Abstract
Background: To study the effects of neuregulin on human umbilical vein endothelial cells (HUVEC) protection. Methods: The HUEVC were cultured and divided into three different groups according to their culture conditions. In negative control group (group A), HUVEC were cultured in dulbecco modified eagle medium (DMEM) supplemented with 25 mmol/L glucose and neuregulin (10 ng/ml, 100 ng/ml, and 1000 ng/ml, respectively) for 48 hr. In experimental group (group B), HUVEC were cultured in DMEM with 75 mmol/L glucose and neuregulin (10 ng/ml, 100 ng/ml, and 1000 ng/ml, respectively) for 48 hr. In positive control group (group C), HUVEC were cultured in DMEM supplemented with 75 mmol/L mannitol, 25 mmol/L glucose, and neuregulin (10 ng/ml, 100 ng/ml, and 1000 ng/ml, respectively) for 48 hr. This study aimed to observe the cell morphology in different HUEVC groups and analyze apoptosis in each group via characterizing and detecting the protein expressions of CD98hc, p38, and JNK. Results: Significant differences in cell morphology were observed in the experimental group when compared with the control groups. In the experimental group, the degree of apoptosis was negatively related to the neuregulin concentration; CD98hc protein concentration was positively related to neuregulin concentration; JNK protein concentration was positively related to neuregulin concentration. However, there was no significant relationship between p38 protein expression and the concentration of glucose in the medium. Conclusion: Neuregulin could inhibit the apoptosis of HUEVC through the activation of MAPK signaling pathway via activating CD98hc expression.
Keywords: Human umbilical vein endothelial cells, high glucose, apoptosis, neuregulin, MAPK signaling pathway
Introduction
Diabetes mellitus (DM), a chronically progressive disease, is a growing public health problem worldwide. In recent years, the number of patients diagnosed with DM is growing rapidly and is expected to increase continuously due to the rapid socio-economic development and improved living standards. According to the statistics obtained by World Health Organization, there are more than 150 million people who have been diagnosed with DM worldwide [1]. The number of patients with DM had reached 246 million by 2007, which is anticipated to be 300 million by 2030 [1]. Currently the number of patients with DM in China is the second largest in the world, which is only smaller than that in India. Based on the statistics obtained from the survey carried out by Chinese Ministry of Health, there are about 3,000 new cases confirmed of DM every day, with an increase of about 1.2 million cases annually [2]. Meanwhile, type 2 DM accounts for about 95% cases. Moreover, cardiovascular complications are considered as the main severe complications of DM, which cause high mortality and poor prognosis.
Neuregulins (NRGs), which are also termed heregulins or acetylcholine receptors, are originally known as splice variants derived from a single gene [3]. Neuregulin-1beta, which is expressed in the cardiac microvascular endothelium, is indispensable for cardiac development, the maintenance of cardiac myofibril structures, survival and growth of cardiomyocyte and functional integrity of the adult heart. Neuregulin1 proteins are members of the epidermal growth factor (EGF) family, which are ligands for the ErbB receptor tyrosine kinases (RTKs). They promote the growth and survival of cardiomyocytes through the activation of ErbB2 and ErbB4 RTKs. Consequently, neuregulins and their receptors are of profound importance in both the developing and maintaining the adult cardiovascular system [4]. A lot of published data demonstrated that exogenous administration of NRG resulted in activation of ErbB receptors which were cardioprotective. Because endothelium appears to be the major source of NRG in heart, many investigators speculated that this endothelial-derived NRG played a key role in the setting of cardiac injuries in vivo.
Apoptosis, known as a programmed cell death resulting from acute cellular injuries, plays an important role in the maintenance of normal cell proliferation. Recent studies demonstrated that vascular endothelial cell apoptosis was closely associated with cardiovascular complications of DM [5]. Endothelial dysfunction has been implicated in the development of atherosclerosis, thrombosis and hypertension. Vascular endothelial cells are crucial in regulating the vasomotor movements and preventing the adhesion of platelets and blood cells, therefore are functional in inhibiting thrombosis and controlling cell growth and proliferation of vascular smooth muscle cells [6]. Atherosclerotic cardiovascular complications of DM induced by hyperglycemia are frequently observed in patients with DM, which is initiated by the certain gene expressions and their subsequent product generations, therefore leading to significant structural changes in vascular endothelial cells.
Hyperglycemia has been recognized as a primary factor in endothelial barrier dysfunction and in the development of micro- and macro-vascular diseases associated with DM, but the underlying biochemical mechanisms remain elusive. Several potential signaling pathways are suspected to be involved in this high-glucose induced endothelial dysfunction, including the up-regulation of adhesion molecules (e.g. vascular cell adhesion molecule-1 and intercellular adhesion molecule-1) expression, down-regulation of adiponectin receptor expression, repression of the expressions of matrix metalloproteinases, oxidative stress, increased glycosylation of non-enzymatic proteins, enhanced secondary lipid metabolism and activated protein kinase C signaling pathway. These potential pathways could partially elucidate the pathology of vascular complications of DM to some extent, but also could be beneficial in identifying novel therapeutic targets for the prevention of cardiovascular complications of DM. Furthermore, establishing and elucidating the underlying mechanisms of cardiovascular complications of DM is of profound importance to set up clinically innovative therapeutic strategies for addressing diabetic complications, improving the prognosis for patients with DM.
Materials and methods
Materials
Neuregulin was provided by Zensun (Shanghai) Sci & Tech Co., Ltd. Human umbilical vein endothelial cells (HUVECs) were purchased from Nanjing Jiancheng Bioengineering Institute. The Cell Counting Kit was purchased from Beyotime Institute of Biotechnology. Annexin-V-FLUOS kit was obtained from Shanghai Roche Pharmaceuticals Ltd. CD98 antibody was purchased from Santa Cruz Biotechnology. p38 antibody and JNK1/2/3 antibodies were obtained from Zhenjiang Hope Biotechnology Co., Ltd.
Methods
The apoptosis of HUVEC was measured by flow cytometry using annexin V and propidium iodide
HUVECs were seeded in six-well plates with a seeding density of 5 × 105 cells/well and were grown to 80% confluence. The cells were classified into three groups: DMEM supplemented with A) 25 mmol/L glucose; B) 75 mmol/L glucose; C) 75 mmol/L mannitol, 25 mmol/L glucose under different neuregulin treatments (10 ng/ml, 100 ng/ml, and 1000 ng/ml, respectively). Cells were grown in an incubator at 37°C with 5% CO2 for 48 h. Cells were washed three times with Phosphate Buffered Saline (PBS), and then harvested with 1 × trypsin solution. The trypsinized cells were centrifuged at 1000 r/min for 3 min and were resuspended in 100 ml Annexin-V-FLUOS solution by pipetting up and down thoroughly. The cells were then incubated at room temperature for 10 min and were washed with PBS for two times. Finally, the concentration of the cell lysates were normalized and diluted using lysis buffer and cells were assessed by flow cytometry under appropriate conditions using CELL Quest Software following manufacturer’s instructions.
The flow cytometry analysis of CD98
HUVECs were plated in 6-well plates for 48 hours and harvested as previously described. Then the trypsinized cells were centrifuged at 1000 r/min for 3 min and resuspended by pipetting. The concentrations of cell lysates were normalized by PBS followed by cell fixation with 75% ethanol for 2 hours. Anti-Human CD98hc FITC was added into the 200 ml cell lysates and maintained in dark for 45 min at room temperature. Flow cytometry experiments were carried out using flow cytometry (Accuri C6, BD, America), followed by analysis with CELL Quest Software.
CD98 expression was observed by confocal microscopy
HUVECs were grown in six-well plates on sterile glass coverslips (5 × 105 cells/well) until 80% confluence was attained. Cells were maintained in an appropriate medium and placed in an incubator at 37°C under 5% CO2 for 48 h. Cells were washed three times with PBS followed by 15-min fixation with 4% paraformaldehyde at 25°C. Cells were then washed twice with PBS, followed by blocking and permeabilizing with PBST [3% H2O2, 0.1% Triton-X, PBS] for 15 min. Cells were then washed with PBS for 5 min. This was followed by an overnight incubation with primary antibody (CD98) at 4°C with shaking. Again, cells were rinsed with PBS for three times followed by treatment with secondary antibody Cy3 (Abcam, UK) at 37°C for 1 h. Finally, cells with immunofluorescent staining were visualized using fluorescence microscope (IX71, OLYMPUS, JAPAN) and images were processed using Image J software (National Institute of Health, Bethesada, Maryland, USA).
Characterizing of the expression of p38 and JNK1/2/3 by Western Blot
HUVECs were grown as previously described in an incubator at 37°C with 5% CO2 for 48 hours and approximately 80% confluence was attained. Then the culture medium was removed, and cells were washed with PBS and added physiological saline or serum-free medium instead. Cells were then lysed with RIPA lysis buffer (250 ml) by pipetting up and down as per manufacturer’s instructions. The cell lysates were shaken gently for 10 min, leaving on ice for 2 h. The cell lysates were centrifuged at 13,300 rpm for 10 min at 4°C and the supernatant was collected. The protein concentration of each sample was examined by Bicinchoninic Acid protein assay (Thermo Scientific) following the manufacturer’s instructions.
25 mg protein was added to an equal volume of 2 × loading buffer [0.5 M Tris-HCl (pH6.8), 20% Glycerol, 10% sodium dodecyl sulphate, 2% 2-mercaptoethanol and 0.1% Bromophenol Blue] and boiled for 5 minutes at 90°C. The denatured proteins were resolved by 8% or 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis using a Mini-Protean electrophoresis system (Bio-Rad, Berkeley, CA, USA). The separated proteins were then transferred onto a nitrocellulose membrane (Bio-Rad) at 100 volts for 1 h using a Mini-TransBlot system (Bio-Rad). The membranes were then blocked with 5% skimmed milk in PBS for detection of proteins, for 1 h at room temperature with shaking. The membranes were incubated with primary antibodies against to p38 and JNK1/2/3, respectively, at 4°C overnight. The blots were then washed three times, for 5 min each, with PBS prior to incubation with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. The blots were washed as before and proteins were visualized using MilliporeTM ImmobilonTM Western Chemiluminescent HRP Substrate system (Millipore Corporation, Billerica, MA, USA) as per manufacturer’s instructions. The blots were developed on photographic film using Kodax SRX SRX2200 (Rochester, NY, USA).
Statistical analysis
Statistical analysis was carried out using SPSS 13.0 software and data were presented as mean ± standard error of the mean (mean ± SEM). The analysis of variance was performed using one-way ANOVA to compare the mean values of different groups, and using t test to compare the mean values between two groups. And the P value less than 0.05 was considered statistically significant.
Results
The changes in morphology of HUVEC after treatments with Neuregulin
Firstly, Figure 1 demonstrated that the HUVECs maintained in medium supplemented with 25 mmol/L glucose and treatments of neuregulin at specific concentrations, respectively. Cells in negative control group A and group B grew well and clear boundaries were visualized, arranging in a pattern of cobblestone-like. In contrast, the number of cells in Group C was significantly reduced with wider cell gaps (Figure 1). Figure 2 was presented to show the cells maintained in medium with 75 mmol/L glucose as well as the addition of neuregulin. In this case, clear boundaries were also observed in cells of group A and B, growing in cobblestone-like pattern. An obvious reduction of HUVECs in group C had been found with enlarged gaps among cells (Figure 2). In the presence of mannitol (75 mmol/L), on the other hand, most of HUVECs with neuregulin (10 ng/L) were observed to have narrower gaps between cells and the cell membranes were even damaged to break down. However, fewer cells in Group B were found to have smaller size with irregular shape with the treatment of 100 ng/L neuregulin. Cells in group C, in contrast, were observed to have a relatively normal cell growth rate with spindle shape as compared to cells in group A and B (Figure 3).
Figure 1.
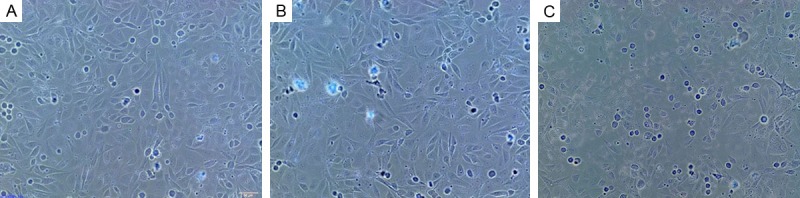
HUVECs in group A and B were observed to grow well with clear boundaries and in a pattern of cobblestone-like. A significant reduction of HUVECs had been found in group C and the gaps among cells were bigger. A. GLU 25 mmol/L+neuregulin 10 ng/L; B. GLU 25 mmol/L+neuregulin 100 ng/L; C. GLU 25 mmol/L+neuregulin 1000 ng/L.
Figure 2.

The cells maintained with neuregulin-intervention were presented with a better morphology and reduced number of dead cells. Therefore, neuregulin had a protective effect on HUVECs proliferation. A. GLU 75 mmol/L+neuregulin 10 ng/L; B. GLU 75 mmol/L+neuregulin 100 ng/L; C. GLU 75 mmol/L+neuregulin 1000 ng/L.
Figure 3.
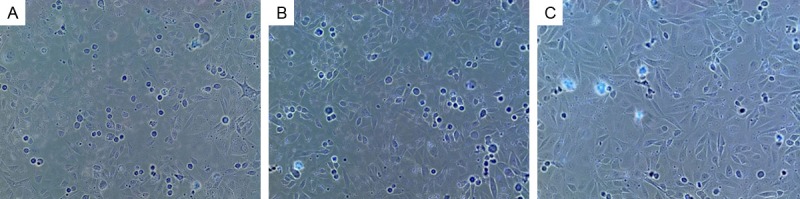
Cells of Group A were observed to have narrower cell gaps and most cells were suspected to have damaged cell membranes. Cells of group B were partially seen to be shrunk with irregular shapes. Group C cells were observed to have relatively high growth rate and maintain the normal spindle shape as compared to cells in Group A and B. A. Mannitol 75 mmol/L+neuregulin 10 ng/L; B. Mannitol 75 mmol/L+neuregulin 100 ng/L; C. Mannitol 75 mmol/L+neuregulin 1000 ng/L.
The effects of neuregulin intervention on HUVEC using annexin V/propidium iodide in flow cytometry analysis
HUVECs were maintained in medium supplemented with 25 mmol/L glucose and the cell proliferation rate was relatively stable. With an increased concentration of neuregulin treatment at 100 ng/L, a significant elevation of osmotic pressure was obtained, resulting in an increase number of apoptotic cells (Figure 4). On the other hand, the initial apoptosis rate of HUVECs was relatively low (26.9%) in the presence of glucose with 75 mmol/L and 10 ng/L neuregulin. However, the addition of neuregulin with a specific concentration (1000 ng/L) would significantly reduce the amount of apoptotic cells from 67.8% (group B, 100 ng/L neuregulin) to 24.2% (group C, 1000 ng/L) (Figure 5). On the other hand, 75 mmol/L mannitol treatment would also repress the apoptosis of HUVECs as the increase of neuregulin concentrations. However, the apoptosis of HUVECs were highly elevated the increase of osmotic pressure in the medium (Figure 6). Therefore it was obvious that neuregulin could exert cardioprotection, to some extent, on HUVECs against the high osmotic effect that caused by the death of cells.
Figure 4.
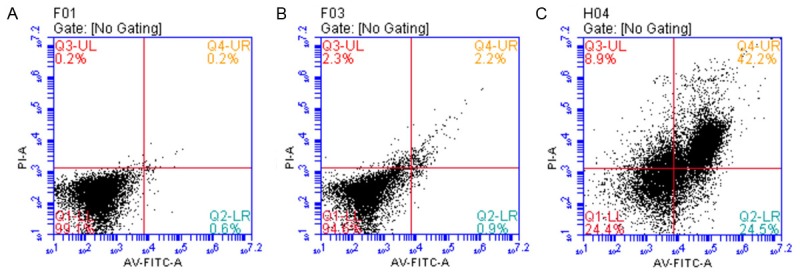
The HUVECs were cultured with the medium supplemented with glucose at 25 mmol/L, which was optimized for cell growth. However, the combination with neuregulin treatments (10, 100, 1000 ng/L, respectively) would all cause an increase of osmotic pressure, leading to an enhancement in cell apoptosis. A. GLU 25 mmol/L+neuregulin 10 ng/L; B. GLU 25 mmol/L+neuregulin 100 ng/L; C. GLU 25 mmol/L+neuregulin 1000 ng/L.
Figure 5.
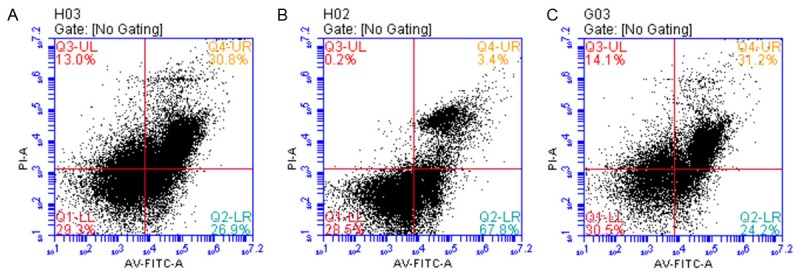
In the presence of relatively high concentration of glucose (75 mmol/L) combined with neuregulin (10 ng/L), the apoptosis rate of cells in group A was 26.9%. As the concentration of neuregulin increased in Group B (100 ng/L), the apoptosis rate was enhanced remarkably to 67.8%. By contrast, neuregulin addition at 100 ng/L exhibited an inhibition on apoptosis of HUVEC (24.2%), probably due to the cardioprotective effect of neuregulin on HUVECs. A. GLU 75 mmol/L+neuregulin 10 ng/L; B. GLU 75 mmol/L+neuregulin 100 ng/L; C. GLU 75 mmol/L+neuregulin 1000 ng/L.
Figure 6.
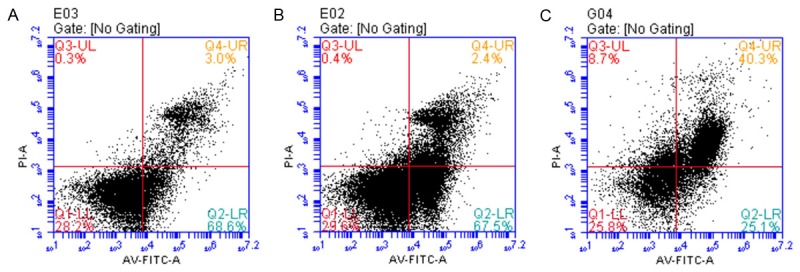
The neuregulin treatments could reduce the amount of apoptotic cells in the presence of manniotol (75 mmol/L). Moreover, the increase of osmotic pressure would also enhance the apoptosis of HUVECs to some extent regardless of the mannitol addition. A. Mannitol 75 mmol/L+neuregulin 10 ng/L; B. Mannitol 75 mmol/L+neuregulin 100 ng/L; C. Mannitol 75 mmol/L+neuregulin 1000 ng/L.
The effect of neuregulin treatment on CD98 expression in HUVEC
The detection and observation of CD98 expression by confocal microscopy
In this study, confocal microscope (IX71, OLYMPUS, JAPAN) was applied to detect and determine the expression level of CD98 proteins with the aid of secondary antibody Cy3 (Abcam, UK). As presented in figures obtained from this experiment, CD98 expressions were stained red and were observed in HUVECs in each experimental group (Figures 7, 8, 9). It had been proved that glucose at 25 mmol/L was an optimal concentration for HUVECs proliferation. As the increase of neuregulin concentrations, the osmotic pressure was elevated, inhibiting the proliferation of HUVEC as well as the expression of CD98 (Figure 7). More importantly, 75 mmol/L glucose would activate the cell apoptosis to some extent and the 100 ng/L neuregulin intervention would promote the CD98 expression notably as showed in Figure 8B. The treatment with 1000 ng/L neuregulin, however, would cause an increase in cell apoptosis, leading to a decrease in CD98 expression (Figure 8). The addition of mannitol, on the other hand, had no significant effect on the CD98 expression as compared with those detected in Figures 8, 9.
Figure 7.
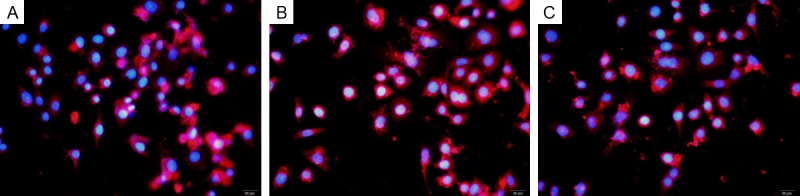
As showed in the pictures obtained from confocal microscopy, CD98 was detected in HUVECs maintained with 25 mmol/L glucose regardless of the presence of neuregulins. Cells stained red for CD98 with blue nuclei. A. GLU 25 mmol/L+neuregulin 10 ng/L; B. GLU 25 mmol/L+neuregulin 100 ng/L; C. GLU 25 mmol/L+neuregulin 1000 ng/L.
Figure 8.

HUVECs were cultured with 75 mmol/L glucose in combination with neuregulin treatments at 10, 100, 1000 ng/L. In comparison with the CD98 expression observed in Figure 7, there was no significant difference found in the expression level of CD98. A. GLU 75 mmol/L+neuregulin 10 ng/L; B. GLU 75 mmol/L+neuregulin 100 ng/L; C. GLU 75 mmol/L+neuregulin 1000 ng/L.
Figure 9.
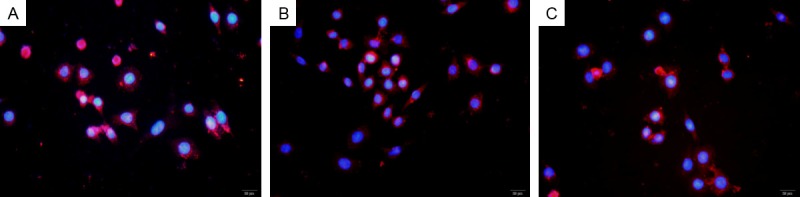
75 mmol/L mannitol was added to examine the effect of mannitol on the expression of CD98 in HUVECs and the results demonstrated that the expression level of CD98 was not altered remarkably as compared with the pictured presented in Figure 8. A. Mannitol 75 mmol/L+neuregulin 10 ng/L; B. Mannitol 75 mmol/L+neuregulin 100 ng/L; C. Mannitol 75 mmol/L+neuregulin 1000 ng/L.
The characterization of CD98 expression by flow cytometry analysis
HUVECs were cultured in medium supplemented with 25 mmol/L glucose, which was ideal for cell growth. Upon the treatment of neuregulin, the proliferation of HUVECs was repressed due to the increase of osmatic pressure and therefore the CD98 expression was also decreased to some extent (Figure 10). When 75 mmol/L glucose was adopted to maintain the HUVECs, more apoptosis was observed. However, neuregulin (100 ng/L) intervention exerted a promotive effect on the expression of CD98 as found in group B cells. In contrast, the treatment of neuregulin at 1000 ng/L enhanced the apoptosis of HUVECs, which could be caused by the increase of osmotic pressure, resulting in a reduction in CD98 expression (Figure 10). In addition, the osmotic pressure was peaked in HUVECs in the presence of 75 mmol/L mannitol and the apoptosis was also enhanced to a large extent regardless of the high concentration of neuregulin treatments (Figure 10).
Figure 10.
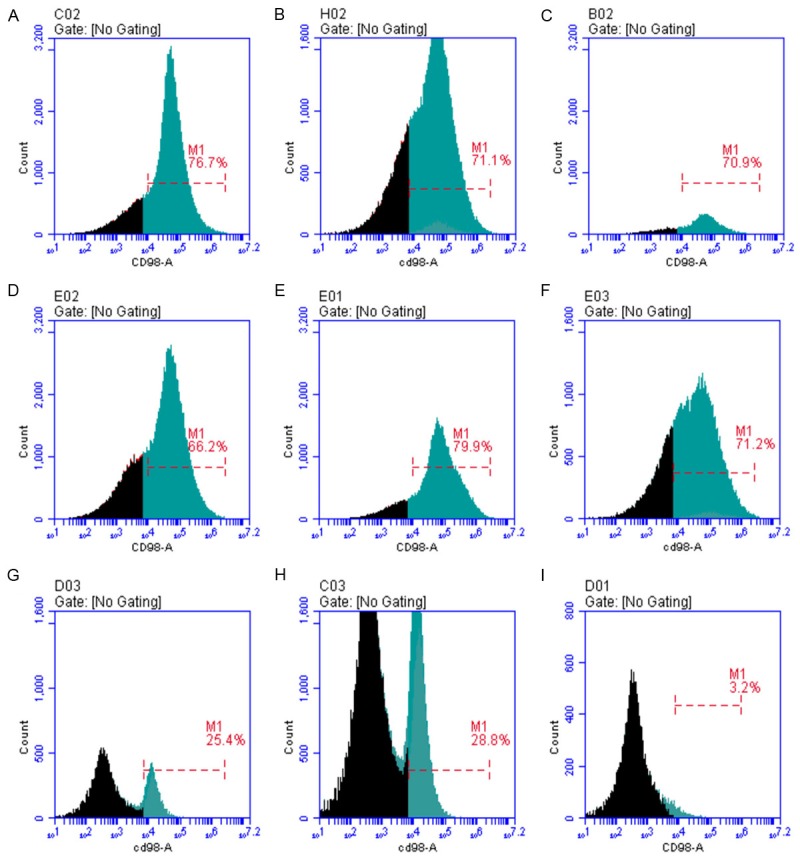
(A-C) HUVECs were cultured in medium supplemented with glucose at 25 mmol/L, which was favored for cell growth. With the addition of neuregulin, osmotic pressure in the medium was increased, leading to a relatively high repression of CD98 expression. (D-F) As compared to the 10 ng/L neuregulin treatment (66.2%), CD98 expression exhibited different degrees as increased the concentrations of neuregulin. It was obvious that CD98 expression was relatively lower than that in Group C, which could be caused by the high osmotic pressure. (G-I) In the presence of mannitol (75 mmol/L), the proliferation of HUVEC was repressed significantly due to the high osmotic pressure. More importantly, the expression level of CD98 was also inhibited, which was positively correlated with the neuregulin concentration. A. GLU 25 mmol/L+neuregulin 10 ng/L; B. GLU 25 mmol/L+neuregulin 100 ng/L; C. GLU 25 mmol/L+neuregulin 1000 ng/L; D. GLU 75 mmol/L+neuregulin 10 ng/L; E. GLU 75 mmol/L+neuregulin 100 ng/L; F. GLU 75 mmol/L+neuregulin 1000 ng/L; G. Mannitol 75 mmol/L+neuregulin 10 ng/L; H. Mannitol 75 mmol/L+neuregulin 100 ng/L; I. Mannitol 75 mmol/L+neuregulin 1000 ng/L.
Characterization of the expression level of p38 and JNK1/2/3 via Western Bolt analysis to determine the effect of neuregulin intervention on HUVEC apoptosis
To examine the effect of neuregulin on HUVEC apoptosis with or without the addition of mannitol (75 mmol/L), western blot analysis was applied to detect and evaluate the expression levels of p38 and JNK1/2/3. P38 expression in each group had no significant difference. In contrast, neuregulin concentration was positively correlated with JNK proteins.
Discussion
Cardiovascular complications of DM and endothelial cell injury
Dysfunction of the endothelium is regarded as a crucial factor in the pathogenesis of vascular disease in DM [7]. Unfortunately, the prevention of the progression of vascular complications of DM remains pessimistic. Vascular endothelium is defined as a structural layer in adult heart, maintaining the mechanical barrier between blood and vascular smooth muscle cells, regulating vascular contraction and expansion, inflammatory cell adhesion and aggregation. Moreover, vascular endothelium exerts regulatory effect on controlling the platelet function and blood clotting function. Endothelial cells could modulate the degree of vascular tension, vascular inflammation and blood coagulation. Several influential factors are involved in these signaling pathways, including nitric oxide, endothelin, prostacyclin and vascular endothelial growth factor (VEGF).
On the other hand, high level of glucose could repress the endothelial function to some extent, resulting in a significant increase in endothelial cell apoptosis [7]. Hyperglycemia is now considered as the essential factor in endothelial dysfunction, resulting in a high mortality and morbidity [8]. Present studies suggested that a variety of signaling pathways could be involved in this high-glucose induced endothelial dysfunction, such as the expression of adhesion molecules (e.g. vascular cell adhesion molecule-1 and intercellular adhesion molecule-1), adiponectin receptor expression, matrix metalloproteinases and so on [9]. Moreover, oxidative stress, non-enzymatic glycation of proteins, secondary lipid metabolism and protein kinase C activation were also suspected to be the initiative factors of the endothelial dysfunction [10]. However, the causes and mechanisms of coronary atherosclerosis remain to be established and determined.
Kaira K had reported that VEGF and CD98 expression was closely related, suggesting that the specific regulatory effect of VEGF on endothelial cell growth. VEGF could be able to promote angiogenesis and increase vascular permeability [11]. In other words, the increase of VEGF expression would exert promotive effects on endothelial cell growth, playing a key role in maintaining the integrity of the vascular endothelium as well as the normal function of endothelial cells. Our experimental results demonstrated that CD98 expression had a significant influence on normal endothelial functions. Therefore, CD98 expression, which was potential to promote the secretion of VEGF, could be essential in the protection of endothelial cells.
The protective effect of neuregulin on HUVEC
NRG was initially found to be expressed in recombinant Escherichia coli with a molecular weight of 7054 Dal. NRG was shown to have a conserved EGF-like domain, which was used for ligand binding and receptor activation. The NRGs used in this study were purchased from Zensun Company, which were able to produce recombinant human NRGs without losing this critical EGF-domain. Moreover, the expression of NRGs was only detected in microvascular endothelial cells in adult heart, but not in the aorta, coronaries or cardiac veins [12]. As previously described, NRG proteins belong to the EGF family, which are ligands for the ErbB RTKs, acting as essential regulators in heart development and normal heart function.
The RTKs family consists of EGFR, ErbB2, ErbB3, and ErbB4 receptors [13]. Recent studies showed that NRG had several isoforms, including NRG-1, NRG-2, and NRG-3. NRG-1 was mainly expressed in the cardiac microvascular endothelium, promoting cell growth and survival of cardio myocytes in cell culture through the activation of ErbB2 and ErbB4. Therefore NRG-1 was the most well studied NRG isoform due to its essential role in the cell proliferation, migration, differentiation and survival [14]. Hence it was suggested that NRG-1/ErbB signaling network could achieve the protective effect on HUVEC via ErbB4-dependent activation of MAPK pathway.
Many evidences demonstrated that the knockout of ErbB2 and ErbB4 genes in embryonic mice could cause deficiencies of heart muscle, leading to the death of mice [12]. More importantly, knockout of ErbB3 gene in embryonic mice would also cause defects in cardiac valves [12]. Therefore, NRGs, which had a profound role in activating the ErbB2 and ErbB4 receptors in cardiomyocytes, were speculated to be crucial in heart development and maintenance of heart functions.
The control of MAPK signaling pathway by NRGs via regulating CD98 expression
Zhao and Bulus et al. illustrated that endothelial-secreted NRGs had an antioxidative role in response to oxidative stress [15,16]. What’s more, NRGs are capable of exerting inhibitory effects on cell apoptosis, promoting cell proliferation by regulating the downstream PI3K/AKT signaling pathways [17-19]. In addition, Izumi found that the use of ErbB2 antibody would significantly reduce the diameter of tumor vessels in ErbB2 positive tumors. It was obvious that VEGF expression was also decreased to a large extent. More importantly, NRGs could promote the expression of VEGF, which was one of essential signaling molecules in JNK/MAPK signaling pathway. The expressions of VEGF and CD98 were positively correlated [11]. On the other hand, CD98 expression was demonstrated to be positively associated with the concentration of NRGs in the present study. As a result, it was suggested that CD98 expression was potentially up-regulated by the concentration of NRGs, which thereby exerted a regulatory effect on the expression of VEGF to activate the JNK/MAPK signaling pathway, and eventually protected the cardiac endothelial cells.
Bulus, N. et al. [20] also discovered that CD98 could activate MAPK signaling pathway, thereby promoting the proliferation of renal tubular epithelial cells. They used three different siRNAs to reduce the expression of CD98. After one week, the inner medullary collecting duct cells that used siRNA-knockdown were all dead. Moreover, the addition of MAPK inhibitor would also exert inhibitory effects on CD98-positive cell growth. It was evidently that CD98 proteins could regulate the cell proliferation via activating the MAPK signaling pathway [21,22].
In this study, results obtained from confocal microscopy demonstrated that CD98 was detected in different HUVEC groups. The cell growth was optimal under normal glucose concentration. However, the increase of NRG concentration would lead to an increase of osmotic pressure, resulting in a significant repression on cell proliferation and CD98 expression. The expression level of CD98 was detected in HUVEC maintained in 75 mmol/L glucose without the intervention of NRG. It was found to be the lowest, which was 65.9%. In contrast, the increase of NRG concentration (10 ng/L, 100 ng/L, 1000 ng/L) would activate the expression of CD98 to different levels, which were 66.2%, 77.9% and 71.2%, respectively. Hence NRG could potentially recover the impaired CD98 expression in HUVEC, thereby promoting the cell proliferation and protecting the cardiac tissues against high glucose -induced injury.
The Western blot analysis had presented a positive correlation between the CD98 expression and JNK expression, whereas no clear association was determined between CD98 and p38. All these together suggested that the NRG-ErbB network could activate the downstream MAPK signaling pathway as well as the JNK subfamily proteins. Consequently, NRG could exert a protective effect on HUVEC via the activation of CD98 expression, which could be beneficial in treating the cardiovascular complications of DM.
Disclosure of conflict of interest
None.
References
- 1.Setacci C, de Donato G, Setacci F, Chisci E. Diabetic patients: epidemiology and global impact. J Cardiovasc Surg (Torino) 2009;50:263–73. [PubMed] [Google Scholar]
- 2.Kirstine Brown F. Current situation of treatment of diabetes management. Sect Endocrinol Foreig Med Sci. 2005;25:172–173. [Google Scholar]
- 3.Wen D, Suggs SV, Karunagaran D, Liu N, Cupples RL, Luo Y, Janssen AM, Ben-Baruch N, Trollinger DB, Jacobsen VL. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994;14:1909–19. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan X, Morgan JP. Neuregulin1 as novel therapy for heart failure. Curr Pharm Des. 2011;17:1808–17. doi: 10.2174/138161211796391010. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti M, Stocca A, Dapas B, Farra R, Uxa L, Bosutti A, Barazzoni R, Bossi F, Giansante C, Tedesco F, Cattin L, Guarnieri G, Grassi G. Inhibitory effects of fenofibrate on apoptosis and cell proliferation in human endothelial cells in high glucose. J Mol Med (Berl) 2008;86:185–95. doi: 10.1007/s00109-007-0257-3. [DOI] [PubMed] [Google Scholar]
- 6.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saboor M, Moinuddin , Ajmal M, Ilyas S. Functional status of vascular endothelium in diabetes mellitus. J Ayub Med Coll Abbottabad. 2014;26:239–43. [PubMed] [Google Scholar]
- 8.Symons JD, Abel ED. Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide. Rev Endocr Metab Disord. 2013;14:59–68. doi: 10.1007/s11154-012-9235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng SY, Chao TH, Li YH, Liu PY, Lee CH, Cho CL, Wu HL, Chen JH. Cilostazol improves high glucose-induced impaired angiogenesis in human endothelial progenitor cells and vascular endothelial cells as well as enhances vasculoangiogenesis in hyperglycemic mice mediated by the adenosine monophosphate-activated protein kinase pathway. J Vasc Surg. 2015 doi: 10.1016/j.jvs.2014.10.103. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaira K, Takahashi T, Abe M, Akamatsu H, Nakagawa K, Ohde Y, Okumura T, Murakami H, Tsuya A, Nakamura Y, Naito T, Kondo H, Nakajima T, Endo M, Yamamoto N. CD98 expression is associated with the grade of malignancy in thymic epithelial tumors. Oncol Rep. 2010;24:861–7. doi: 10.3892/or.2010.861. [DOI] [PubMed] [Google Scholar]
- 12.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–4. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MR, Linthicum FH Jr. Expression of neuregulin and activation of erbB receptors in vestibular schwannomas: possible autocrine loop stimulation. Otol Neurotol. 2004;25:155–9. doi: 10.1097/00129492-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, Lorell BH. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–12. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- 15.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–46. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–9. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 17.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–9. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–37. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 20.Bulus N, Feral C, Pozzi A, Zent R. CD98 increases renal epithelial cell proliferation by activating MAPKs. PLoS One. 2012;7:e40026. doi: 10.1371/journal.pone.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prager GW, Feral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007;282:24477–84. doi: 10.1074/jbc.M702877200. [DOI] [PubMed] [Google Scholar]
- 22.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci U S A. 2005;102:355–60. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]


