Abstract
Objective: To study the immune mechanism of nourishing kidney and eliminating toxicity decoction (NKETD) on Chronic Hepatitis B (CHB), we detected the serum concentrations of IFN-γ (the characteristic cytokine of Th1), IL-17A (the characteristic cytokine of Th17) and the quantitative proportion of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg in HBV transgenic mice. Methods: The HBV transgenic mice were randomly divided into six groups: high-dose group, middle-dose group, low-dose group, lamivudine group, model control group and normal mice control group. The serum concentrations of IFN-γ and IL-17A in mice were measured by ELISA method and the ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg was detected by Flow Cytometry Method (FCM). Results: The decoction could increase the serum concentration of IFN-γ and decrease that of IL-17A in HBV transgenic mice. The higher the dose was, the more significantly the concentration of IFN-γ increased. And high-dose decoction could decrease the serum concentration of IL-17A in HBV transgenic mice significantly and continuously while middle-dose and low-dose decoction had no significant effects. However, there wasn’t statistically significant variation on the ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg in HBV transgenic mice. Conclusion: The decoction could treat CHB by regulating the immune function by promoting the generation of Th1 and/or enhancing its function while inhibiting Th17. The immune regulation by decoction had more significant effects than lamivudine.
Keywords: Nourishing kidney and eliminating toxicity decoction, Chronic Hepatitis B, IFN-γ, IL-17A, ratio of CD+4CD+25 foxp3 Treg to CD+4 Treg
Introduction
Hepatitis B virus (HBV) infection is still a global health problem. About 400 million people worldwide are chronically infected with the virus, of whom 500,000 die each year from Chronic Hepatitis B (CHB) [1-3]. Interferon and nucleoside analogues (such as lamivudine) have a certain degree of efficacy in treating CHB. But the efficacy of these drugs is limited. For example, short term treatment of lamivudine can inhibit HBV replication by blocking the viral polymerase activity, while long term treatment would induce variations in the YMDD motif of the HBV polymerase and consequently lamivudine resistant strains were detected [4].
The key to eliminating HBV lies in specific cellular immunity [5]. It was suggested that HBV clearance required cellular and humoral immune responses, and non-HBV-specific immune response was possibly associated with liver damage and appeared to initiate the process of hepatic fibrosis [6]. T helper type 1 (Th1) lymphocytes played a pivotal role in virus clearance and liver damage by secreting interferon-gamma (IFN-γ) [7]. Recently, a novel and unique subset of IL-17A producing CD4+ Th (Th17) cell highly enriched in liver of CHB patients has been described as a potential to exacerbate liver damage [8]. In addition, CD4+CD25 + foxp3+ regulatory T (Treg) cell appeared to restrain immune reactivity contributing to limit liver damage [6]. In conclusion, accumulating evidence suggested that the imbalance of Th1, Th17 and Treg played an important role in leading to immune tolerance of HBV [6,9].
The HBV transgenic mice selected in the experiment are mainly used for studying the immunological pathogenesis of HBV and evaluating therapeutic efficacy of anti-HBV drugs, which are a kind of relatively ideal animal model for evaluating and comparing anti-HVB drugs [10].Traditional Chinese Medicine is not apt to increase the risk of drug resistance and its therapeutic efficacy is more stable [11]. The NKETD is one of the Traditional Chinese Medicines and a large number of clinical trials have proved its excellent therapeutic efficacy [11,12].
In this study, we detected the serum concentrations of IFN-γ, IL-17A and the quantitative proportion of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg in HBV transgenic mice to analyze the immune mechanism of the NKETD in treating CHB, and consequently provided a certain theoretical significance for the clinical application of the NKETD.
Material and methods
Chemicals and reagents
170 g NKETD: parasitic loranthus: 20 g; semen cuscutae: 20 g; tu-chung: 10 g; phoenix-tail fern: 20 g; salvia miltiorrhizae: 20 g; gardenia fructus: 20 g; phyllanthus ruinaria: 30 g; radix fici hirtae: 20 g; pulp of cornus: 10 g; All the above medicinal materials were purchased from No. 1 Hospital Affiliated to Guangzhou University of Traditional Chinese Medicine and have been identified by pharmaceutical department.
Mouse IFN-γ Elisa Kit (Joyee Biological Technology Co., Ltd., Shanghai); Mouse IL-17A Elisa Kit (Joyee Biological Technology Co., Ltd., Shanghai); Phosphate Buffer Solution (Double Helix Biological Technology Co., Ltd., Shanghai); Mouse Peripheral Blood CD+ 4CD+ 25 foxp3 Treg Kit (eBioscience, US); Blood Cytolysate (BD Medical Instrument Co., Ltd., Shanghai); Lamivudine: 100 mg/tablet (Glaxo Smith Kline Limited); Normal saline: 250 ml: 2.25 g (Sichuan Kelun Pharmaceutical Co., Ltd., China). iMark Automatic Microplate Reader (Bio-RAD; US); FCM (BD Medical Instrument Co., Ltd., Shanghai).
Experimental animals
50 HBV transgenic mice; 10 normal mice (serum tests HBV-DNA negative). These were provided by Zhuangzhou Air Force Hospital Liver Disease Research Center.
Animal treatment
50 HBV transgenic mice were randomly divided into model control group, lamivudine group, high-dose decoction group, middle-dose decoction group and low-dose decoction group. Then take another 10 normal mice as normal control group.
Body surface area converting method combined with actual situation was applied for calculating feeding volume. The feeding volume was set as follows: male mice (> 25 g): 0.25 ml/day; female mice or male mice (< 25 g): 0.20 ml/day; concentrations of high-dose, middle-dose and low-dose groups were set respectively as 2 g, 1 g, 0.5 g/ml. Lamivudine group: 0.02 mg (lamivudine)/g (weight of mice). Model control group and normal control group: normal saline 0.2 ml/d or 0.25 ml/d.
Specimen collection
Collect the blood behind the eyeballs at 30th day and 60th day respectively. Get 200 μl whole blood and 100 μl whole blood respectively from each mouse. The 200 μl whole blood was for ELISA experiment. The 100 μl whole blood was for immediate FCM detection.
Concentration detection of IFN-γ and IL-17A
Details of the techniques are provided in the Supplementary Methods I. In brief, add standard solutions (100 μl/hole) of different concentrations into corresponding holes, and add buffer solutions (50 μl/hole) and serum samples (50 μl/hole) into sample holes for sample analysis. After series of sample preparation, the OD450 value of these samples was immediately measured by ELIASA. The standard curve and its function formula can be evaluated through Microsoft Excel software.
The experimental preparations and detection procedures for IL-17A are the same as IFN-γ.
Detection procedure of the Ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg
Details of the techniques are provided in the Supplementary Methods II. In brief, Add CD4 antibodies (2 μl) and CD25 antibodies (2 μl) into flow-type tube, then add mouse anticoagulant whole blood (100 μl) respectively and let them react in a dark place at 4°C for half an hour. After series of sample preparation, these samples were immediately detected by FCM.
Statistical analysis
All data were reported as mean ± standard derivation and analyzed using the Student’s t test. The value of P < 0.05 was regarded as statistically significant. The data were analyzed statistically by SPSS 17.0 multivariate statistical analysis software.
Results and discussion
Treatment of NKETD decreased the serum IFN-γ concentration
The standard curve of IFN-γ standard solution is y = 398.42x2 + 153.11x - 9.3986, R2 = 0.9994. Since the serum IFN-γ concentration in the model control group increased significantly (P < 0.05) compared to that in normal control group, we suggested that HBV infection could induce the secretion of IFN-γ and consequently these cytokines could promote the immune system. After treatment of NKETD, the serum IFN-γ concentrations in high-dose, middle-dose and low-dose group all increased significantly (P < 0.05) compared to that in the model control group respectively. It was suggested that NKETD could further introduce the secretion of serum IFN-γ in HBV transgenic mice. All the serum IFN-γ concentrations in the three groups increased significantly (P < 0.05) compared to that in lamivudine group. However, the serum IFN-γ concentration in lamivudine group exhibited no significant variation compared to that in model control group, which suggested that NKETD had an advantage in treating CHB in the respect of immune function relative to lamivudine (classical anti-HBV drug). IFN-γ was the characteristic cytokine of Th1 [13], which suggested that NKETD could promote the generation of Th1 and/or enhance its function (Figure 1). Substantial evidence existed to support that Th1-type cytokines were selectively secreted during HBV infection, and Th1-mediated effects contributed to liver cell injury and eradication of HBV infection [7,14,15]. Our findings from this study further supported that Th1-mediated effects were highly relevant to HBV infection.
Figure 1.
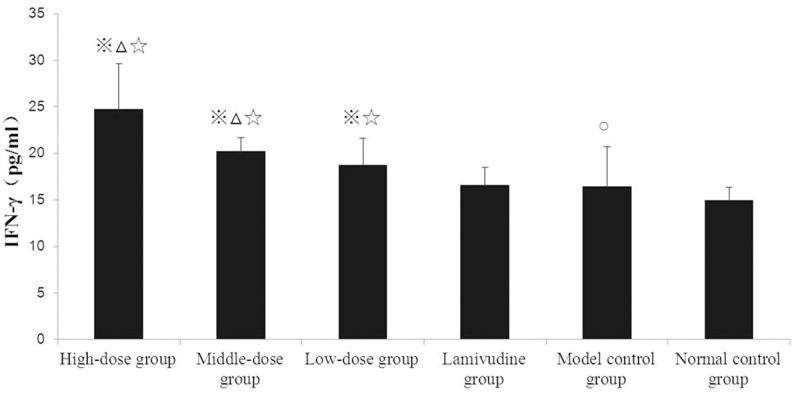
Comparison of Serum IFN-γ Concentration in Each Group at 30th day. Notes: 1. ※P < 0.05 compared to model control group; 2. ΔP < 0.05 compared to low-dose group; 3. ○P < 0.05 compared to normal control group; 4. ☆P < 0.05compared to lamivudine group.
Treatment of NKETD decreased the serum IL-17A concentration
(1) At 30th day, the standard curve of IL-17A standard solution is y = 73.818x2 + 133.2x - 10.4, R2 = 0.999. The comparisons among groups at 30th day: The serum IL-17A concentration in model control group increased significantly (P < 0.05) compared to that in normal control group. The serum IL-17A concentrations in high-dose, middle-dose, low-dose group and lamivudine group all decreased significantly (P < 0.05) compared to that in model control group respectively. The serum IL-17A concentrations in high-dose and middle-dose group decreased significantly (P < 0.05) compared to that in low-dose group respectively. The serum IL-17A concentrations in high-dose and middle-dose group decreased significantly (P < 0.05) compared to that in lamivudine group respectively (Figure 2).
Figure 2.
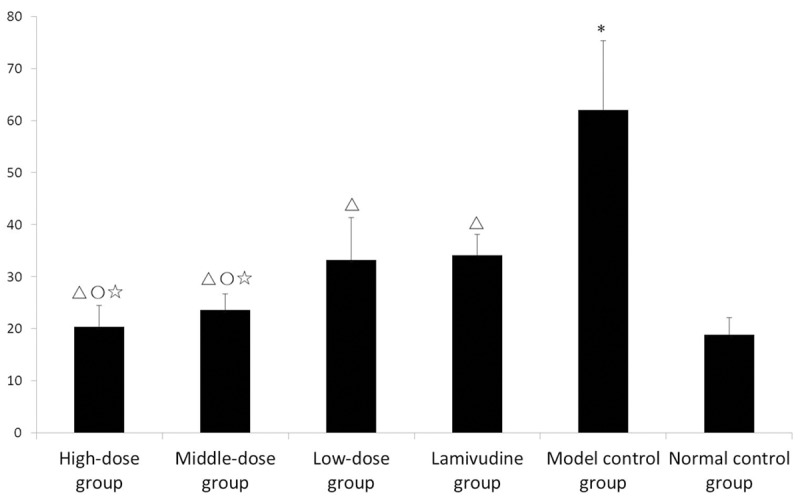
Comparison of Serum IL-17A Concentration in Each Group at 30th day (X̅ ± s). Notes: 1. *P < 0.05 compared to normal control group; 2. ΔP < 0.05 compared to model control group; 3. ○P < 0.05 compared to low-dose group; 4. ☆P < 0.05 compared to lamivudine group.
(2) At 60th day, the standard curve of IL-17A standard solution is y = 153.32x2 + 114.35x - 8.7218, R2 = 0.999. The comparisons among groups at 60th day: The serum IL-17A concentration in model control group increased significantly (P < 0.05) compared to that in normal control group. The serum IL-17A concentrations in high-dose, middle-dose, low-dose group and lamivudine group all decreased significantly (P < 0.05) compared to that in model control group respectively. The serum IL-17A concentrations in high-dose and middle-dose decreased significantly (P < 0.05) compared to that in low-dose group respectively. The serum IL-17A concentrations in high-dose and middle-dose decreased significantly (P < 0.05) compared to that in lamivudine group respectively (Figure 3).
Figure 3.
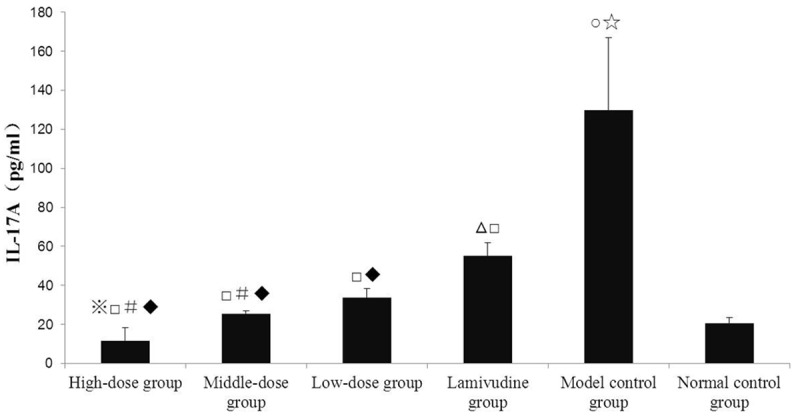
Comparison of serum IL-17A concentration in each group at 60th day was showed and serum IL-17A concentration in each group at 60th Day was compared to that at 30th day in Figure 2. Notes: 1. ※P < 0.05 compared to high-dose group in Figure 2; 2. ΔP < 0.05 compared to lamivudine group in Figure 2; 3. ○P < 0.05 compared to model control group in Figure 2; 4. ☆P < 0.05 compared to normal control group in Figure 2; 5. □P < 0.05 compared to model control group in Figure 2; 6. #P < 0.05 compared to low-dose group in Figure 2; 7. ♦P < 0.05 each level of decoction compared to lamivudine group in Figure 3.
(3) Comparisons between 30th day and 60th day in the same group: The serum IL-17A concentration in high-dose group at 60th day decreased significantly (P < 0.05) compared to that at 30th day. The serum IL-17A concentration in lamivudine group at 60th day increased significantly (P < 0.05) compared to that at 30th day. The serum IL-17A concentration in model control group at 60th day increased significantly (P < 0.05) compared to that at 30th day (Figure 3).
By comparing model control group with normal control group, the serum IL-17A concentration in the former increased significantly, which suggested that HBV infection could significantly promote the secretion of IL-17A. Compared to the serum IL-17A concentration in model group, all these concentrations of groups significantly decreased to different degrees after the NKETD treatment or lamivudine treatment, which could be caused by immune regulation of NKETD or effect of lamivudine on HBV respectively. The higher the concentration of the NKETD was, the more significantly the serum IL-17A concentration decreased. Although the low-dose group exhibited no significant variation compared to lamivudine group, the serum IL-17A concentrations in high-dose and middle-dose group significantly decreased, which also suggested that the NKETD had an advantage in treating CHB in the respect of immune function relative to lamivudine. It was suggested that IL-17A was associated with neutrophil-mediated liver damage during HBV infection and IL-17A might exacerbate persistent liver inflammation and hepatic injury [6]. In addition, IL-17A was the characteristic cytokine of Th17 [8], which was increased in patients with hepatitis B and contributed to further deterioration of disease progression [16]. Our observation confirmed the interesting interaction between IL-17A and HBV infection. However, the detailed immune mechanism during HBV infection remained to be elucidated.
Change of Ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg was not statistically significant
The results of FCM were performed in Supplementary Figures 1-6. The ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg in model control group was not statistically significant (P > 0.05) compared to that in normal control group, but it tended to decreased. And the ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg in high-dose and middle-dose group increased slightly compared to that in model control group, however, it was not statistically significant (P > 0.05) (Table 1). CD4+ T lymphocytes served a critical role in immune regulation, which was regarded as a highly heterogeneous population of cells characterized by the ability to secrete various types of cytokines and to assist other immune cells [17]. However, it was suggested that a particular process like tissue damage could not be explained by single cytokine, because there were complex pathophysiological cytokine networks in tissue lesions [6,18], and NKETD was reported to be associated with Th1 and Th17, which indicated that the immune mechanism of NKETD in treating CHB was worthy of further investigation.
Table 1.
The Ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg in Each Group at 60th day (X̅)
| Group | n | Ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg (%) |
|---|---|---|
| High-dose group | 8 | 5.99 |
| Middle-dose group | 8 | 5.92 |
| Low-dose group | 9 | 5.57 |
| Lamivudine group | 7 | 4.88 |
| Model control group | 6 | 5.84 |
| Normal control group | 8 | 6.05 |
Notes: 1. n: the number of the mouse in the group.
Traditional Chinese Medicine is regarded as maintenance therapy and has increasingly become popular in the west, which has been explored widely [19]. Our findings from this study have confirmed that Traditional Chinese Medicine can exhibit more effective than some interferon and nucleoside analogues in some respects.
Conclusion
The NKETD could regulate the immune function in treating CHB probably by promoting the generation of Th1 and/or enhancing its function while inhibiting the function of Th17. It had a more significant effect than lamivudine on immune regulation.
Supplementary methods
Supplementary methods I: concentration detection of IFN-γ and IL-17A
Prepare IFN-γ standard solution and dissolve it into distilled water: 1000 pg/ml, 500 pg/ml, 250 pg/ml, 125 pg/ml and 62.5 pg/ml. The concentrations of IL-17A standard: 62.5 pg/ml, 31.25 pg/ml, 15.63 pg/ml, 7.813 pg/ml and 3.906 pg/ml. Add standard solutions (100 μl/hole) of different concentrations into corresponding holes, add buffer solutions (50 μl/hole) for sample analysis into sample holes, add serum samples (50 μl/hole) into each sample hole, and then incubate them at room temperature of 25 to 28°C for two hours. After that, add diluted cleaning solutions (300 μl/hole), keep at least 15-second standing and pour the solution inside out. Clean the troughs 5 times. Put the plate on the thick absorbent paper and pat dry after rinsing the plate for the last time. Then add biotinylated antibody treatment fluid (100 μl/hole), seal the holes with gummed paper and incubate at room temperature for an hour. Add diluted cleaning solutions (300 μl/hole), keep at least 15-second standing and pour the solution inside out. Clean the troughs 5 times. Put the plate on the thick absorbent paper and pat dry after rinsing the plate for the last time. Then add enzyme bonder treatment fluid (100 μl/hole), seal the holes with gummed paper and incubate at room temperature for an hour. Add diluted cleaning solutions (300 μl/hole) in, keep at least 15-second standing and pour the solution inside out. Clean the troughs 5 times. Put the plate on the thick absorbent paper and pat dry after rinsing the plate for the last time. Then add TMB chromogenic agent (100 μl/hole) and incubate in a dark place at room temperature for 20 minutes. Then add stop buffer (50 μl/hole) and immediately put the plate into the ELIASA to measure OD450 value. The standard curve and its function formula can be evaluated through Microsoft Excel software and then their concentrations can be calculated respectively according to the standard curve.
The experimental preparations and detection procedures for IL-17A and IFN-γ are the same
Supplementary methods II: detection procedure of the ratio of CD+ 4CD+ 25 foxp3 Treg to CD+ 4 Treg
Add CD4 antibodies (2 μl) and CD25 antibodies (2 μl) into flow-type tube, then add mouse anticoagulant whole blood (100 μl) respectively and let them react in a dark place at 4°C for half an hour. Later, add diluted erythrocyte lysate (1 ml) (dissolved in ultrapure water, the volume ratio of it to ultrapure water is 1:5) and let them react for 5 minutes. Centrifuge them after adding PBS buffer solution (2 ml) (1500 rpm/min, 5 min/time, centrifugal temperature: 20°C, similarly hereinafter). After centrifugation, pour most of solution inside the flow-type tube out, add PBS buffer solution (2 ml) and re-centrifuge. Then pour the solution out, add intraprep permeabilization reagent (1 ml) (v/v (eBioscience Fixation/Permeabilization Concentrate: eBioscience Fixation/Permeabilization Diluent) = 1:3) and let them react for 6 hours. Add staining buffer (2 ml) (eBioscience Permeabilization Buffer dissolved in ultrapure water, v:v = 1:9) and then centrifuge. After centrifugation, pour the solution inside the flow-type tube out, add PBS buffer solution (2 ml), and then re-centrifuge. Pour the solution out, add Anti-Mouse CD16/32 (1 μl/tube) and let them react in a dark place at 4°C for 15 minutes. Then add foxp3 antibody (2 μl/tube) and let them react in a dark place at 4°C for 30 minutes. Add staining buffer (2 ml) and centrifuge. After centrifugation, pour the solution inside the flow-type tube out, add PBS buffer solution (2 ml) and re-centrifuge. Then, pour the solution out, add staining buffer (400 μl/tube), and use FCM to detect.
Acknowledgements
We acknowledge the National Natural Science Foundation of China (Grant No. 81173255) for financial support. We thank the Laboratory of National Key Discipline of Clinical Foundation of Chinese Medicine and the ‘Program 211’ of Guangdong province at the Guangzhou University of Chinese Medicine for providing some laboratory facilities used in this study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:13–21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 4.Degertekin B, Hussain M, Tan J, Oberhelman K, Lok AS. Sensitivity and accuracy of an updated line probe assay (HBV DR v. 3) in detecting mutations associated with hepatitis B antiviral resistance. J Hepatol. 2009;50:42–48. doi: 10.1016/j.jhep.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J, Blum HE, Thimme R. T-cell responses in hepatitis B and C virus infection: similarities and differences. Emerging Microbes Infections. 2013;2:e15. doi: 10.1038/emi.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye YF, Xie XJ, Yu JW, Zhou L, Xie HY, Jiang GP, Yu XB, Zhang WJ, Wu JA, Zheng SS. Involvement of Th17 and Th1 Effector Responses in Patients with Hepatitis B. J Clin Immunol. 2010;30:546–555. doi: 10.1007/s10875-010-9416-3. [DOI] [PubMed] [Google Scholar]
- 7.Penna A, Del Prete G, Cavalli A, Bertoletti A, D’Elios MM, Sorrentino R, D’Amato M, Boni C, Pilli M, Fiaccadori F, Ferrari C. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology. 1997;25:1022–1027. doi: 10.1002/hep.510250438. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, Shi F, Shi M, Wang HF, Wang FS. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- 9.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbar SK, Onji M. Hepatitis B virus (HBV)-transgenic mice as an investigative tool to study immunopathology during HBV infection. Int J Exp Pathol. 1998;79:279–291. doi: 10.1046/j.1365-2613.1998.740406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng JH, Shen Q, Liu YM, Xu QY, Wu Y, Wang D. Effect of nourishing the kidney and clearing toxicity decoction on phenotypes and functions of dendritic cells derived from peripheral blood monocytes of chronic hepatitis B patients. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases. 2012;22:348–351. [Google Scholar]
- 12.Huang Y, Shen Q, Liu YM, Peng JH, Xu QY, Zeng ZQ, Yuan Y, li XP. Clinical observation of chronic hepatitis B based on the nourishing kidney and eliminating toxicity decoction. Journal of New Chinese Medicine. 2010;42:31–32. [Google Scholar]
- 13.Xu WR, Yang GW, Xu YM, Zhang Q, Fu Q, Yu J, Yu MW, Zhao WS, Yang Z, Hu FS, Han D, Wang XM. The possibility of traditional chinese medicine as maintenance therapy for advanced nonsmall cell lung cancer. Evid Based Complement Alternat Med. 2014;2014:278917. doi: 10.1155/2014/278917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J Immunol. 2002;168:6165–6172. doi: 10.4049/jimmunol.168.12.6165. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Ohta A. A critical role for antigen-specific Th1 cells in acute liver injury in mice. J Immunol. 1999;162:6503–6509. [PubMed] [Google Scholar]
- 16.Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396–403. doi: 10.1111/j.1365-2893.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 17.Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, Tang Z, Fu YX, Wang FS. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 18.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 19.Qin XY, Cui J, Zhang Y. Coeloglossum viride var. bracteatum extract protects against amyloid toxicity in rat prefrontal cortex neurons. Int J Clin Exp Med. 2010;3:88–94. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


