Abstract
Objective: To investigate the mechanisms for reversing drug resistance of cisplatin (DDP) by Hsp90 inhibitors (geldanamycin (GA), 17-AAG, 17-DMAG) in human ovarian cancer. Methods: Cell proliferation rate in DDP resistant human ovarian cancer cell line SKOV3/DDP and its parent cell line SKOV3 after treatment with Hsp90 inhibitors and/or DDP were tested by MTT assay, and the reversing fold (RF) of DDP by Hsp90 inhibitors was calculated. Cell cycle and cell apoptosis status after treatment were analyzed by flow cytometry. The expression of multiple drug resistance related genes was analyzed by RT-PCR and Western-blot. Results: All three tested Hsp90 inhibitors synergistically inhibited the cell proliferation of SKOV3 with DDP and enhanced the sensitivity of SKOV3/DDP cells to DDP. The RF of DDP by Hsp90 inhibitors were all more than two fold. GA caused cell cycle arrest in G2/M phasein SKOV3 cells. 17-AAG increased cell apoptosis but did not change cell cycle in SKOV3/DDP cells. The mRNA and protein expression levels of various drug resistant related genes including LRP, GST-π, p53, bcl-2, survivin, ERCC1, XRCC1, BRCA1 and BRCA2 were more dramatically altered by Hsp90 inhibitors and DDP in combination compared to Hsp90 inhibitors or DDP treatment alone. Conclusions: Exposure of SKOV3/DDP cells to Hsp90 inhibitors and DDP in combination results in synergistic cytotoxic and pro-apoptotic effects. Hsp90 inhibitors reverse the drug resistance of SKOV3/DDP cells to DDP by modifying the expression of multiple drug resistance related genes.
Keywords: Ovarian cancer, heat shock protein 90, Hsp90 inhibitors, cisplatin, resistance genes
Introduction
Ovarian cancer is the leading cause of death in gynecologic cancers. Platinum-based drugs are the first line and major therapy for ovarian cancer. Though the combined chemotherapy based on platinum after tumor cytoreductive surgery could enhance the recovery rate of ovarian cancer patients, only 70%-80% ovarian cancer is responsive to this combined chemotherapy. Also, drug resistance to platinum is easy to occur in the early phase of chemotherapy treatment, which greatly reduces the sensitivity of ovarian cancer patients to chemotherapy and causes recurrence and metastasis leading to poor prognosis. Thus, to study the drug resistance mechanisms of platinum and explore resistance reversing drugs with a high efficacy and a low toxicity are hot topics in the field.
As a molecular chaperone protein, heat shock protein 90 (Hsp90) could adjust the confirmation, activation, maturation and stability of client proteins. In cancers there are many mutated and/or overexpressed signal transduction proteins. Hsp90 inhibitors could ubiquitinate these proteins and then cause their degradation in proteasomes to achieve their anti-cancer effects. Currently Hsp90 inhibitors include geldanamycin (GA) and its derivatives, 17-dimethylaminoethyl-amino-17-demethoxy-geldanamycin (17-AAG), 17-dimethylaminoethyl-amino-17-demethoxy-geldanamycin (17-DMAG). GA is a benzoquinone ansamycin antibiotic that was first isolated from the extract of Streptomyces hygroscopicus in 1970. It is a nature inhibitor for Hsp90 and blocks its chaperone function by binding to the ADP/ATP-binding pocket of the protein. This binding specifically inhibits the interactions of Hsp90 with many tumor-associated proteins, decreases their stabilities and promotes their degradation, and thus inhibits the signaling pathway of tumor cell growth [1]. 17-AAG was discovered and entered into clinic trials in 1999. 17-AAG could induce cell apoptosis and cause cell cycle arrest in G2/M phase in various cancers [2]. It could also block the activation of ERK1/2 in the RAS/RAF/MEK/ERK signaling pathway or decrease the DNA repair ability to reduce the drug resistance in cancer therapy. 17-DMAG is also a specific inhibitor for Hsp90 that has a better water solubility than 17-AAG. 17-DMAG could inhibit the function of Hsp90 by decreasing the expression of survivin and livin, and it could induce cell apoptosis in gastric cancer through induction of STAT signaling and mutation of p53 [3]. Though 17-DMAG could not decrease the expression level of Hsp90, it could cause the reduction of Akt, Met and HER-2 in ovarian cancer [2,4]. Jhaveri K et al reported that 17-DMAG significantly inhibits the cell proliferation of ovarian cancer, cervical cancer and breast cancer [5].
Currently it is known that GA and its derivatives could inhibit the cell proliferation and induce cell apoptosis in ovarian cancer. In our previous study we also found that GA inhibits the cell proliferation in SKOV3 ovarian cancer cells as well as causes cell apoptosis by cell cycle arrest in G2/M phase. The mechanism could be due to reduced expression of Akt and raf-1 [4]. However, there is no report about whether GA and its derivatives can reverse the drug resistance of DDP in ovarian cancer and the underlying molecular mechanisms. Thus the present study will address this question.
Materials and methods
Reagents
GA and 17-AAG were purchased from Alexis Biochemicals (Farmingdale, NY), whereas 17-DMAG was get from BioVision Inc. (Milpitas, California). Cisplatin (DDP) was purchase from Sigma (St. Louis, MO). LRP and GST-π antibody were purchased from abcam (Cambridge, England). Mouse Bcl-2 monoclonal antibody was purchased from Dakopatts (Glostrup, Denmark). Mouse P53 monoclonal antibody (DO-1) was purchased from Oncogene. Rabbit survivin monoclonal antibody and rabbit Hsp90α monoclonal antibody were purchased from Selleckchem (Houston, TX). Rabbit β-Actin polyclonal antibody was purchase from Invitrogen (Grand Island, NY). Rabbit and mouse GAPDH polyclonal antibodies were purchased from GeneTex Inc. (Irvine, CA). RT-PCR reverse transcription kit was purchased from Fermentas (Pittsburgh, PA).
Cell culture
DDP resistant ovarian cancer cell line SKOV3/DDP and its parent cell line SKOV3 were preserved at research center in Fourth Hospital of Hebei Medical University (Shijiazhuang, China). SKOV3 and SKOV3/DDP cells within the log phase of growth were cultured in RPMI1640 and plated in 96-well plate at a density of 5 × 104/mL. Experimental groups were treated with GA (20, 40, 200, 400 and 2000 nmol/L), DDP (3, 6 and 9 μg/mL), GA (40 nmol/L and 400 nmol/L) + DDP (3 μg/mL), 17-DMAG (2, 4, 8, 16 and 32 μmol/L), DDP (1, 2, 4, 8 and 16 μg/mL), 17-DMAG (6 μmol/L) + DDP (1, 2, 4, 8 and 16 μg/mL), 17-AAG (1.2, 2.4, 4.8, 9.6 and 19.2 μg/ml), DDP (2, 4, 8, 16 and 32 μg/mL), 17-AAG + DDP (SKOV3: 17-AAG 1.2, 2.4, 4.8, 9.6, 19.2 μg/ml + 2 μg/ml DDP, SKOV3/DDP: 17-AAG 1.2, 2.4, 4.8, 9.6, 19.2 μg/ml + 3 μg/ml DDP). SKOV3 cells without drug treatments were used as control.
MTT assay
Cells were incubated with or without drugs for 24, 48 and 72 h, MTT (5 mg/mL) were then added into the media. After further incubation for another 4 h, cell culture media was aspirated and DMSO was added to dissolve the formed crystal. The absorbance (OD) of each well was measured with a plate reader (UV visible spectrophotometer 2010HT2 type, Thermo Fisher Scientific, Austria) at 490 nm. The cell inhibition rate was calculated as (OD control-OD experimental)/OD control × 100%. The experiment was repeated three times. IC50 and the reversing factor for DDP + GA or its derivatives were calculated. The interaction index of two combined drugs was evaluated by q value as follow[6]: q=EA+B/(EA + EB - EA × EB), where EA is the effect of GA, EB is the effect of DDP, EA+B is the effect of combined use of two drugs. If q>0.85, the effects of the two drugs are additive, whereas q<0.85, the effects of the two drugs are antagonistic.
Detection of cell cycle and apoptosis by flow cytometry
Cells were incubated with or without drugs for 48 h, collected and washed twice with D-hanks, 70% ethanol for 2 h and then then treated with RNase for 1 h at 37°C. After staining with PI for 30 min at 4°C and filtered with strainer, single cell suspension was analyzed on a flow cytometry (FACS CaliburTM, BD Bioscience) with Multicycle AV for cell cycle and Expo ADC for apoptosis, respectively.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was extracted with Trizol (Invitrogen, Grand Island, NY) according to the manufacturer’s manual. The concentration and purity of RNA was determined by NanoDrop. The RNA with a ratio of OD260/OD280 among 1.8-2.0 was used for following experiments. One μg RNA was reverse transcribed with oligo(dT)18, dNTP, RevertAidTMM-MuLV Reverse Transcriptase to synthesize cDNA. The synthesized cDNA was used as template to amplify target genes with primers in Table 1. The PCR products were electrophoresed in a 1.5% agarose gel and analyzed with gel-pro analysis 3.1 for the specific DNA bands of target genes.
Table 1.
Primers for RT-PCR
| Primer | Sequence (5’-3’) | Product size (bp) | Tm (°C) |
|---|---|---|---|
| β-actin F | CCGACAGGATGCAGAAGGAGAT | 238 | 58 |
| β-actin R | GTCAAGAAAGGGTGTAACGCAACT | ||
| LRP F | AGTCAGAAGCCGAGAAAG | 356 | 52 |
| LRP R | CCCAGCCACAGCAAGGT | ||
| GST-π F | CAGGAGGGCTCACTCAAAG | 369 | 55 |
| GST-π R | GATCAGCAGCAAGTCCAGCAG | ||
| GAPDH F | CCGACAGGATGCAGAAGGAGAT | 238 | 58 |
| GAPDH R | GTCAAGAAAGGGTGTAACGCAACT | ||
| Hsp90α F | GCAGCAAAGAAACACCTGGAG | 302 | 52 |
| Hsp90α R | TCCATGCGTGATGTGTCGTC | ||
| P53 F | TTCCGAGAGCTGAATGAGGC | 332 | 58 |
| P53 R | GTGCAGGCCAACTTGTTCAG | ||
| Bcl-2 F | CTGTGAAGCAGAAGTCTGGGAA | 188 | 58 |
| Bcl-2 R | CAGCATGATCCTCTGTCAAGTT | ||
| Survivin F | GGACCACCGCATCTCTACAT | 234 | 58 |
| Survivin R | CCAGTCCCTCCCTGAATCTG | ||
| ERCC1 F | CGCCGAATATGCCATCTCAC | 492 | 59 |
| ERCC1 R | ATCAGGAGGTCCGCTGGTTT | ||
| XRCC1 F | CAGGTTCCAGCAGTGAGGAG | 492 | 59 |
| XRCC1 R | TGAAGGCTGTGACGTATCGG | ||
| BRCA1 F | GGATTTATCTGCTCTTCGCGTT | 514 | 59 |
| BRCA1 R | TTGTATCCGCTGCTTTGTCCT | ||
| BRCA2 F | GAAGCGTGAGGGGACAGATT | 399 | 58 |
| BRCA2 R | GATTGGTACAGCGGCAGAGT |
Western blot analysis
SKOV3 and SKOV3/DDP cells were washed twice with PBS and lysed for whole cell extracts. Protein was resolved on 10% SDS-PAGE and transferred to PVDF membrane. Following blocking with 5% non fat milk, the blots were incubated with primary antibodies (1:600). The signal was detected using an ECL system.
Statistical analysis
All the data were analyzed with SPSS 13.0. Data are expressed as means ± SD. One-way ANOVA and Student’s t-test were used for statistical analysis, with P<0.05 accepted as significant difference.
Results
The synergistic inhibition effects of Hsp90 inhibitors and DDP on SKOV3/DDP cells
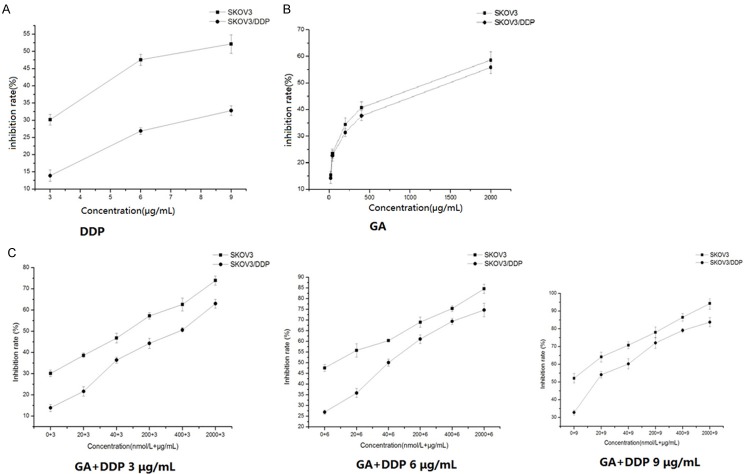
To test the effects of Hsp90 inhibitors on the drug resistance of DDP on ovarian cancer cells, DDP resistant human ovarian cancer cells SKOV3/DDP and its parent cell line SKOV3 were treated with various concentrations of GA or its derivatives including 17-AAG and 17-DMAG in the presence or absence of DDP. As expected, we found that the inhibition effect of DDP was more significant in SKOV3 than SKOV3/DDP cells (Figure 1A). GA could dose-dependently inhibit the cell proliferation in both SKOV3 and SKOV3/DDP cells, but there was no difference in the inhibition effects at the same dose between these two cell lines (Figure 1B). Interestingly, GA dose-dependently reduced the difference of inhibition effects caused by DDP between SKOV3 and SKOV3/DDP cells (Figure 1C). Specifically, the IC50 for SKOV3/DDP cells was 14.08 μg/ml when treated with DDP alone, whereas it decreased to 6.27 μg/ml when treated together with 40 nmol/L GA and further decreased to 2.55 μg/ml when treated together with 400 nmol/L GA (Table 2). The RF was 2.24 when treated with 40 nmol/L GA and it was further increased to 5.52 when treated with 400 nmol/L GA. The interaction index q value for 40 nmol/L GA combined with DDP on SKOV3 was 1.006, whereas the q value on SKOV3/DDP was 1.091; when treated with 400 nmol/L GA, the interaction index q value on SKOV3 was 1.070, and the q value on SKOV3/DDP was 1.092. Furthermore, the inhibition effects of cell proliferation by DDP, 17-DMAG and 17-AAG were time dependent (Figures 2A, 2B, 3A and 3B). Similarly, 17-DMAG dose-dependently reduced the difference of inhibition effects caused by DDP between SKOV3 and SKOV3/DDP cells (Figures 2C and 3C). Specifically, the IC50 for SKOV3/DDP cells was 6.65 μg/ml when treated with DDP alone, whereas it decreased to 2.19 μg/ml when treated together with 6 μmol/L 17-DMAG, thus the RF was 2.19 (Table 2). The interaction index q value for 17-DMAG combined with DDP on SKOV3 was 0.941, whereas the q value on SKOV3/DDP was 0.999. The IC50 for SKOV3/DDP cells was 32.19 μg/ml when treated with DDP alone, whereas it decreased to 13.66 μg/ml when treated together with 5.85 μg/ml17-AAG, thus the RF was 2.35. The q value for 17-AAG combined with DDP on SKOV3 was 0.927, whereas the q value on SKOV3/DDP was 1.452. Together, the combination of GA and its derivatives with DDP on SKOV3 and SKOV3/DDP results in q values higher than 0.85, which demonstrated that the combination of these drugs has a additive inhibition effect on cell proliferation.
Figure 1.
The inhibitory rate of DDP, GA and GA combined with DDP on the proliferation of SKOV3 and SKOV3/DDP cells. Cells were treated with different concentrations of DDP (A), GA (B) and GA (C) combined with DDP (3, 6, 9 μg/mL) for 24 h. The cell viability was determined in by MTT assay.
Table 2.
The q value of the combination of Hsp90 inhibitors and DDP in SKOV3 and SKOV3/DDP cells
| GA + DDP | 6 μmol/L17-DMAG + 3 μg/mL DDP | 5.85 μg/ml17-AAG + 3 μg/ml DDP | ||
|---|---|---|---|---|
|
|
||||
| 40 nmol/LGA +3 μg/ml DDP | 400 nmol/LGA + 3 μg/ml DDP | |||
| IC50 of DDP | 14.08 | 14.08 | 6.65 | 32.19 |
| IC50 of combination | 6.27 | 2.55 | 2.19 | 13.66 |
| Reversing Index (RF) | 2.24 | 5.52 | 2.19 | 2.35 |
| q value (SKOV3) | 1.006 | 1.070 | 0.941 | 0.927 |
| q value (SKOV3/DDP) | 1.091 | 1.092 | 0.999 | 1.452 |
Figure 2.
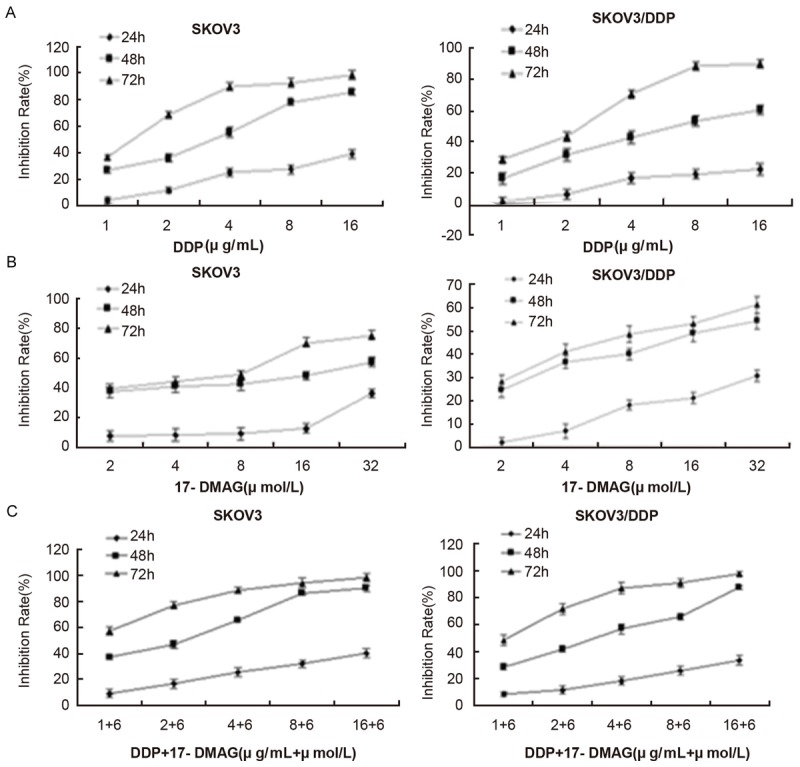
The inhibitory rate of DDP, 17-DMAG and 17-DMAG combined with DDP on the proliferation of SKOV3 and SKOV3/DDP cells. Cells were treated with different concentrations of DDP (A), 17-DMAG (B) and 17-DMAG (C) (6 μmol/L) combined with DDP for 24, 48 and 72 h. The cell viability was determined in by MTT assay.
Figure 3.
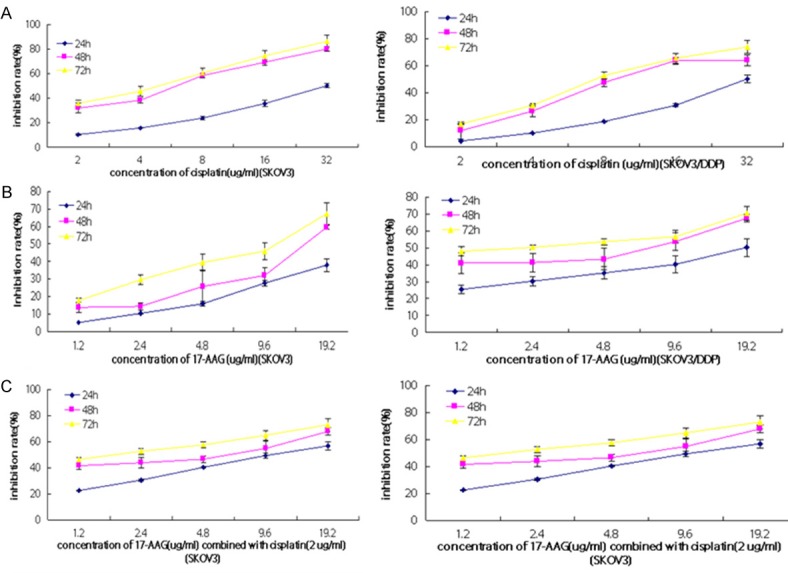
The inhibitory rate of DDP, 17-AAG and 17-AAG combined with DDP on the proliferation of SKOV3 and SKOV3/DDP cells. Cells were treated with different concentrations of DDP (A), 17-AAG (B) and 17-AAG (C) combined with DDP (2 μg/mL) for 24, 48 and 72 h. The cell viability was determined in by MTT assay.
The effects of Hsp90 inhibitors and DDP on cell apoptosis and cell cycle in SKOV3/DDP cells
To test the mechanisms for the inhibition effects of cell proliferation by Hsp90 inhibitors and DDP on ovarian cancer cells, cell cycle and cell apoptosis were analyzed by flow cytometry. After incubation with various concentrations of GA for 48h, the apoptotic rate of SKOV3 cells and the cell number in G2/M phase were increased with the drug concentration, which demonstrated that GA could cause cell cycle arrest in G2/M phase (Figure 4). For apoptosis, in SKOV3 cells, 17-AAG + DDP greatly increased the cell apoptotic rate compared to control group, whereas DDP or 17-AAG treatment did not increase the cell apoptotic rate (Figures 5, 6). In SKOV3/DDP cells, 17-AAG treatment increased the cell apoptosis, and this effect was even greater when used together with DDP. For cell cycle, compared to control group, the percentage of cells in the pre-DNA replecative G0/G1 phase and S pahse from treated groups were similar in SKOV3 cells, whereas the percentage of cells in the G2/M phase were significantly increased (Figure 6). In SKOV3/DDP cells, compared to control group, the percentage of cells in the pre-DNA replecative G0/G1 phase, S pahse, G2/M pahse from treated groups were all similar (Figure 6). Thus, 17-AAG enhances the sensitivity of SKOV3/DDP cells to DDP by increasing cell apoptosis, but it does not change cell cycle of ovarian cancer cells.
Figure 4.
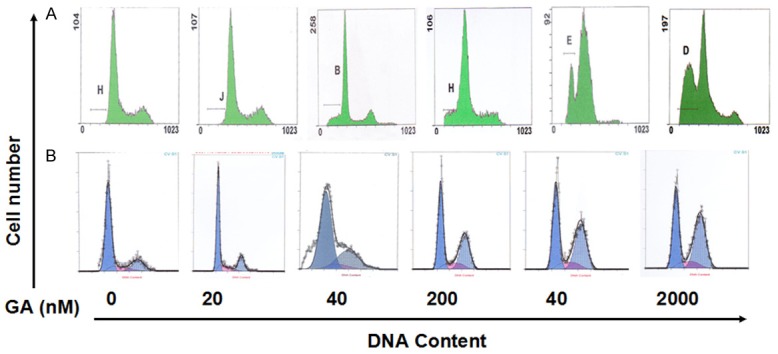
Flow cytometry detection of cell apoptosis and cell cycle in SKOV3 cells treated with GA. The apoptotic rate (A) and cell cycle (B) of SKOV3 cells after treated with GA (0-2000 nmol/L) for 48 h were detected by flow cytometry.
Figure 5.
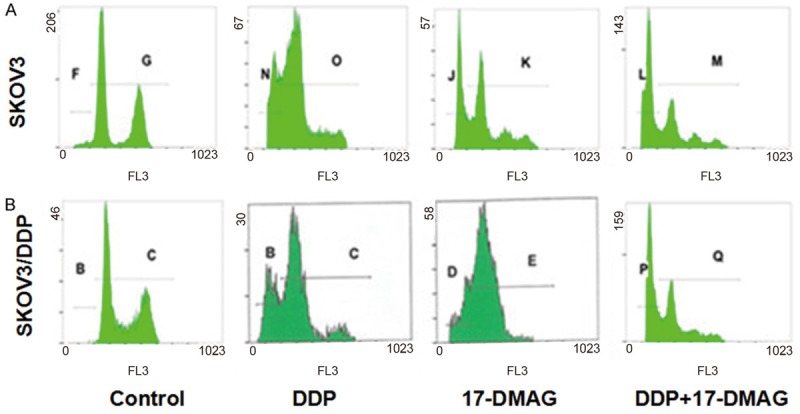
Flow cytometry detection of cell apoptosis and cell cycle in SKOV3 cells treated with 17-DMAG + DDP. The apoptotic rate and cell cycle of SKOV3 (A) and SKOV3/DDP (B) cells after treated with 1.5 μg/mL DDP and/or 6 μmol/L 17-DMAG for 48 h.
Figure 6.
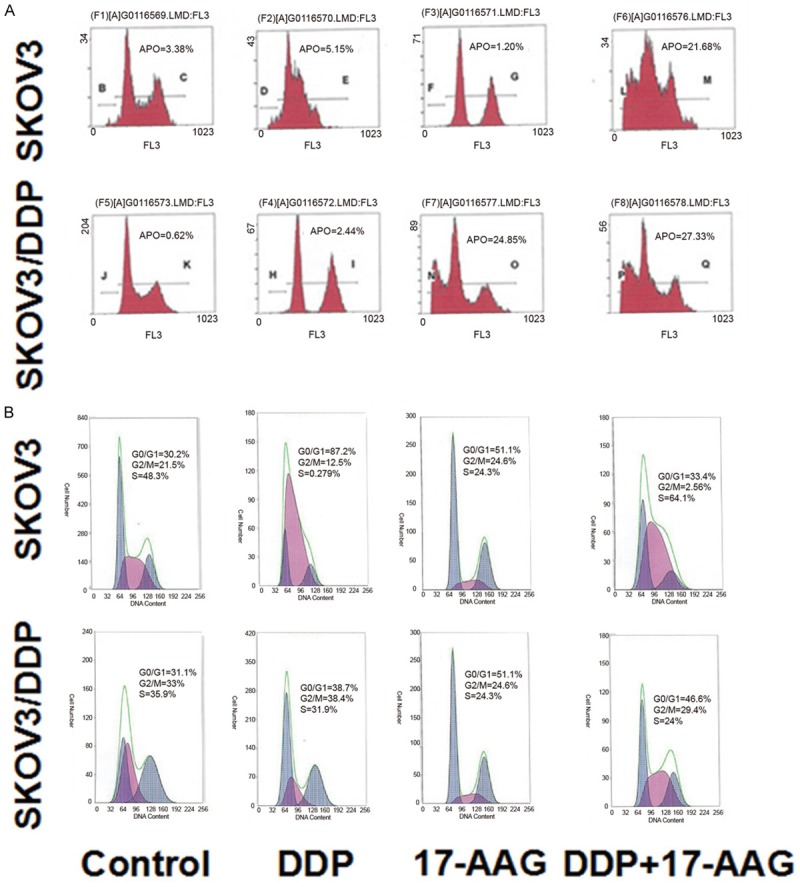
Flow cytometry detection of cell apoptosis and cell cycle in SKOV3 cells and SKOV3/DDP cells treated with 17-AAG and/or DDP. The apoptotic rate (A) and cell cycle (B) of SKOV3 and SKOV3/DDP cells after treated with 3 μg/ml DDP and/or 5.85 μg/ml 17-AAG for 48 h.
mRNA expression of multiple drug resistance related genes in human ovarian cancer cells
The drug resistance of tumor cells to chemotherapeutic drugs is the major reason for the failure of chemotherapy. The molecular mechanisms are related to many drug resistant associated enzymes and proteins, such as P-glycoprotein (P-gp), Multidrug resistance-associated protein (MRP), lung resistance-associated protein (LRP), Glutathione- S- transferase- pi (GST-π), DNA Topoisomerase II (Topo- II) [2]. To further determine the exact mechanism for DDP resistance in ovarian cancer cell, the mRNA expression of several known drug resistance related genes were examined. After 48h incubation with or without drugs, we detected the mRNA expression of LRP and GST-π. We found that DDP only decreased the mRNA expression of LRP and GST-π only in SKOV3 but not SKOV3/DDP group. GA significantly decreased LRP and GST-π compared to control group in SKOV3 and SKOV3/DDP cells, whereas GA + DDP treatment further enhanced the reduction of LRP and GST-π (Figure 7). The results showed that the mRNA expression levels of Hsp90α were similar among DDP, 17-DMAG and 17-DMAG + DDP treated SKOV3 and SKOV3/DDP cells (Figure 8). The mRNA expression levels of p53, Bcl-2 and survivin were significantly higher in untreated control group of SKOV3/DDP compared to SKOV3 cells. Compared to control group, DDP treatment increased the mRNA expression of p53, Bcl-2 and survivin, whereas treatment with 17-DMAG or 17-DMAG + DDP decreased their expression. There was no significant difference between 17-DMAG and 17-DMAG + DDP group in the mRNA expression level of p53, Bcl-2 and survivin. The mRNA expression levels of BRCA1 and BRCA2 were greatly enhanced by DDP, 17-AAG and 17-AAG + DDP treatment in SKOV3 and SKOV3/DDP cells (Figure 9), the increase is more obvious in SKOV3/DDP cells and the induction by 17-AAG + DDP is greater than DDP or 17-AAG alone. The mRNA expression levels of excision repair cross-complementation group 1 (ERCC1) and X-ray repair cross-complementing protein 1 (XRCC1) were decreased by 17-AAG treatment compared to control group in SKOV3 and SKOV3/DDP cells, 17-AAG + DDP further decreased the expression levels of ERCC1 and XRCC1 (Figure 9). Together, these results demonstrated that Hsp90 inhibitors could alter the mRNA expression of multiple drug resistance related genes.
Figure 7.
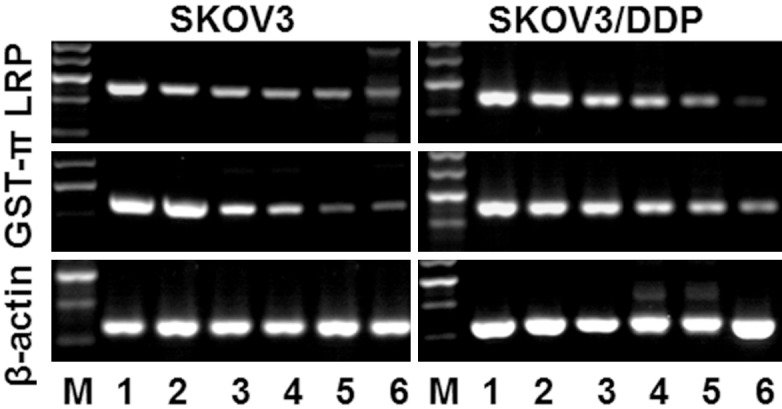
The mRNA expressions of LRP and GST-π mRNA in SKOV3 and SKOV3-DDP cells. Treated with GA and DDP and their combination for 48h and were analyzed by RT-PCR. M: Marker; 1: control; 2:3 μg/ml DDP; 3: 40 nmol/L GA; 4: 400 nmol/L GA; 5: 3 µg/L DDP + 40 nmol/LGA; 6: 3 µg/L DDP + 400 nmol/L GA.
Figure 8.
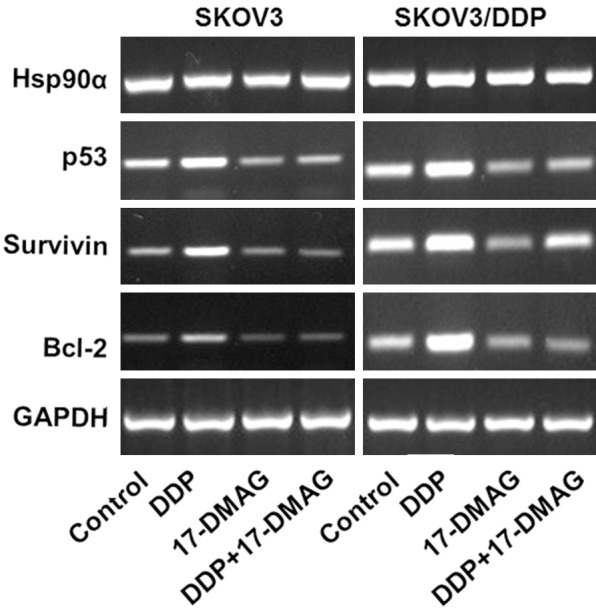
The expressions of Hsp90α, p53, Bcl-2 and Survivin mRNA in SKOV3 and SKOV3-DDP cells. Treated with 17-DMAG, DDP alone or the combination and were analyzed by RT-PCR.
Figure 9.
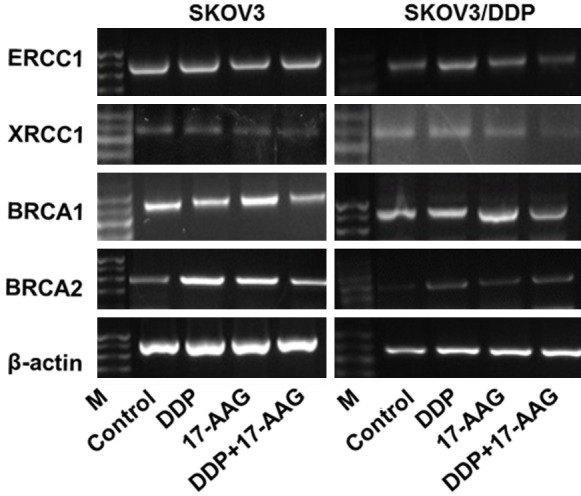
The expression of BRCA1, BRCA2, ERCC1 and XRCC1 mRNA in SKOV3 and SKOV3/DDP cells. Treated with 17-AAG, DDP alone or the combination and were analyzed by RT-PCR. M: Marker.
Protein expression of multiple drug resistance related genes in human ovarian cancer cells
To further evaluate the changes of the multiple drug resistance related genes, we examined the protein levels of these genes. In SKOV3 cells GA or DDP alone decreased the protein expression levels of LRP and GST-π (Figure 10). In SKOV3/DDP cells GA also decreased the protein expression levels of LRP and GST-π, whereas GA + DDP further increased the reduction levels. However, DDP alone had no effects. The protein expression of Hsp90α, bcl-2 and survivin were not changed by treating with 17-DMAG or DDP alone. DDP alone increased but 17-DMAG alone or 17-DMAG + DDP decreased the protein expression of p53 in SKOV3 and SKOV3/DDP cells compared to untreated control group. However, there was no difference in the protein expression of p53 between 17-DMAG alone and 17-DMAG + DDP group. The protein expression levels of p53, bcl-2 and survivin were lower in untreated SKOV3 cells compared untreated SKOV3/DDP cells (Figure 11). Together, these data show that the protein level of multiple drug resistance related genes were changed by Hsp90 inhibitors together with DDP treatment.
Figure 10.
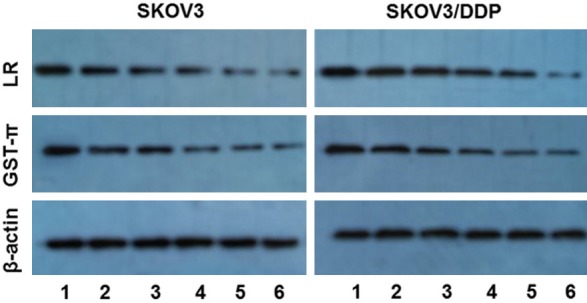
The protein expressions of LRP and GST-π mRNA in SKOV3 and SKOV3-DDP cells. Treated with GA and DDP and their combination for 48h and were analyzed by Western-blot. 1: control; 2: 3 μg/ml DDP; 3: 40 nmol/L GA; 4: 400 nmol/L GA; 5: 3 μg/L DDP + 40 nmol/LGA; 6: 3 μg/L DDP + 400 nmol/L GA.
Figure 11.
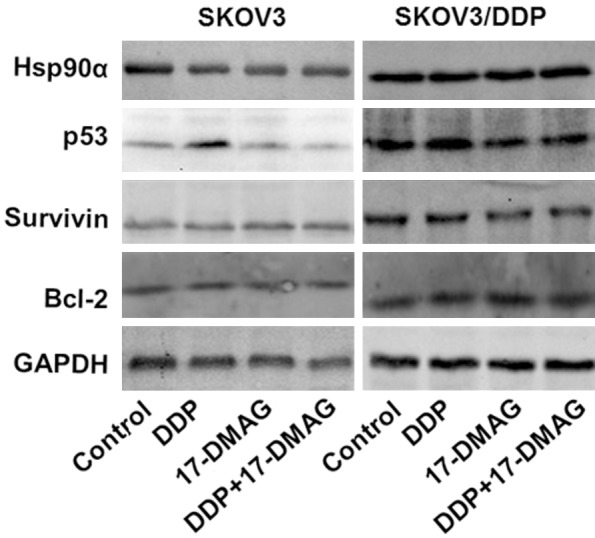
The protein expressions of Hsp90α, p53, Bcl-2 and Survivin mRNA in SKOV3 and SKOV3-DDP cells. Treated with 17-DMAG, DDP alone or the combination and were analyzed by Western-blot.
Discussion
The present study demonstrated that GA, 17-DMAG, 17-AAG remarkably inhibited the cell proliferation of SKOV3 and SKOV3/DDP cells in a dose-dependent manner. However, there was no difference between SKOV3 cells and SKOV3/DDP cells when they treated with the same dose of GA, 17-DMAG or 17-AAG. DDP also dramatically inhibited the cell proliferation of SKOV3 and SKOV3/DDP cells in a dose-dependent manner, but the inhibition rate was significantly higher in SKOV3 cells than SKOV3/DDP cells. Importantly, the combination of GA, 17-DMAG or 17-AAG with DDP potentiated the inhibition effects.
LRP, a drug resistance-associated protein that can cause resistance to platinum drugs and alkylating agent, was discovered from non-small cell lung cancer cell line SW-1573/2R120 in 1993 [7]. LRP blocks the nuclear pores to prevent antineoplastic drugs from entering into the nuclei, transports antineoplastic drugs into cytoplasmic vesicles and pumps the drugs out of cells to decrease the intracellular drug concentration, thus cause drug resistance [8]. LRP is increased in tumor tissues compared to normal tissue, but the expression level is dependent on specific tissue. The overexpression of LRP can predict the sensitivity of cancer patients to chemotherapy and prognosis, so that it could be an important index for screening chemotherapy drugs and evaluating the prognosis [9]. The expression of LRP in ovarian epithelial cancer is about 78%, which is obviously higher than benign tumors. This suggests that the expression of LRP could be related to the advance of ovarian cancer. Furthermore, LRP is an independent marker for prediction of the efficacy and prognosis in ovarian cancer [10]. The gene amplification or protein overexpression of LRP is an important mechanism for multi-drug resistance. Thus evaluation of the LRP expression by different methods provides a good reference for determining a specific chemotherapy in clinic. The current work detected the mRNA and protein expression of LRP after GA, DDP and GA + DDP treatment for 48 h in SKOV3 and SKOV3/DDP cells by Western blot and RT-PCR. The results show that GA but not DDP decreased the expression of LRP, whereas GA + DDP treatment further decreased the mRNA and protein expression of LRP. This data suggests that GA could increase the sensitivity of ovarian cancer cells to DDP by reducing the expression of LRP at mRNA and protein levels.
GSTs are a protein superfamily with many physiological functions, consisting of two isogenic or heterogenic subunits. There are three isoforms: α, μ and π. They are widely distributed in various organs with tissue-specificity for isoforms [11]. GST-π is the major isoform expressed in ovarian tumors and closely related to tumor drug resistance. GST-π could catalyze the binding of antineoplastic drugs with GST to decrease the cytotoxicity. Also, GST-π could bind with lipophilic drugs to increase their water solubility and thus facilitate their excretion. Moreover, GST-π has the peroxidase activity which could reduce the oxidative damage of chemotherapeutic drugs to normal cells. Immunohistochemisty analysis indicated that the expression of GST-π is highly increased in ovarian cancer compared to normal ovarian tissue or benign ovarian tumors, and it is correlated with clinic stages but not the cell differentiation status [12]. GST-π is associated with the drug resistance of platinum drugs and alkylating reagents. In addition, the gene expression of GST-π is closely related to the advance and prognosis of ovarian cancer patients treating with cisplatin [13]. The current data detected the mRNA and protein expression of GST-π after GA, DDP and GA + DDP treatment for 48h in SKOV3 and SKOV3/DDP cells by Western blot and RT-PCR. The results show that GA but not DDP decreased the expression of GST-π, whereas GA + DDP treatment further decreased the mRNA and protein expression of GST-π. This data suggests that GA could increase the sensitivity of ovarian cancer cells to DDP by reducing the expression of GST-π at mRNA and protein levels.
p53, bcl-2 and survivin are important genes involved in apoptosis. They are involved in the regulation of cell cycle, life span and apoptosis. There are two types p53, wild-type p53 (wtp53) and mutation p53 (mtp53). mtp53 is related to the initiation and drug resistance of tumor. It is reported that the level of mtp53 is dramatically increased in drug resistant cells results from chemotherapy of cisplatin and Taxol in stage IIIc ovarian cancer tumor cells, which demonstrated that the drug resistance of chemotherapy in ovarian cancer is related to the mutation of p53 [14]. At the same time, mtp53 is an important client protein for hsp90. The mutation of wtp53 results in a change in conformation with exposed binding sites and increased affinity to hsp90. After binding with hsp90, the stability of p53 is increased and thus mtp53 is avoided from the degradation of protease and is accumulated, whereas mtp53 loses the control of cell cycle. However, one study showed that the expression levels of survivin, cyclin D1 and NF-κB are decreased but p53 is increased in primary liver cancer cells treated by 17-DMAG.
Bcl-2 is an important anti-apoptotic gene that is increased in drug-resistant tumor cells. However, this drug induced bcl-2 has lost its original role of anti-apoptosis [15]. Bcl-2 is over expressed in cisplatin-resistant ovarian tumor cells, and when inhibiting this induction by drugs or gene silencing the drug resistance could be reversed [16]. In drug resistant cells, the gene expression of bcl-2 is increased and thus the anti-apoptotic effect is increased, which results in reduced cell apoptosis and drug resistance. Cisplatin could induce ovarian cancer cell apoptosis by reducing the expression of bcl-2, whereas over expression of bcl-2 inhibits cell apoptosis and leads to drug-resistance to cisplatin [17].
Survivin is a recently discovered anti-apoptotic gene. The gene expression of survivin is highly related to the cell proliferation of ovarian cancer, and the drug-resistance in ovarian cancer is related to the anti-apoptotic role of survivin. Reducing the expression of survivin could enhance the sensitivity of tumor cells to cisplatin induced apoptosis [18].
Hsp90 inhibitors including GA could reduce the induction of the protein expression levels of Bcl-2, Bcl-xL and Bax, mitochondria membrane potential loss, cytochrome C release, the activation of caspase 8, caspase 9 and caspase 3, cleavage of PARP-1 and the induction of p53 protein levels [15]. 17-DMAG could decrease the expression of survivin and livin by inhibiting the function of Hsp90, as well as induce cell apoptosis through activating STAT signaling pathway and causing p53 mutation. The results showed that 17-DMAG (or 17-AAG) and DDP in combination could significantly reduce the expression of resistance genes of p53, bcl-2 and survivin mRNA in SKOV3 and SKOV3/DDP cells. Moreover, 17-DMAG ( or 17-AAG) + DDP treatment further decreased the mRNA and protein expression of p53 in SKOV3/DDP cells, which suggests that 17-DMAG could increase the sensitivity of ovarian cancer cells to DDP by reducing the expression of P53 and survivin at mRNA and protein levels.
The enhancement of cell signaling pathway and DNA repair ability is a hot topic in the mechanisms for drug-resistance in ovarian cancer. The abnormal cell signaling pathway includes PI3K/Akt cell signaling, ERK1/2 cell signaling, STAT3 cell signaling, NF-κB cell signaling and so on. Platium durgs play their role of cytotoxicity through formation of intra-strand heavy platium-DNA adduct and crosslinking between two DNA strands. DNA repair pathway that occurs during genomic DNA recombination and DNA repair in crosslinking could remove these adducts to cause drug resistance. Thus the current study investigated the effects of 17-AAG on DNA repair genes ERCC1 and XRCC1. 17-AAG has a better anti-tumor effects and a lower toxicity compared to GA. 17-AAG could inhibit cell signaling pathway such as RAF/MEK/ERK, PI3K/Akt and p38/MAPK [19]. PI3K/Akt signaling pathway is highly active in ovarian cancer, and 17-AAG with cisplatin or taxol could induce cell apoptosis by inhibiting PI3K/Akt signaling in some ovarian cancer cell lines.
ERCC1 is a key factor involved in the procedure of nucleotide excision repair (NER) which is responsible for the formation of platinum-DNA adduct and the sensitivity of tumor cells to platium-based chemotherapy [20]. Overexpression of ERCC1 inhibits the efficacy of platinum and leads to drug resistance. The mRNA expression level of ERCC1 in platinum resistant tumor cells is increased, whereas tumor cells with a low level of ERCC1 mRNA have higher sensitivity in clinic. Thus, reducing the gene expression of ERCC1 can increase the sensitivity to cisplatin. The expression of ERCC1 could be served as a prognosis index for cancer patients who are treated with cisplatin-based therapy [21]. XRCC1 participates in DNA base excision repair pathway. The polymorphism of XRCC1 increases the incidences of gastric cancer and lung cancer and the DNA repair ability [22]. XRCC1 can adjust the efficacy and toxicity of cytotoxic drugs. It is single strand break repair factor that is related to non-homologous end joining [23]. It is demonstrated that XRCC1 encoded protein is the key for repairing DNA damage induced by DDP and Carboplatin (CBP) [23]. XRCC1 mediated DNA repair can remarkably affect the efficacy of tumor therapy and its polymorphism could predict the clinic efficacy for platinum based therapy [23].
The key function of BRCA1 and BRCA2 is repairing gene mutation through homologous recombination [20]. BRCA1 and BRCA2 are susceptible genes in breast cancer, the germline mutation of them is the most common genetic deficiency that can cause hereditary ovarian cancer [24]. Expression of functional breast cancer susceptibility gene 1 (BRCA1) is associated with resistance to platinum-based chemotherapeutics and poly (ADP ribose) polymerase (PARP) inhibitors in human breast and ovarian cancers [25]. BRCA1 and BRCA2 are tumor suppressors that are critical for resolving double-strand DNA breaks (DSBs) and interstrand crosslinks (ICLs) by homologous recombination (HR). 17-AAG can lead to the ubiquitination and degradation of BRCA1 by proteasome, thus dampen the DNA repair ability after irradiation and platinum-based chemotherapeutics to cause the loss of drug resistance. BRCA1 can modulate the checkpoint and repair reaction related to DNA damage. Hsp90 inhibitors can increase the sensitivity of BRCA1 to malignant tumors. 17-AAG can dose-dependently decrease the protein level of BRCA2, increase Hsp90 and enhance the specific anti-tumor activity of PARP inhibitors [26].
The present work demonstrated that GA may reverse the drug resistance of ovarian cancer cells to DDP through reducing the expression of LRP, GST-π. Similarly, 17-DMAG and 17-AAG may reverse the drug resistance of ovarian cancer cells to DDP through the reducing the expression of p53, bcl-2 and survivin. 17-AAG may also reverse the drug resistance of ovarian cancer cells to DDP through the increasing the expression of BRCA1 and BRCA2, and decreasing the expression of XRCC1 and ERCC1.
In summary, GA and its derivatives can synergistically inhibit the cell proliferation of SKOV3 and SKOV3/DDP with DDP. Also they can partially reverse the drug resistance of DDP in ovarian cancer cells through multiple resistance-associated genes such as LRP, GST-π and p53.
Disclosure of conflict of interest
None.
References
- 1.Jilani K, Qadri SM, Lang F. Geldanamycin-induced phosphatidylserine translocation in the erythrocyte membrane. Cell Physiol Biochem. 2013;32:1600–9. doi: 10.1159/000356596. [DOI] [PubMed] [Google Scholar]
- 2.Geng M, Wang L, Chen X, Cao R, Li P. The association between chemosensitivity and Pgp, GST-π and Topo II expression in gastric cancer. Diagn Pathol. 2013;8:198. doi: 10.1186/1746-1596-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang ZM, Wang MH, Yang XM. The Cytotoxic Effects of Geldanamycin on Human Ovarian Cancer SKOV3 Cells. Tumor. 2011;31:899–905. [Google Scholar]
- 4.Bodzek P, Partyka R, Damasiewicz-Bodzek A. Antibadies against Hsp60 and Hsp65 in the sera of woman with ovarian cancer. J Ovarian Res. 2014;7:30. doi: 10.1186/1757-2215-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CS, Kim YJ, Lee SA, Myung SC, Kim W. Combined effect of Hsp90 inhibitor geldanamycin and parthenolide via reactive oxygen species-mediated apoptotic process on epithelial ovarian cancer cells. Basic Clin Pharmacol Toxicol. 2012;111:173–81. doi: 10.1111/j.1742-7843.2012.00883.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Qin SK, Chen BA, Chen HY. Experimental study on antitumor effect of arsenic trioxide in combination with cisplatin or doxorubicin on hepatocellular carcinoma. World J Gastroenterol. 2001;7:702–5. doi: 10.3748/wjg.v7.i5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yakirevich E, Sabo E, Naroditsky I, Sova Y, Lavie O, Resnick MB. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Gynecol Oncol. 2006;100:152–9. doi: 10.1016/j.ygyno.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Sedláková I, Laco J, Tošner J, Caltová K, Cervinka M, Rezáč A, Spaček J, Skapinec P. Proteins of resistance and drug resistance in ovarian carcinoma patients. Klin Onkol. 2012;25:457–63. [PubMed] [Google Scholar]
- 9.Lv J, Tian Y. Effect of Src tyrosine kinase inhibition on the drug-resistance as well as MDR1 and LRP expression of the human cis-platinum-resistant lung cancer cell line A549/DDP. Zhongguo Fei Ai Za Zhi. 2012;15:501–6. doi: 10.3779/j.issn.1009-3419.2012.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Masuyama H, Nobumoto E, Zhang G, Hiramatsu Y. The inhibition of constitutive androstane receptor-mediated pathway enhances the effects of anticancer agents in ovarian cancer cells. Biochem Pharmacol. 2014;90:356–66. doi: 10.1016/j.bcp.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Sabatino MA, Geroni C, Ganzinelli M, Ceruti R, Broggini M. Zebularine partially reverses GST methylation in prostate cancer cells and restores sensitivity to the DNA minor groove binder brostallicin. Epigenetics. 2013;8:656–65. doi: 10.4161/epi.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu D, Shi HC, Wang ZX, Gu XW, Zeng YJ. Multidrug resistance-associated biomarkers PGP, GST-pi, Topo-II and LRP as prognostic factors in primary ovarian carcinoma. Br J Biomed Sci. 2011;68:69–74. doi: 10.1080/09674845.2011.11730326. [DOI] [PubMed] [Google Scholar]
- 13.Economopoulos KP, Serqentanis TN, Vlahos NF. Glutanthione S-transferase M1, T1 and P1 polymorphisms and ovarian cancer risk: a meta-analysis. Int J Gynecol Cancer. 2010;20:732–737. doi: 10.1111/IGC.0b013e3181dedeb5. [DOI] [PubMed] [Google Scholar]
- 14.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid Med Cell Longev. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elstrand MB, Stavnes HT, Tropé CG, Davidson B. Heat shock protein 90 is a putative therapeutic target in patients with recurrent advanced-stage ovarian catcinoma with serous effusions. Hum Pathol. 2012;43:529–35. doi: 10.1016/j.humpath.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Babu A, Wang Q, Muralidharan R, Shanker M, Munshi A, Ramesh R. Chitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/Plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Mol Pharm. 2014;11:2720–33. doi: 10.1021/mp500259e. [DOI] [PubMed] [Google Scholar]
- 17.Yang HL, Lin KY, Juan YC, Kumar KJ, Way TD, Shen PC, Chen SC, Hseu YC. The anti-cancer activity of Antrodia camphorate against human ovarian carcinoma (SKOV3) cells via modulation of HER-2/neu signaling pathway. J Ethnopharmacol. 2013;148:254–65. doi: 10.1016/j.jep.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Gąsowska-Bodnar A, Bodnar L, Dąbek A, Cichowicz M, Jerzak M, Cierniak S, Kozłowski W, Baranowski W. Survivin expression as a prognostic factor in patients with epithelial ovarian cancer or primary peritoneal cancer treat with neoadjuvant chemotherapy. Int J Gynecol Cancer. 2014;24:687–96. doi: 10.1097/IGC.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 19.Hadley KE, Hendricks DT. Use of NQ01 status as a selective biomarker for oesophageal squamous cell carcinomas with greatersensitivity to 17-AAG. BMC Cancer. 2014;14:334. doi: 10.1186/1471-2407-14-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaanan A, Dalban C, Emile JF, Blons H, Fléjou JF, Goumard C, Istanbullu M, Calmel C, Alhazmi K, Validire P, Louvet C, de Gramont A, Laurent-Puig P, Taïeb J, Praz F. ERCC1, XRCC1 and GSTP1 Single Nucleotide Polymorphisms and Survival of Patients with Colon Cancer Receiving Oxaliplatin-Based Adjuvant Chemotherapy. J Cancer. 2014;5:425–32. doi: 10.7150/jca.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudás J, Schartinger VH, Romani A, Schweigl G, Kordsmeyer K, Marta PI, Url C, Kral F, Riechelmann H. Cell cycle association and hypoxia regulation of excision repair cross complementation group 1 protein (ERCC1) in tumor cells of head and neck cancer. Tumour Biol. 2014;35:7807–19. doi: 10.1007/s13277-014-2001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan H, Kunutsor SK, Kauhanen J, Kurl S, Gorodeski EZ, Adler AI, Butler J, Laukkanen JA. Fasting plasma glucose and incident heart failure risk: a population-based cohort study and new meta-analysis. J Card Fail. 2014;20:584–92. doi: 10.1016/j.cardfail.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Lin F, Lin K, Xie X, Zhou C. Increasec ERCC1 protein expression is associated with suboptimal debulking in advanced epithelial ovarian cancer. Anticancer Res. 2010;30:2447–52. [PubMed] [Google Scholar]
- 24.Varga D, Deniz M, Schwentner L, Wiesmüller L. Ovarian cancer: in search of better marker systems based on DNA repair defects. Int J Mol Sci. 2013;14:640–73. doi: 10.3390/ijms14010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stecklein SR, Kumaraswamy E, Behbod F, Wang W, Chaguturu V, Harlan-Williams LM, Jensen RA. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc Natl Acad Sci U S A. 2012;109:13650–5. doi: 10.1073/pnas.1203326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerji U, Sain N, Sharp SY, Valenti M, Asad Y, Ruddle R, Raynaud F, Walton M, Eccles SA, Judson I, Jackman AL, Workman P. An in vitro and in vivo study of the combination of the heat shock protein inhibitor 17-allylamino-17-demethoxygeldanamycin and carboplatin in human ovarian cancer models. Cancer Chemother Pharmacol. 2008;62:769–78. doi: 10.1007/s00280-007-0662-x. [DOI] [PubMed] [Google Scholar]