Abstract
The aim of the present study was to investigate the long-term survival of patients with refractory acute promyelocytic leukemia (APL) that were administered alternately with compound realgar natural indigo tablet (CRNIT) treatment and chemotherapy. In total, 31 patients with refractory APL were administered with CRNIT treatment alternately with chemotherapy. The complete remission (CR) and relapse rates were estimated by bone marrow (BM) examination. The expression of the promyelocytic leukemia-retinoic acid receptor α (PML-RARα) fusion protein and the apoptosis rate in the retinoic acid (RA)-resistant NB4-R1 cell line administered with CRNIT treatment in vitro were measured by western blot analysis and flow cytometry, respectively. The patients were followed up for 12–60 months, with a median follow-up time of 43 months. The total continuous CR rate was 90.32% (28/31), and the duration of response was between 10.3 and 60 months (median, 42.4 months). The total relapse rate was 9.68% (3/31), and the median time of relapse was 13 months (range, 8–27 months). During the treatment with CRNITs, there was no evident BM depression and only limited side-effects were experienced. Additionally, in vitro cell molecular biology results revealed that CRNIT treatment resulted in a marked induction of apoptosis and degradation of the PML-RARα fusion protein. The present results revealed that CRNIT treatment in combination with chemotherapy is an effective and feasible therapy for the treatment of patients with refractory APL.
Keywords: compound realgar natural indigo tablets, chemotherapy, acute promyelocytic leukemia, PML-RARα, apoptosis
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myelogenous leukemia (AML). Despite being a relatively rare condition, APL is extremely malignant due to the rapid spontaneous evolution of the disease and the occurrence of sudden hemorrhages (1,2). At the genetic level, APL is characterized by a specific chromosomal rearrangement between the retinoic acid receptor α (RARα) on chromosome 17, and a number of partners, including the promyelocytic leukemia (PML), PLZF, NPM, STAT5b and NuMA genes. The majority of patients (98%) present with the 15;17 translocation, which results in a fusion between the RARα gene and the PML gene on chromosome 15 (3). Retinoic acid (RA)-based chemotherapy is the standard treatment regimen administered for APL (4,5). However, this regimen only prolongs survival, and numerous patients develop resistance to the administered agents and eventually succumb to APL or RA-associated toxicity and side-effects, particularly the fatal retinoic acid syndrome (6). Although arsenic trioxide (ATO) was identified as an effective drug in the treatment of APL, this agent works only through a peripheral intravenous route.
Compound realgar natural indigo tablets (CRNITs) are another type of arsenic compound that are often used to treat dermatosis. CRNITs have also been used against certain other disease, including chronic bronchitis, bronchial asthma, herpes zoster and pterygium in Traditional Chinese Medicine, with the principal of using a toxic substance to inhibit a toxic disease (7). In the 1990s, CRNIT treatment was introduced to the therapeutic regimens for APL and demonstrated a marked effectiveness in the Northeastern region of China (8). The clinical complete remission (CR) rate achieved with orally administered CRNIT treatment (3.75–7.5 g/day) 30 days was reported as between 98.3 and 100% (9). In addition, 88.52% of the patients demonstrated a survival time of >3 years, and the majority of patients experienced neither bone marrow (BM) depression nor other severe clinical side-effects during the treatment (10).
The present study reports the clinical outcome of CRNIT treatment in 31 patients with refractory APL, and also reports the cell molecular biology results obtained from the administration of CRNIT treatment to the RA-resistant APL NB4-R1 cell line. The aim of the present study was to assess the clinical efficacy and safety of CRNIT treatment applied alternately with chemotherapy in patients with refractory APL, and indicated that CRNITs may be a promising agent for the treatment of APL.
Materials and methods
Patients
Patients were eligible for inclusion in the present study if they were: Admitted to The First Affiliated Hospital, Xi'an Jiaotong University (Xi'an, Shaanxi, China) between June 2008 and June 2013; diagnosed on the basis of clinical data, such as history, symptoms and physical findings, and peripheral blood and BM examinations, according to the French-American-British classification (11); and not in remission following two courses of standard treatment, or experienced recrudescence within 6 months subsequent to remission. A final number of 31 patients with refractory APL were included in the present study. The main clinical data of the patients at the time of diagnosis are summarized in Table I.
Table I.
Clinical data of the patients at the time of diagnosis.
| Patient | Gender | Age, years | Hb level, g/l | WBC count, n x109 | Platelet count, n x1012 | APL cells in BM, % | t (15;17) PML-RARα | Post-remission therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 33 | 68 | 0.7 | 8.3 | 40.5 | + | CRNIT+DA+HA |
| 2 | M | 40 | 112 | 1.1 | 7.9 | 52.7 | + | CRNIT+DA+HA+MA |
| 3 | F | 35 | 101 | 42.5 | 4.0 | 39.9 | + | CRNIT+DA |
| 4 | M | 42 | 83 | 51.9 | 56.4 | 60.1 | + | CRNIT+TA+MA+MEA |
| 5 | F | 26 | 119 | 4.5 | 79.1 | 70.5 | + | CRNIT+HA |
| 6 | F | 51 | 104 | 17.8 | 16.6 | 81.1 | + | CRNIT+DA+HA |
| 7 | F | 48 | 99 | 64.7 | 22.3 | 31.6 | ND | CRNIT+DA+HA |
| 8 | F | 39 | 126 | 2.7 | 76.3 | 39.8 | + | CRNIT+DA+HA+TEA |
| 9 | M | 39 | 142 | 0.9 | 24.0 | 64.2 | + | CRNIT+DA+TA |
| 10 | M | 40 | 125 | 3.9 | 188.0 | 40.7 | ND | CRNIT+DA+HA+TA+IDA |
| 11 | M | 35 | 133 | 7.7 | 101.0 | 33.5 | – | CRNIT+HA+IDA |
| 12 | M | 32 | 77 | 48.1 | 12.4 | 92.7 | + | CRNIT+MA+TA+MA+TA+MA+TEA |
| 13 | M | 46 | 98 | 3.3 | 49.5 | 51.2 | ND | CRNIT+MEA+IDA |
| 14 | F | 23 | 100 | 10.2 | 122.7 | 39.8 | + | CRNIT+HA+DA+MA+ MEA+TA |
| 15 | M | 24 | 122 | 6.2 | 80.5 | 65.2 | + | CRNIT+MEA+TA+TA+MA |
| 16 | M | 49 | 131 | 3.1 | 102.4 | 33.8 | ND | CRNIT+DA+DA+MA+ TA+IDA |
| 17 | F | 50 | 69 | 50.5 | 24.6 | 86.5 | + | CRNIT+DA+HA |
| 18 | F | 17 | 111 | 4.0 | 42.1 | 42.9 | – | CRNIT+MA+MA+TA |
| 19 | F | 31 | 110 | 4.6 | 89.5 | 49.7 | + | CRNIT+HA+TA+MEA |
| 20 | F | 29 | 65 | 0.6 | 13.5 | 54.4 | + | CRNIT+TA+IDA |
| 21 | M | 44 | 127 | 1.7 | 18.8 | 67.2 | + | CRNIT+HA+MA |
| 22 | M | 35 | 104 | 4.3 | 100.0 | 78.0 | + | CRNIT+IDA+HA |
| 23 | F | 55 | 65 | 32.4 | 37.8 | 95.5 | + | CRNIT+TA+MEA+DA |
| 24 | M | 31 | 106 | 2.9 | 76.0 | 83.8 | + | CRNIT+DA+HA+MA+TA |
| 25 | F | 20 | 115 | 3.4 | 46.4 | 43.6 | ND | CRNIT+TA+HA+DA |
| 26 | F | 33 | 114 | 10.5 | 57.9 | 58.8 | + | CRNIT+HA+HA+TA+IDA |
| 27 | M | 42 | 98 | 7.7 | 52.0 | 67.1 | + | CRNIT+DA+MA+HA |
| 28 | M | 36 | 102 | 15.2 | 74.0 | 70.6 | + | CRNIT+HA+TA+HA+DA |
| 29 | M | 52 | 90 | 20.0 | 8.8 | 35.0 | + | CRNIT+MEA+TA+MA |
| 30 | F | 40 | 76 | 17.6 | 15.4 | 32.8 | + | CRNIT+MEA+TEA+TA |
| 31 | M | 48 | 123 | 6.7 | 81.0 | 46.6 | + | CRNIT+MA+TA+HA |
M, male; F, female; Hb, hemaglobin level; WBC, white blood cell count; APL, acute promyelocytic leukemia; BM, bone marrow; PML-RARα, promyelocytic leukemia-retinoic acid receptor α; ND, not detected; CRNIT, compound realgar natural indigo tablet; DA, daunomycin and Ara-C; MA, mitoxantrone and Ara-C; HA, homoharringtonine and Ara-C; TA, pirarubicin and Ara-C; IDA, idarubicin and Ara-C; TEA, pirarubicin, etoposide and Ara-C; MEA, mitoxantrone, etoposide and Ara-C.
Written informed consent was obtained from the patients or their families upon admission hospital and prior to the initial treatment. This study was approved by the Medical Ethics Committee of Xi'an Jiaotong University (Xi'an, China).
Treatment schema
Compound realgar natural indigo tablets
CRNITs were composed of realgar, indigo naturalis, savia miltiorrhiza and radix pseudostellariae. The tablets were administered at a daily dose of 3.75–7.5 g, which was taken over three doses subsequent to meals.
Combination chemotherapy regimens
The combination chemotherapy regimens administered in the present study were as follows: DA, 40 mg/day daunomycin (days 1–3) and 150 mg/day Ara-C (days 1–7) administered by intravenous drip over the 7-day course of treatment; MA, 6 mg/day mitoxantrone (days 1–3) and 100 mg/day Ara-C (days 1–7) administered by intravenous drip over the 7-day course of treatment; HA, 4 mg/day homoharringtonine (days 1–7) and 200 mg/day Ara-C (days 1–7) administered by intravenous drip over the 7-day course of treatment; TA, 25 mg/day pirarubicin (days 1–3) and 100 mg/day Ara-C (days 1–7) administered by intravenous drip over the 7-day course of treatment; IDA, 10 mg/day idarubicin (days 1–3) and 100 mg/day Ara-C (days 1–7) administered by intravenous drip over the 7-day course of treatment; TEA, 20 mg/day pirarubicin (days 1–3), 100 mg/day etoposide (days 4–5) and 100 mg/day Ara-C (days 1–5) administered by intravenous drip over the 5-day course of treatment; and MEA, 4 mg/day mitoxantrone (days 1–3), 100 mg/day etoposide (days 1–3) and 100 mg/day Ara-C (days 1–7) administered by intravenous drip over the 5-day course of treatment.
Combination chemotherapy was applied alternately with CRNIT treatment. The initial interval between chemotherapy regimens was 30 days, but extended to 3–4 months following 10 chemotherapeutic cycles. The maintenance therapy lasted ≥2 years.
Complication assessment and management
Central nervous system leukemia
A routine cerebrospinal fluid (CSF) examination was performed subsequent to admission. The patients were also administered with an intrathecal injection of 10 mg methotrexate and 5 mg dexamethasone once a week, for a total of 4 doses.
Infection and bleeding
Anti-infective therapy was administered to patients with APL that experienced infection or fever. In addition, the isolation of these patients and disinfection of the hospital were considered important. Nursing was increased, particularly for the oral cavity and the perineum. Patients with an evident tendency for bleeding were administered with hemostatic therapy (tranexamic acid injection of 0.75 g/day, intravenous drip), and once the patients were diagnosed with disseminated intravascular coagulation (DIC), small doses of heparin (20,000 units/day, intravenous drip) were added to the therapy regimen. Platelets or fresh frozen plasma infusion were also administered when necessary.
Assessment of response
BM aspiration was performed prior to each course of treatment. Samples of BM fluid (0.2 ml) were taken and, subsequent to smear and Wright's stain, the cell morphology was observed under microscopy with oil immersion (cedar oil, C3277; Sigma-Aldrich, St. Louis, MO, USA). The identification of one of the following three criteria was considered to indicate recurrence: i) Combined proportion of myeloblasts and progranulocytes in the BM was >5 and ≤20%, and a CR myelogram was not achieved subsequent to one course of effective anti-leukemia treatment; ii) proportion of myeloblasts and progranulocytes in the BM was >20%; and iii) extramedullary leukemia cells.
Effect of CRNIT treatment on the APL NB4-R1 cell line
Apoptosis assay
The NB4-R1 cell line is a RA-resistant cell line that was previously isolated from the RA-sensitive human APL cell line NB4. The NB4-R1 cell line expresses wild-type RARα and PML/RARα at the mRNA and protein levels (12). The NB4-R1 cells were cultured in RPMI-1640 medium (Gibco Life Technologies, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a humidified incubator containing a 5% CO2 atmosphere. The cells were divided into two groups, the control group, which consisted of untreated NB4-R1 cells, and the experimental group, which was administered with 34 mg/l CRNIT treatment.
A double-labeling system was used to distinguish between apoptosis and necrosis. In total, 1×106 cells were harvested and washed twice with pre-chilled phosphate-buffered saline (PBS; 0.1 mol/l; pH 7.2). In addition, 100 µl incubation buffer, 2 µl Annexin V-5(6)-carboxyfluorescein-N-hydroxysuccinimide ester (FLUOS) and 2 µl propidium iodide (PI; Roche, Mannheim, Germany) were added directly to the culture medium or to the cell suspension. The mixture was incubated for 15 min at room temperature in the dark. Subsequently, 500 µl PBS was added to each sample tube and the samples were analyzed using fluorescence-activated cell sorting (Becton Dickinson, Franklin Lakes, NJ, USA) using Cell Quest Research Software (Becton Dickinson) equipped with a 427 nm argon laser light source, 489 nm band pass filter (FL1-H) and 582 nm band pass filter (FL2-H). Electronic compensation of the instrument was performed to exclude overlapping of the emission spectra. A total of 10,000 events were acquired. The cells were gated and a dual-parameter dot plot of FL1-H (x-axis; FLUOS fluorescence) vs. FL2-H (y-axis; PI fluorescence) revealed a logarithmic fluorescence intensity.
PML-RARα fusion protein assay
Western blot analysis was performed as previously described (13). Briefly, the cells were lysed in RIPA buffer in the presence of a proteinase inhibitor cocktail (Sigma-Aldrich), which included 10 µg/ml phenylmethyl sulfonyl fluoride, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 100 µg/ml Pefabloc SC and 100 µg/ml chymostatin. A total of 20 µg protein was separated by 12% SDS-PAGE and transferred to a nitrocellulose membrane. The rabbit polyclonal anti-RARα IgG antibody (dilution, 1:700; catalog no. sc-551; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse monoclonal anti-GAPDH IgG1 antibody (dilution, 1:1,000; catalog no. sc-365062; Santa Cruz Biotechnology, Inc.) were used to confirm the expression level of the PML-RARα fusion protein.
Statistical analysis
All cell molecular biology experiments were performed at least three times. Statistical analysis was performed using SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Treatment efficacy
The patients were followed up for 12–60 months, with the median follow-up time being 43 months. In total, 28 out of 31 patients achieved continuous CR (CCR) following CRNIT treatment in combination with other chemotherapy regimens (Table II), the CCR rate was 90.32%, and the duration of response was between 10.3 and 60 months (median, 42.4 months). Three patients, consisting of patients 12, 17 and 23, relapsed at months 8, 13 and 27, respectively, due to treatment being interrupted in months 6, 10 and 23 of the treatment course, respectively. Among these three patients, patient 12 succumbed to AML at month 10, and patients 17 and 23 were subsequently administered with CRNIT and combination chemotherapy, and achieved CR again. At the time of writing, patients 17 and 23 remain in clinical CR, and all remaining 30 patients survived. Immediately subsequent to CR, the presence of PML-RARα transcripts was examined using fluorescence in situ hybridization in 26 patients. All patients demonstrated expression of PML-RARα, with the exception of patient 29.
Table II.
Remission induction with CRNIT and combination chemotherapy.
| Patient | Treatment result | Total doses of CRNIT, g | Time to CR, days | Time of sustained remission, months |
|---|---|---|---|---|
| 1 | CR | 3.75 | 31 | 10.3 |
| 2 | CR | 6.00 | 31 | 28.0 |
| 3 | CR | 6.00 | 32 | 36.5 |
| 4 | CR | 7.50 | 37 | 37.0 |
| 5 | CR | 7.50 | 57 | 55.0 |
| 6 | CR | 7.50 | 59 | 41.0 |
| 7 | CR | 6.75 | 28 | 56.6 |
| 8 | CR | 6.75 | 30 | 34.0 |
| 9 | CR | 5.25 | 34 | 50.9 |
| 10 | CR | 5.25 | 38 | 38.7 |
| 11 | CR | 7.50 | 40 | 40.8 |
| 12 | NR | 7.50 | 37 | NA |
| 13 | CR | 6.00 | 42 | 60.0 |
| 14 | CR | 6.75 | 49 | 37.0 |
| 15 | CR | 6.75 | 50 | 42.4 |
| 16 | CR | 6.00 | 36 | 41.0 |
| 17 | NR | 6.00 | 30 | NA |
| 18 | CR | 6.00 | 27 | 49.7 |
| 19 | CR | 6.00 | 33 | 31.0 |
| 20 | CR | 7.50 | 52 | 57.1 |
| 21 | CR | 7.50 | 56 | 42.0 |
| 22 | CR | 7.50 | 54 | 47.8 |
| 23 | NR | 7.50 | 60 | NA |
| 24 | CR | 7.50 | 34 | 39.6 |
| 25 | CR | 6.75 | 37 | 53.0 |
| 26 | CR | 4.50 | 44 | 58.5 |
| 27 | CR | 4.50 | 28 | 47.2 |
| 28 | CR | 5.25 | 39 | 52.6 |
| 29 | CR | 5.25 | 53 | 54.2 |
| 30 | CR | 7.50 | 54 | 59.1 |
| 31 | CR | 6.00 | 30 | 44.4 |
NA, not assessed; CRNIT, compound realgar natural indigo tablet; CR, complete remission; NR, no remission.
Complications
In total, 4 out of 31 patients demonstrated significant CSF abnormalities, and one patient experienced cerebral hemorrhage. Subsequent to treatment with intrathecal injection, a routine CSF examination of this patient revealed a normal result. The remaining patients did not experience severe infection, bleeding or DIC.
Side-effects
Out of the 31 patients with APL, 6 demonstrated an increased peripheral WBC count, with peak values of 14.2–187.0×109 cells/l (normal range, 4–10×109 cells/l). The time to reach the peak WBC numbers subsequent to the initiation of CRNIT treatment was 10–42 days (median, 22 days; Fig. 1). This situation appears to be similar to the clinical picture observed following RA treatment in patients with APL. However, the proportion of patients demonstrating hyperleukocytosis is much higher during RA remission induction, with 80–90% of patients developing the condition (14). No significant changes in hemoglobin (Hb) and platelet levels were observed in 24 patients, but 7 patients, consisting of patients 1, 4, 12, 17, 20, 23 and 30, demonstrated a slightly decreased Hb and platelet level following low-dose chemotherapy. Therefore, the administration of CRNIT treatment was not associated with significant BM suppression.
Figure 1.
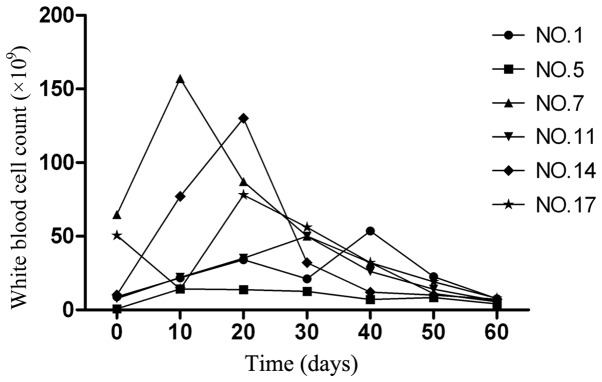
Dynamic changes of peripheral white blood cell counts during compound realgar natural indigo tablet treatment in six patients that demonstrated hyperleukocytosis. NO., patient number.
The principal side-effects of CRNIT treatment are summarized in Table III. The results revealed that the most common side-effects were gastrointestinal symptoms, including nausea, vomiting and loss of appetite, and dermatological symptoms, including itching or erythematous skin changes.
Table III.
Main side-effects due to the administration of rifampin and combination chemotherapy (n=31).
| Side-effects | Frequency, n (%) |
|---|---|
| Skin dryness, itching or erythematous changes | 7 (22.6) |
| Headache | 2 (6.5) |
| EKG change | 4 (12.9) |
| Nausea, vomiting or loss of appetite | 8 (25.8) |
| Liver function | |
| (1) GPT increased | 2 (6.5) |
| (2) GOT increased | 3 (9.7) |
| (3) AKP increased | 1 (3.2) |
| (4) Total bilirubin increased | 1 (3.2) |
| Toothache | 1 (3.2) |
| Oral ulcer | 1 (3.2) |
| Hemorrhage of teeth, nose or skin | 2 (6.5) |
EKG, electrocardiogram; GPT, glutamate pyruvate transaminase; GOT, glutamate oxaloacetate transaminase; AKP, alkaline phosphatase.
In addition, liver function tests revealed moderate alterations, such as increased serum hepatic enzyme levels in 7 patients, consisting of patients 4, 12, 16, 20, 23, 27 and 29. Electrocardiogram changes, including T-wave changes, were observed in 4 patients, consisting of patients 6, 16, 23 and 31. Other side-effects, usually of a mild nature, were encountered in isolated cases. All these manifestations, however, were tolerated by the patients or disappeared rapidly with symptomatic treatment so that no discontinuation of the drug was required during the induction of remission.
CRNIT treatment in NB4-R1 cells resulted in the induction of apoptosis and degradation of the PML-RARα fusion protein in vitro
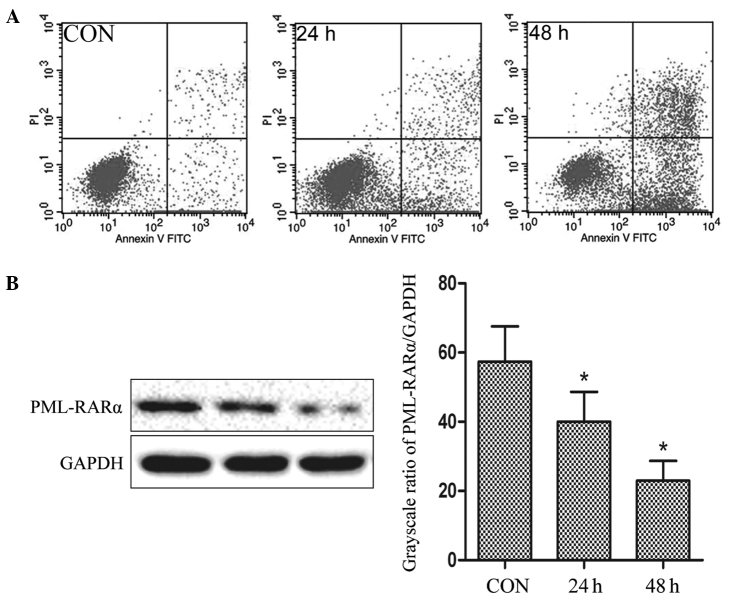
Subsequent to the administration of 34 mg/l CRNIT treatment, flow cytometry data revealed that 4.6±0.14% of the NB4-R1 cells administered with CRNIT treatment for 24 h were in the upper right (UR) quadrant and 18.45±0.82% of the cells were in lower right (LR) quadrant. Also, 13.02±0.62% of the NB4-R1 cells administered with CRNIT treatment for 48 h were in the LR quadrant and 53.24±0.98% of the cells were in the UR quadrant. By contrast, only 1.59±0.22% and 4.26±0.41% of the untreated control cells were in the LR and UR quadrants, respectively (Fig. 2A).
Figure 2.
Role of compound realgar natural indigo tablet treatment on the NB4-R1 cell line in vitro. (A) Flow cytometry analysis of the apoptosis in NB4-R1 cells. (B) Western blot analysis of the expression of the PML-RARα fusion protein in NB4-R1 cells. CON, control; FITC, fluorescein isothiocyanate; PML-RARα, promyelocytic leukemia-retinoic acid receptor α.
Representative western blot analysis and comparative grayscale ratios are shown in Fig. 2B. Compared with the expression in the control group (57.35±10.21%), the expression of the PML-RARα fusion protein was downregulated by 17.34 and 34.41% in the cells administered with CRNIT treatment for 24 h and 48 h, respectively (40.01±8.64% and 22.94±5.73%; P<0.05). The data indicated that the administration of CRNIT treatment may result in the degradation of the PML-RARα fusion protein in NB4-R1 cells.
Discussion
Realgar, indigo naturalis, savia miltiorrhiza and radix pseudostellariae are clinically active antitumor compounds (15–18). In 2006, the Phase II Clinical Trials Cooperative Group of China reported a similar curative effect between the administration of RA and CRNIT treatment in patients with incipient APL (19). However, few clinical studies have been performed investigating the efficacy and safety of CRNIT treatment in patients with refractory APL.
In the present study, 28 out of 31 patients with refractory APL achieved CCR during the administration of CRNIT treatment in combination with other chemotherapy regimens, with a CCR rate of 90.32%. This result has particular clinical significance as previous studies have suggested that patients with refractory APL patients possess a relatively poor prognosis, achieving CCR rates of ~80% (20,21). According to a previous multicentric study in China, the remission rate achieved with the administration of RA and chemotherapy alone or in combination in patients at first relapse was 40%, and even the CR duration was generally short (22). CRNIT may demonstrate no significant cross-resistance with the drugs that are currently used drugs for the treatment of APL, as all patients in the present study received RA and chemotherapy during the previous induction of remission or post-remissional therapy.
As an oral therapy, clinical observations revealed that the currently used dose of 3.75–7.5 g/day CRNIT treatment was, in general, well tolerated and associated with only mild to moderate side-effects. Although neutropenia and thrombocytopenia were observed in a subset of patients, neither BM suppression nor bleeding was problematic in the heavily pre-treated group of patients. Overall, the present data provide strong support for future investigation into the administration of CRNIT treatment in combination with the other agents currently used to treat APL.
The high CR rate reported in the present study was due to the rational combination of CRNIT treatment with chemotherapy. The present in vitro experiment revealed that realgar may induce apoptosis and degradation of the PML-RARα fusion protein in NB4-R1 cells, which was consistent with previous studies (23). CRNITs contain the core components of realgar and indigo naturalis, which act synergistically. Although the compounds do not directly induce apoptosis, the synergistic effects strengthen the anti-APL function of realgar. In addition, salvia miltiorrhiza and radix pseudostellariae may promote the recovery of normal hematopoietic function, and prevent DIC, bleeding and infection. Additionally, salvia miltiorrhiza and radix pseudostellariae were revealed to be able to protect the function of the heart, liver, kidney and other visceral organs (24). Lan et al also confirmed that the mechanism of CRNIT treatment involved multiple components and targets (17). It was concluded that the combination of multiple drugs may not only enhance the CR rate, but also reduce incidence of complications and drug toxicity.
Notably, 2 patients in the present study that did not possess the t (15;17) chromosome translocation or express the PML-RARα fusion gene transcripts, patients 11 and 18, demonstrated an excellent response to CRNIT treatment, which was different from the curative effect of ATO. The latter was reported to yield a relatively poor result in patients without the PML-RARα fusion protein (24). It is possible that PML-RARα fusion gene transcripts was not the only target of CRNITs, and CRNITs may exert anti-leukemia effects through other methods, not only through the breakdown of the PML-RARα fusion gene or protein. Therefore, the patients that lack the t (15,17) chromosome translocation demonstrated an extremely high CCR rate. Therefore, it was concluded that CRNIT treatment possesses considerable potential as a future therapy for APL.
It should be noted that the long-term effect and specific mechanism of CRNITs requires additional investigation, despite the studies investigating long-term CRNIT administration as a second-line agent, and the use of CRNIT treatment should be reserved for patients with APL refractory to RA and conventional chemotherapy (20,21,25). If the relative safety of the long-term use of CRNITs is assured in the future, CRNIT may be incorporated into a multidrug protocol for de novo patients.
Acknowledgements
This study was supported by the Fundamental Research Funds for the Central Universities, Shaanxi Province Science and Technology Development Fund (grant no., 2012KTCL03-12). The authors thank Dr. Xinyang Wang for his technological assistance.
References
- 1.Dos Santos GA, Kats L, Pandolfi PP. Synergy against PML-RARa: targeting transcription, proteolysis, differentiation and self-renewal in acute promyelocytic leukemia. J Exp Med. 2013;210:2793–2802. doi: 10.1084/jem.20131121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- 3.Lallemand-Breitenbach V, Zhu J, Chen Z, de Thé H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18:36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Adés L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: The European APL Group experience. Blood. 2010;115:1690–1696. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 5.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. New Engl J Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 6.Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: Characteristics, outcome and prognostic factors. Blood. 2009;113:775–783. doi: 10.1182/blood-2008-07-168617. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Lu C, Jiang M, et al. Traditional chinese medicine-based network pharmacology could lead to new multicompound drug discovery. Evid Based Complement Alternat Med. 2012;2012:149762. doi: 10.1155/2012/149762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SL, Guo A, Xiang Y, et al. Clinical study nf the treatment of acute promyelocytic leukemia mainly with composite indigo naturalis tablets. Zhonghua Xue Ye Xue Za Zhi. 1995;1:26–28. (In Chinese) [Google Scholar]
- 9.Xiang Y, Huang S, Guo A, et al. The analysis of therapeutic efficiency on treating acute promyelocytic leukemia (APL) with Compound Huangdai Tablets. Zhonghua Xue Ye Xue Za Zhi. 2000;13:11–12. (In Chinese) [Google Scholar]
- 10.Xiang Y, Huang S, Guo A, et al. The influence on long-term survey of the patients with acute promyelocytic leukemia treated alternatively with compound huang dai tablets and chemotherapy. Zhonghua Xue Ye Xue Za Zhi. 2003;16:11–12. (In Chinese) [Google Scholar]
- 11.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenauer A, Raelson JV, Nervi C, Eydoux P, DeBlasio A, Miller WH., Jr Alterations in expression, binding to ligand and DNA and transcriptional activity of rearranged and wild-type retinoid receptors in retinoid-resistant acute promyelocytic leukemia cell lines. Blood. 1996;88:2671–2682. [PubMed] [Google Scholar]
- 13.Liu Y, He P, Zhang M, et al. Silencing of the human SET gene in vitro with lentivirus-mediated RNA interference. Mol Med Rep. 2013;7:843–847. doi: 10.3892/mmr.2013.1275. [DOI] [PubMed] [Google Scholar]
- 14.Roberts TF, Sprague K, Schenkein D, Miller KB, Relias V. Hyperleukocytosis during induction therapy with arsenic trioxide for relapsed acute promyelocytic leukemia associated with central nervous system infarction. Blood. 2000;96:4000–4001. [PubMed] [Google Scholar]
- 15.Wu J, Shao Y, Liu J, Chen G, Ho PC. The medicinal use of realgar (As4 S4) and its recent development as an anticancer agent. J Ethnopharmacol. 2011;135:595–602. doi: 10.1016/j.jep.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, Li Y, Lu Y, et al. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int J Cancer. 2011;129:1042–1052. doi: 10.1002/ijc.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang HN, Pang JH, Yang SH, et al. Inhibitory effect of indigo naturalis on tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human umbilical vein endothelial cells. Molecules. 2010;15:6423–6435. doi: 10.3390/molecules15096423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Coorperation Group of Phase II Clinical Trial of Compound Huangdai Tablet, corp-author. Phase II clinical trial of compound Huangdai tablet in newly diagnosed acute promyelocytic leukemia. Chin J Hematol. 2006;27:801–804. [Google Scholar]
- 20.Gong JX, Meng JB, Ma Y. Effects of all-trans retinoic acid and compound huangdai tablet sequential maintenance treatment on the long-term efficacy of acute promyelocytic leukemia patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1473–1476. (In Chinese) [PubMed] [Google Scholar]
- 21.Xiang Y, Chang XH, Cheng YB. Effect of post-remission therapy mainly with compound huangdai tablet on long-term survival of patients with acute promyelocytic leukemia. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:1253–1256. (In Chinese) [PubMed] [Google Scholar]
- 22.Sun GL. Treatment of acute promyelocytic leukemia (APL) with all-trans retinoic acid (ATRA): A report of five-year experience. Zhonghua Zhong Liu Za Zhi. 1993;15:125–129. (In Chinese) [PubMed] [Google Scholar]
- 23.Chen S, Fang Y, Ma L, Liu S, Li X. Realgar-induced apoptosis and differentiation in all-trans retinoic acid (ATRA)-sensitive NB4 and ATRA-resistant MR2 cells. Int J Oncol. 2012;40:1089–1096. doi: 10.3892/ijo.2011.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE. Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel. 2010;13:50–65. [PubMed] [Google Scholar]
- 25.Tang D, Zhang Z, Gao Y, Wei Y, Han L. Protective effects of serum containing Ginkgo biloba extract on glomerulosclerosis in rat mesangial cells. J Ethnopharmacol. 2009;124:26–33. doi: 10.1016/j.jep.2009.04.017. [DOI] [PubMed] [Google Scholar]