitro and reduce the concentration of IL-2, which is necessary for the induction of LAK activity (25). A prior study revealed that IL-2 could induce peripheral blood mononuclear cells to produce TNF, which improves resultant antitumor effects (26). The injection of solasodine hydrochloride to tumor-bearing mice may increase the antitumor activity of LAK cells (27). The treatment of malignant tumors by the in vivo activation of LAK cells from lymphocytes, the expansion of a LAK cell population and the activation of macrophages to produce large amounts of TNF, can inhibit the growth of tumor cells and prevent tumor invasion and metastasis (28). Consequently, this approach can significantly improve the quality of life and prolong the survival of cancer patients.
In order to investigate the impact of low doses of radiation on LAK cells, the effects of different doses of radiation on the culture and anti-tumor activity of the cells were studied in the present study. This may provide basic information for the further study of LAK cell therapy.
References
- 1.Heymann D, Rédini F. Targeted therapies for bone sarcomas. Bonekey Rep. 2013;2:378. doi: 10.1038/bonekey.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoch M, Ali S, Agrawal S, Wang C, Khurana JS. Extraskeletal osteosarcoma: a case report and review of the literature. J Radiol Case Rep. 2013;7:15–23. doi: 10.3941/jrcr.v7i7.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma - A case report and review of literature. Indian J Surg Oncol. 2013;4:374–377. doi: 10.1007/s13193-013-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puri A, Pruthi M, Gulia A. Outcomes after limb sparing resection in primary malignant pelvic tumors. Eur J Surg Oncol. 2014;40:27–33. doi: 10.1016/j.ejso.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Tao LJ, Zhou XD, Shen CC, et al. Tetrandrine induces apoptosis and triggers a caspase cascade in U2-OS and MG-63 cells through the intrinsic and extrinsic pathways. Mol Med Rep. 2014;9:345–349. doi: 10.3892/mmr.2013.1761. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z, Tao L, Shen C, Liu B, Yang Z, Tao H. Silencing of Barkor/ATG14 sensitizes osteosarcoma cells to cisplatin-induced apoptosis. Int J Mol Med. 2014;33:271–276. doi: 10.3892/ijmm.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uno K, Tsukuda M, Kushida K, et al. Clinical experience with In-111 labeled LAK cells and TILS for tumor localization. Prog Clin Biol Res. 1990;355:239–245. [PubMed] [Google Scholar]
- 8.Luksch R, Perotti D, Cefalo G, et al. Immunomodulation in a treatment program including pre- and post-operative interleukin-2 and chemotherapy for childhood osteosarcoma. Tumori. 2003;89:263–268. doi: 10.1177/030089160308900306. [DOI] [PubMed] [Google Scholar]
- 9.Herrero-Martín D, Osuna D, Ordóñez JL, et al. Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. Br J Cancer. 2009;101:80–90. doi: 10.1038/sj.bjc.6605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Yoon DS, Choi HJ, Hahm DH, Oh SM. Phosphorylation of IκBα at serine 32 by T-lymphokine-activated killer cell-originated protein kinase is essential for chemoresistance against doxorubicin in cervical cancer cells. J Biol Chem. 2013;288:3585–3593. doi: 10.1074/jbc.M112.422170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennings VA, Ilett EJ, Scott KJ, et al. Lymphokine-activated killer and dendritic cell carriage enhances oncolytic reovirus therapy for ovarian cancer by overcoming antibody neutralization in ascites. Int J Cancer. 2014;134:1091–1101. doi: 10.1002/ijc.28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjota A, Zhang YQ, Piedmonte MR, Lee CL. Adoptive immunotherapy using lymphokine-activated killer cells and recombinant interleukin-2 in preventing and treating spontaneous pulmonary metastases of syngeneic Dunning rat prostate tumor. J Urol. 1991;146:177–183. doi: 10.1016/s0022-5347(17)37748-0. [DOI] [PubMed] [Google Scholar]
- 13.Gillette J, Nielsen-Preiss S. Cancer stem cells: seeds of growth in osteosarcoma. Cancer Biol Ther. 2009;8:553–554. doi: 10.4161/cbt.8.6.8142. [DOI] [PubMed] [Google Scholar]
- 14.Tomuleasa C, Soritau O, Brie I, et al. Mesenchymal stem cell irradiation in culture engages differential effect of hyper-fractionated radiotherapy for head and neck cancers. J BUON. 2010;15:348–356. [PubMed] [Google Scholar]
- 15.Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res. 2010;5:78. doi: 10.1186/1749-799X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34:1381–1386. [PubMed] [Google Scholar]
- 18.Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247–263. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang SY, Shi Y. Reaction of low dose ionizing radiation on amplification in vitro and antitumor effect of TIL cells. Chinese Journal of Cancer Prevention and Treatment. 2009;16:1552–1553. [Google Scholar]
- 20.Fu Q, Zhang LS, Zhu SP, Liu XH. Effects of low-dose radiation on LAK cells to kill tumor target cells. Chinese Journal of Radiological Medicine and Protection. 1995;15:321. [Google Scholar]
- 21.Wang YC, Shi EX, Ding XL. Killing effect on lung cancer cell line A549 by lymphokine-activated killer cells activated by dendritic cells. Northern China National Defense Medicine. 2009;21:11–12. [Google Scholar]
- 22.Yanagawa H, Sone S, Fukuta K, Nishioka Y, Ogura T. Local adoptive immunotherapy using lymphokine-activated killer cells and interleukin-2 against malignant pleural mesothelioma: Report of two cases. Jpn J Clin Oncol. 1991;21:377–383. [PubMed] [Google Scholar]
- 23.Mou QJ, Wang J, Cui WF, Wang ZJ. Proliferation and cytotoxic activity comparison of three sources of CIK cells. Shandong Medicine. 2010;50:7–9. [Google Scholar]
- 24.Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989;70:175–182. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- 25.Balasa B, Yun R, Belmar NA, et al. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol Immunother. 2015;64:61–73. doi: 10.1007/s00262-014-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin P, Wang E, Provenzano M, et al. Molecular signatures induced by interleukin-2 on peripheral blood mononuclear cells and T cell subsets. J Transl Med. 2006;4:26. doi: 10.1186/1479-5876-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinxia Ai, Huanbo Cui. The effect of SBHL on the tumor growth and immune functions in S180-bearing mice. Zhong Guo Mian Yi Xue Za Zhi. 2010;26:26–29. (In Chinese) [Google Scholar]
- 28.Parhar RS, Ernst P, Sheth KV, al-Sedairy ST. Anti-tumor cytotoxic potential and effect on human bone marrow GM-CFU of human LAK cells generated in response to various cytokines. Eur Cytokine Netw. 1992;3:299–306. [PubMed] [Google Scholar]


