Abstract
Aim: To document the clinicopathological characteristics and analyze the possible reasons for misdiagnosis or missed diagnosis of hepatoid adenocarcinoma of the stomach (HAS), using data from a single center. Methods: We retrospectively analyzed 19 patients initially diagnosed as HAS and 7 patients initially diagnosed as common gastric cancer with high levels of serum α-fetoprotein (AFP). All had undergone surgical treatment, except 3 patients only had biopsies at our hospital. Immunohistochemistry for AFP and Hepatocyte antigen was performed. Final diagnosis for these 26 patients were made after HE and immunohistochemistry slides reviewed by 2 experienced pathologists. Prognostic factors were determined by univariate analysis. Results: Nineteen cases were confirmed to be HAS. A total of 4 out of 19 cases initially diagnosed as HAS and 4 out of 7 cases initially diagnosed as common gastric adenocarcinoma were misdiagnosed/missed diagnosed, thus, the misdiagnosis/missed diagnosis rate was 30.8% (8/26). The incidence of HAS among gastric cancer in our center was 0.19% (19/9915). Sixteen (84.2%) patients showed T stages greater than T2, 12 (70.6%) patients had positive lymph nodes in 17 available patients and 3 (15.8%) of the patients with tumors presented liver metastasis at the time of diagnosis. Histologically, cytoplasmic staining types included 10 cases of eosinophilic, 1 case of clear, 5 cases of clear mixed with eosinophilic and 3 cases of basophilic. Fourteen (73.7%) patients expressed AFP, whereas only 6 (31.6%) were hepatocyte-positive. Univariate analysis showed that N stage (HR 2.429, P=0.007) and tumor AFP expression (HR 0.428, P=0.036) were significantly associated with disease-free survival. The median overall survival time was 12.0 months, and the median disease-free survival time was 7.0 months. Four (80%) of 5 N0 patients and 2 (50%) of 4 N1 patients survived without progression, but no N2-3 patients survived. Conclusion: HAS remains easily being misdiagnosed/missed diagnosed based on a pathological examination, probably because the condition is rare and has various cytoplasmic types. Although the survival rate for HAS is poor, a curative effect may be achieved for N0 or N1 cases.
Keywords: Stomach, hepatoid adenocarcinoma, α-fetoprotein (AFP), diagnosis, survival
Introduction
Gastric cancer has become the second most common type of tumor worldwide [1]. In this study, we focused on a special subtype of gastric cancer, hepatoid adenocarcinoma of the stomach (HAS). This disease has an extremely low incidence rate (0.3% of all gastric tumors) but is associated with a notably worse prognosis than other gastric tumors [2]. HAS was first reported by Ishikura as a case report in 1985 [3]. Since then, a consensus has been reached that the condition is a special type of carcinoma that histologically resembles hepatocellular carcinoma (HCC). HAS is typically associated with high level of α-fetoprotein (AFP) in the serum [4]. Several studies have reported that HAS can easily be misdiagnosed/missed diagnosed based on a pathological analysis. Here, we aimed to document the clinicopathological characteristics and analyze the possible reasons for misdiagnosis or missed diagnosis of hepatoid adenocarcinoma of the stomach (HAS) at our center.
Materials and methods
Patients and diagnostic criteria
Between January 1999 and January 2014, a total of 9915 cases of gastric cancer were surgically treated at our center. We selected gastric cancer patients with an initial pathological diagnosis of HAS (19 cases) or with a serum AFP level above 100 ng/ml (7 cases) to be included in this study (Table 1). All had undergone surgical treatment, except 3 patients only had biopsies at our hospital. The cohort included 4 women and 22 men with a mean age of 62.5 years (range, 41-80 years). This study was approved by the Institutional Review Board of Cancer Hospital and Institute, Chinese Academy of Medical Sciences and Peking Union Medical College.
Table 1.
Data of 26 patients diagnosed previously as HAS or common adenocarcinoma with high serum AFP level
| No. | Age | Sex | Previous diagnosis | Serum AFP (ng/ml) | Confirmed diagonsis | Hepatoid area ratio | IHC | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AFP | Hepatocyte antigen | |||||||
| 1 | 57 | Female | HAS mainly | NA | HAS | 90% | ++ | - |
| 2 | 58 | Male | HAS minor | NA | HAS | 10% | + | - |
| 3 | 74 | Male | HAS minor | 555.2 | HAS | 10% | ++ | + |
| 4 | 69 | Male | HAS partial | 81 | HAS | 60% | - | + |
| 5 | 70 | Male | HAS partial | 550 | HAS | 90% | + | - |
| 6 | 64 | Female | HAS partial | NA | HAS | 90% | + | + |
| 7 | 71 | Male | HAS partial | NA | HAS | 50% | - | - |
| 8 | 61 | Male | HAS partial | NA | HAS | 30% | - | - |
| 9 | 67 | Male | HAS partial | NA | HAS | 90% | - | + |
| 10 | 63 | Male | HAS partial | NA | HAS | 30% | ++ | - |
| 11 | 59 | Male | HAS major | NA | HAS | 30% | + | - |
| 12 | 58 | Male | HAS major | NA | HAS | 90% | - | - |
| 13 | 41 | Male | HAS major | 4375 | HAS | 90% | ++ | - |
| 14 | 71 | Male | HAS major | NA | HAS | 80% | + | + |
| 15 | 53 | Male | HAS major | NA | HAS | 10% | ++ | - |
| 16 | 58 | Male | HAS major | 2.01 | common | NA | - | - |
| 17 | 78 | Male | HAS partial | NA | common | NA | - | + |
| 18 | 56 | Female | HAS minor | NA | common | NA | - | + |
| 19 | 80 | Male | HAS partial | NA | common | NA | - | + |
| 20 | 66 | Male | common | 382.3 | HAS | 70% | +++ | + |
| 21 | 60 | Male | common | 2813 | HAS | 90% | + | - |
| 22 | 49 | Male | common | 268.8 | HAS | 90% | + | - |
| 23 | 54 | Female | common | 499.6 | HAS | 90% | ++ | - |
| 24 | 61 | Male | common | 919.5 | common | NA | - | - |
| 25 | 66 | Male | common | 1611 | common | NA | - | - |
| 26 | 62 | Male | common | 280.8 | common | NA | - | - |
IHC, immunohistochemistry; HAS, hepatoid adenocarcinoma; NA, not associated.
Archival formalin-fixed and paraffin-embedded tumor blocks were used to perform immunohistochemistry of AFP and Hepatocyte antigen (HepPar1) in all cases (Table 1). Assessments of maximal tumor size were obtained from previous pathology reports. Histological characteristics were performed independently by two senior pathologists. The pathological diagnosis of HAS was solely determined by morphological characteristics, regardless of serum or tissue AFP levels [5]. The morphological diagnostic criteria included the existence of a solid area that was distinct from the glandular area, with polygonal cells with rich cytoplasm that were arranged in a medullary or trabecular manner. Survival data were obtained by reviewing clinical or follow-up records. Additionally, preoperative and postoperative chemotherapy regimens were assessed.
Surgical procedure
If the tumor was located at the cardia and infiltrated in the lower part of the esophagus, a resection with esophageal-stomach anastomosis under the bow was performed. Otherwise, a total gastric resection or distal gastric resection was performed. Liver metastases were resected if an R0 resection could be achieved; otherwise, patients were treated with an ethanol injection.
Statistical analyses
All statistical analyses were performed using the software program IBM SPSS statistics 21 (IBM Corp., Armonk, NY, United States). Disease-free survival and overall survival times were estimated using the Kaplan-Meier method. Univariate tests were performed using the Cox regression method.
Results
Confirmed diagnosis and histological characteristics of the patient cohort
To analyze the misdiagnoses of HAS, we selected patients with an initial pathological diagnosis of HAS and patients with a serum AFP level greater than 100 ng/ml but without an initial HAS diagnosis. Serum AFP was tested in 3.5% (347/9915) of all surgically treated gastric cancer patients; 10 of these patients, including 3 that were initially diagnosed as HAS, had a serum AFP level greater than 100 ng/ml. After reviewing, 4 out of 19 patients initially diagnosed as HAS were confirmed as common adenocarcinoma and 4 out of 7 patients with an elevated serum AFP level initially diagnosed as common adenocarcinoma were confirmed as HAS. Thus, the misdiagnosis/missed diagnosis rate was 30.8% (8/26). The incidence of HAS in all of the primary gastric cancer cases was 0.19% (19/9915). Microscopically, 3 cases had basophilic cytoplasm, 10 cases had eosinophilic cytoplasm, 1 case of clear and 5 cases had clear mixed with eosinophilic cytoplasm (Figure 1). One patient with clear mixed with eosinophilic staining (patient 20) and the 3 cases with basophilic staining (patients 21, 22, 23) were initially diagnosed as common adenocarcinoma (missed diagnosis). Two patients (patients 5 and 24) had high serum AFP levels before undergoing neoadjuvant chemotherapy and serum AFP levels decreased after treatment. Microscopically, neither of them had obvious tumor regression. Patient 5 had hepatoid adenocarcinoma of the stomach (Figure 2A), and patient 24 had common adenocarcinoma of the stomach (Figure 2B). Unfortunately, the biopsies before the neoadjuvant chemotherapy were unavailable, as they were performed in another hospital. One patient (patient 21) was diagnosed with common adenocarcinoma by liver biopsy and was later confirmed as having HAS (Figures 3 and 6). Four cases misdiagnosed as HAS (patients 17, 18, 19 and 20) were diagnosed as common adenocarcinoma after review; 3 of these cases were positive for hepatocyte antigen (Figure 4). A total of 10 patients had a serum AFP level greater than 100 ng/ml, including 7 confirmed as HAS with positive tumor AFP expression (Figure 5A and 5B), and 3 patients confirmed as common adenocarcinoma with negative tumor AFP expression (Figure 5C and 5D).
Figure 1.
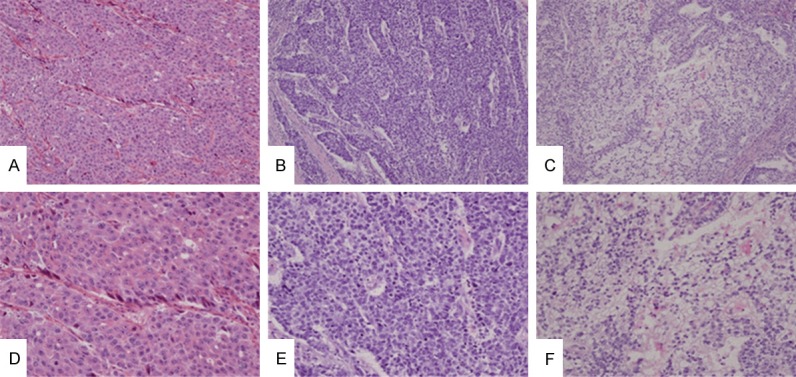
Hepatoid adenocarcinoma of stomach has large and polygonal cells with abundant eosinophilic (A and D), basophilic (B and E), or clear (C and F) cytoplasms. These cells are often arranged in a trabecular pattern. These features are indistinguishable from those of hepatocellular carcinomas (A-C, ×100; D-F, ×200, H&E) (D is the higher power of A, E is the higher power of B and F is the higher power of C, respectively).
Figure 2.
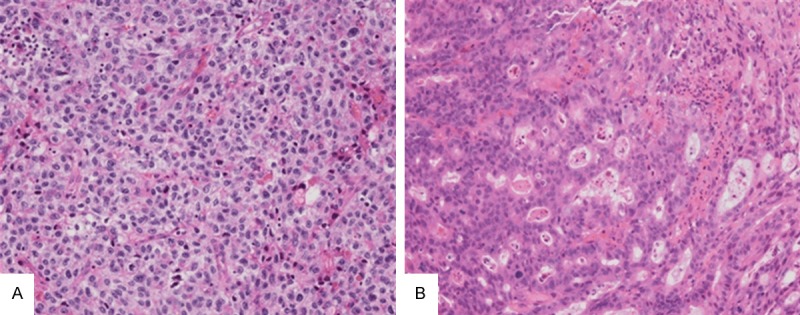
Two cases of stomach adenocarcinoma had neoajuvant chemotherapy. Neither of them had obvious tumor regression. A Shows a hepatoid adenocarcinoma of stomach, which had large and polygonal cells with abundant clear and weakly eosinophilic cytoplasms, arranging in a trabecular pattern. B Shows a conventional adenocarcinoma of stomach, which had high serum AFP level before neoajuvant chemotherapy, and a decreased serum AFP level after neoajuvant chemotherapy, but abundant sampling did not find any hepatoid adenocarcinoma components. The biopsy before neoajuvant chemotherapy was not available to be reviewed because it was taken in a local hospital (×200, H&E).
Figure 3.
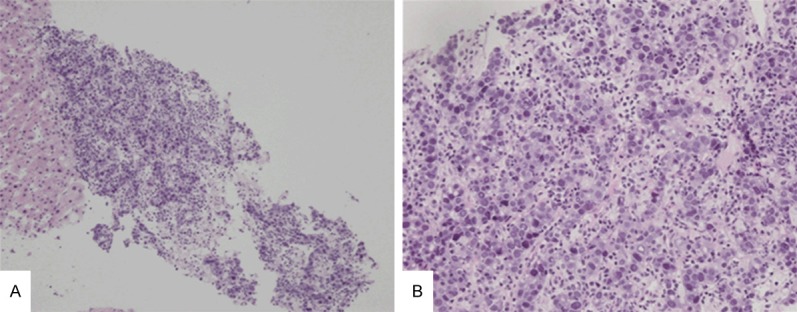
A liver biopsy of liver metastasis lesion of hepatoid adenocarcinoma of stomach. The tumor has large and polygonal cells with abundant basophilic cytoplasms, arranging in a trabecular pattern. (A, ×100; B, ×200, H&E) (B is the higher power of A).
Figure 6.
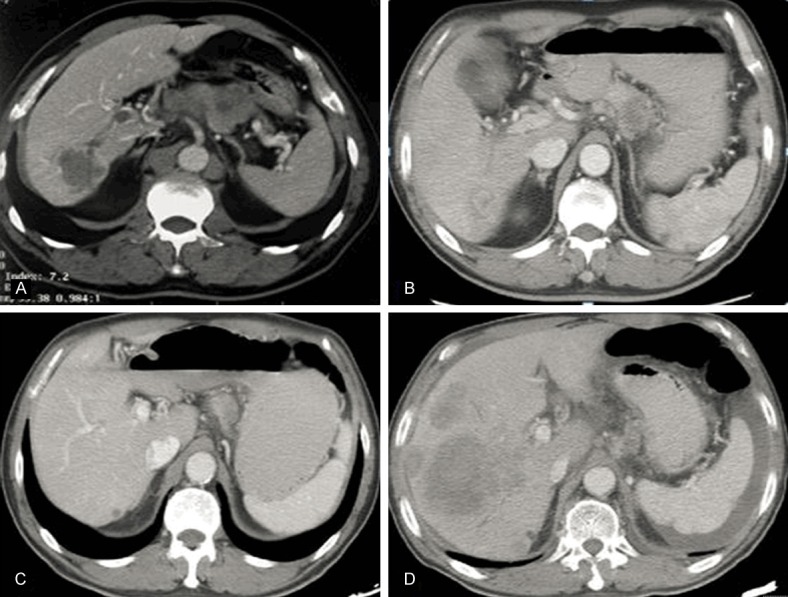
A case of hepatoid adenocarcinoma with liver metastasis and perigastric lymph node enlargement before chemotherapy (A). Both the liver metastasis and perigastric lymph node shrunk at 2 months after chemotherapy with ABRAXANE plus S-1 (B). (C) The tumor responded well to the therapy at 14 months after chemotherapy with ABRAXANE plus S-1 for 9 cycles and Folfri for 10 cycles (C). The tumor progressed after radiotherapy at 17 months (D).
Figure 4.
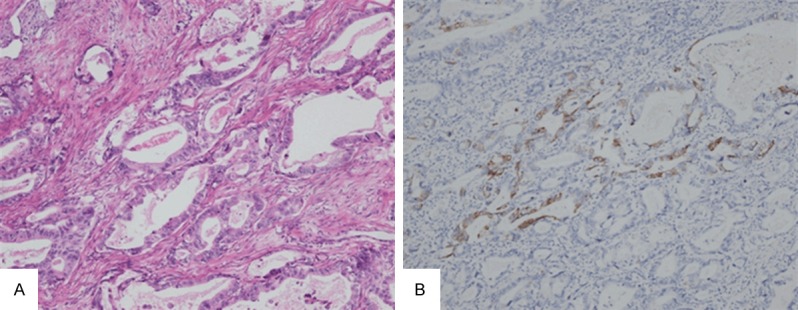
A gastric adenocarcinoma is mainly composed with glands, previously diagnosed as hepatoid adenocarcinoma probably because of weak AFP staining. But after repeating AFP immunohistochemistry (it was truly negative), it was diagnosed as common adenocarcinoma of stomach. It has focal Hepatocyte antigen staining (A, ×200, H&E; B, ×200, immunohistochemistry for Hepatocyte antigen).
Figure 5.
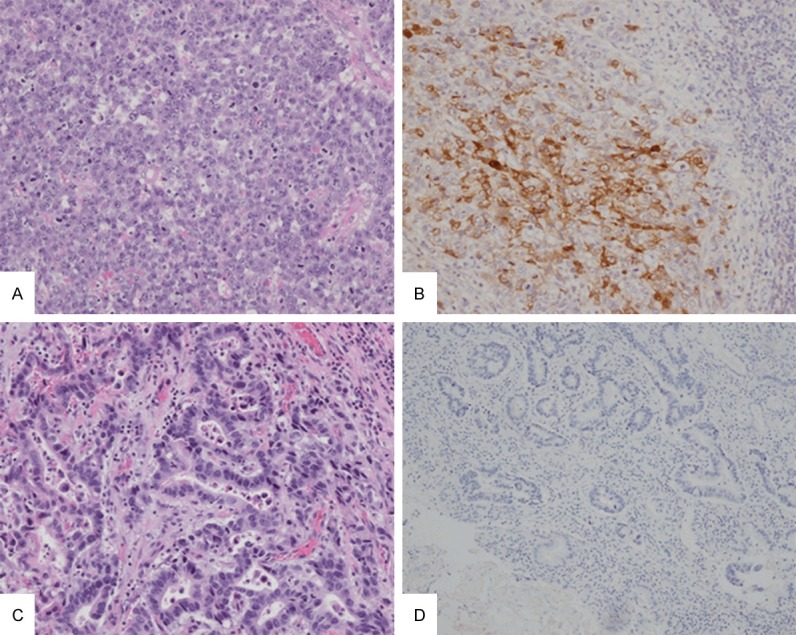
Both of hepatoid adenocarcinoma and common adenocarcinoma of stomach can have increased serum AFP level. A and B show a hepatoid adenocarcinoma with both increased serum AFP level and positive AFP staining. C and D show a morphologically common stomach adenocarcinoma with negative AFP staining, although it had increased serum AFP level (A and C, ×200, H&E; B and D, ×200, immunohistochemistry for AFP).
Clinicopathological characteristics
Table 2 summarizes the clinicopathological characteristics of the 19 patients with a final pathological diagnosis of HAS. These patients included 16 men and 3 women (ratio, 5.33:1). The tumors were located in the following regions: 8 (42.1%) cardia, 7 (36.8%) body and 4 (21.1%) antrum. Angiolymatic invasion was observed in 10 (55.6%) of the 18 available patients. Sixteen (84.2%) patients had T stages greater than T2. Twelve (70.6%) patients had lymph node metastasis in 17 available patients. Three (15.8%) of the patients presented liver metastasis at the time of diagnosis. Immunohistochemically, 14 (73.7%) of these patients expressed AFP, whereas only 6 (31.6%) were hepatocyte-positive. For two patients (cases 12 and 21), the primary tumors were not removed, so these two cases were excluded from further analysis. The mean age of the remaining 17 patients was 62.1 years, and there was no significant correlation between age and tumor recurrence (95% CI 0.980-1.146, P=0.147). The mean tumor size was 6.6 cm (range, 2.5-11 cm), and there was no significant correlation between tumor size and tumor recurrence (95% CI 0.751-1.382, P=0.906). Only one patient was diagnosed by health examination without any symptoms. The remaining patients had symptoms, including abdominal pain, dysphagia, fatigue and abdominal distention. Curative resection was conducted on 16 patients, including 7 cases of resection with esophageal-stomach anastomosis under the bow, 5 cases of total gastrectomy, 3 cases of distal gastrectomy and 1 case of subtotal gastrectomy plus liver metastasis resection. One patient with liver metastasis was treated with distal gastrectomy plus ethanol injection. Twelve patients were treated with adjuvant chemotherapy, but there was no significant correlation between adjuvant chemotherapy and tumor recurrence (95% CI 0.162-1.758, P=0.302).
Table 2.
Data of 19 patients confirmed as HAS
| No. | Location of tumor | Tumor size (cm) | T stage | Lymph-vascular invasion | Lymph node positive/total | Treatment | Site of progress |
|---|---|---|---|---|---|---|---|
| 1 | Cardia | 6 | 3 | + | 0/38 | R0 resection + OXA, CF, 5-Fu, 6 cycles | NA |
| 2 | Cardia | 10 | 2 | - | 4/31 | R0 resection + cetuximab+CDDP,Xeloda 4 cycles | Local |
| 3 | Cardia | 8 | 3 | + | 0/21 | R0 resection | Local |
| 4 | Cardia | 2.5 | 4a | - | 2/11 | R0 resection + Paclitaxel liposome, OXA, CF, 5-Fu 6 cycles | Liver |
| 5 | Body | 6 | 3 | - | 5/18 | Neoadjuvant DDP, 5-Fu 2 cycles+ R0 resection + TXT, L-OHP, 5Fu 6 cycles | Liver |
| 6 | Cardia | 5 | 3 | + | 1/11 | R0 resection + OXA, CF, 5-Fu, 6 cycles | NA |
| 7 | Antrum | 5 | 3 | + | 11/17 | R0 resection | Neck |
| 8 | Body | 5.5 | 4a | - | 7/27 | R0 resection + OXA, CF, 5-Fu, 6 cycles | Disseminated |
| 9 | Cardia | 8 | 3 | + | 20/27 | R0 resection | Liver |
| 10 | Antrum | 6.2 | 4a | + | 0/31 | R0 resection + Traditional medicine | NA |
| 11 | Body | 6 | 3 | + | 4/23 | R0 resection + OXA, CF, 5-Fu, 6 cycles | Local |
| 12 | Body | 20 | 4a | NA | NA | exploration + OXA, CF, 5-Fu, 6 cycles | Liver |
| 13 | Antrum | 8 | 4a | - | 3/20 | R2 resection +TAI (THP,CF,MMC,5FU) | Liver |
| 14 | Cardia | 5 | 2 | + | 4/21 | R0 resection | Local |
| 15 | Body | 6 | 2 | + | 2/19 | R0 resection +TXT, L-OHP, CF, 5-Fu 6 cycles | NA |
| 20 | Body | 11 | 4a | + | 4/27 | R0 resection + OXA, S-1 6 cycles | Liver |
| 21 | Body | NA | 4a | - | NA | ABRAXANE, S-1 9 cycles + Folfri 10 cycles + Radiotherapy (50Gy/2Gy/25F,6mv-X 95%PTV) | Liver |
| 22 | Cardia | 8 | 3 | - | 0/28 | R0 resection + Docetaxel,OXA,5Fu,CF 10 cycles | NA |
| 23 | Antrum | 6 | 3 | - | 0/35 | R0 resection + OXA, CF, 5-Fu, 6 cycles | NA |
NA, not associated.
Follow-up
Survival data were obtained for all patients. One of the two patients who did not receive resection had tumor progression after chemotherapy (OXA, CF and 5-Fu; 6 cycles) and died 6 months after surgery (patient 12). The other patient (patient 21) had a tumor that was not resected and achieved a partial response after chemotherapy with a regimen of ABRAXANE plus S-1 for 9 cycles and Folfri for 10 cycles; however, after additional radiotherapy (50 Gy/2 Gy/25 F, 6mv-X 95% PTV), liver metastases progressed at 17 months, and the patient died at 20 months (Figure 6). The remaining 17 patients were used for further analyses. The overall and disease-free survival curves are shown in Figure 7. The median overall survival time was 12.0 months, and the median disease-free survival time was 7.0 months. Univariate analysis showed that N stage (HR 2.429, 95% CI 1.271-4.641, P=0.007), and tissue AFP (HR 0.428, 95% CI 0.194-0.945, P=0.036) were significantly associated with disease-free survival. There were 80% (4/5) of N0 and 50% (2/4) of N1 patients survived without progression, and no N2-3 patients survived.
Figure 7.
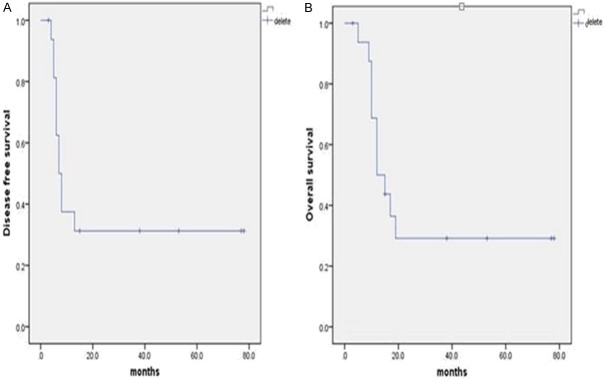
Disease-free (A) and overall (B) survival curves for the 17 resected patients.
We found that 41.7% (5/12) of patients who received adjuvant chemotherapy and 20% (1/5) of patients who did not receive adjuvant therapy remained disease-free, but there was no significant correlation between adjuvant therapy and tumor recurrence (HR 0.534, 95% CI 0.162-1.758, P=0.302). One patient (patient 10) suffered an intestinal obstruction after resection and could not tolerate chemotherapy; therefore, we administered only traditional Chinese medicinal therapy (Fu Fang Ban Mao capsules) to the patient, and he has survived without progression for 40 months since resection.
Discussion
Since the first report of AFP-producing gastric cancer (AFPGC) in 1970 [6], it has been indicated that AFPGC includes four histological subtypes: hepatoid, enteroblastic, yolk sac tumor and common adenocarcinoma type [7]. In 1985, Ishikura reported the first case of HAS [3]. In the next year, he reported seven similar cases, and indicated this kind of tumor mainly occurred at elderly people, often occurred at antrum, had distinct hepatoid differentiation, usually had liver metatastis and had poor prognosis. At the same time, he expanded the definition of hepatoid adnocarcinoma to those had hepatoid differentiation but without AFP produce [8]. Approximately 46% of HAS cases do not produce AFP. The diagnosis of HAS solely depends on morphology, regardless of serum AFP levels [9]. In addition to the stomach, hepatoid adenocarcinoma has been detected in a variety of organs, including the ovaries, renal pelvis, ampulla of Vater, lung, and pancreas [4,10].
The average age of the HAS patients in our cohort was 62.1 years, and the male-to-female ratio was 5.33:1. These results support the notion that HAS is more common in elderly men [11].
Although HAS is the most common extrahepatic tumor that morphologically mimics HCCs, its incidence among all primary gastric cancers is extremely low, with a range of 0.1%-1% [12,13]. Because of its rarity, this special subtype of gastric cancer remains relatively unfamiliar to clinicians and pathologists. Consequently, HAS is often misdiagnosed or missed diagnosed. In addition to their histological similarity to HCC, elevated serum AFP levels in gastric cancer patients also suggest HAS [14]; however, tests for serum AFP levels are not routine at many centers [2,12,13]. In our center, only 3.5% of gastric cancer patients received a test for serum AFP. If all gastric cancer patients underwent serum AFP tests, the actual incidence rate of HAS would likely be greater than the current estimate of 0.19%. In the present cohort, we found 4 cases of previously missed diagnosed among 7 patients with serum AFP levels greater than 100 ng/ml. Another reason for missed diagnosis is that cytoplasm stainings of HAS are various, occasionally showing eosinophilic, clear or basophilic staining [5,15]. In this cohort, we found 3 cases of HAS with basophilic cytoplasms. All of them were missed diagnosed. Because of serum AFP levels greater than 100 ng/ml, they were reviewed. HCCs vary architecturally and cytologically. The different architectural patterns and cytological variants frequently occur in combination. The architectural patterns include trabecular pattern, pseudoglandular or acinar pattern, and compact pattern. Tumor cells of clear cell variants have clear cytoplasm owing to the presence of abundant glycogen. In well and moderately differentiated HCCs, the tumor cells have abundant eosinophilic cytoplasm. But in poorly differentiated HCCs, the tumor cells show an increased nucleus: cytoplasm ratio, increased pleomorphism, solid growing pattern without distinct sinusoid-like spaces and frequently basophilic cytoplasm. But the literature only emphasized the eosinophilic cytoplasm in HAS, resulting in the missed diagnosis of HAS with clear or basophilic cytoplasm, especially the latter.
Our data showed that positive tumor AFP staining were in 73.7% (14/19) of patients, consistent with previous studies [2,16,17]. Immunohistochemically, AFP often presents as disseminated-positive in hepatoid areas, whereas in adenocarcinoma areas, AFP is weak or foci-positive [18]. It has been reported that about 90% of all HCCs are positive for Hepatocyte antigen and usually diffuse positive [19], but HASs are usually negative [4]. In our study, Hepatocyte antigen were only focally staining even if positive in HASs. So AFP and Hepatocyte antigen can be used to help to differentiate HAS from HCC and common adenocarcinoma of stomach.
HAS is a special type of gastric cancer that frequently metastasizes to the liver [13]. In our cohort, the liver was the most common first site of progression (53.8%, 7/13), followed by local (30.8%, 4/13), neck (7.7%, 1/13) and peritoneal dissemination (7.7%, 1/13). Our data show that the most common site of tumor was the cardia (42.1%, 8/19), followed by the body (36.8%, 7/19) and antrum (21.1%, 4/19); these findings differ from previous studies, in which the antrum was the primary site of metastasis [20]. In addition to improved overall survival, we show improved responses to treatment compared to previous studies [2,17], with both 3- and 5-year survival rates reached 30%. In the univariate analysis, we found that lymph node status was significantly related to survival, and after R0 resection, patients with a negative lymph node or an N1 classification had a higher chance of achieving a cure, compared with N2 and above [21]. The long-term outcomes achieved at our center are encouraging, and suggested that early diagnosis and R0 resection are the best way to improve prognoses.
Adjuvant chemotherapy is recommended for HAS; however, there is currently no standard treatment regimen, and HAS usually reacts poorly to regular regimens for common gastric adenocarcinoma [12,22]. Previous studies provided a good explanation for the poor response that HAS lacks thymidine phosphorylase (TP) but expresses abundant dihydropyrimidine dehydrogenase (DPD). The former is essential for the activation of fluorouracil, whereas the latter is responsible for the degradation of fluorouracil [23]. Although statistical robustness is low, some case reports have described several different treatment regimens that have been relatively better at slowing the progression of HAS, including cisplatin plus fluorouracil and epirubicin or irinotecan combined with mitomycin C [24,25]. In this group, although the most used regimen, OXA + CF +5-Fu, seemed to react well and six patients survived without progression, we think the most important favorable factor is the early N stage. The N stage of all of the six patients was N0 or N1. While for the N0 or N1 stage of patients, R0 resection may be the key of treatment.
Conclusions
HAS is a rare type of gastric adenocarcinoma, with an incidence of 0.19% among all gastric cancers. However, the disease has remained relatively unfamiliar to clinicians and pathologists, and the misdiagnosis/missed diagnosis rate remains high. R0 resection is the most important curative method for HAS.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No.), Wu Jie Ping Medical Fundation (No. 320.6750.14137) and the Beijing Hope Run Special Fund (No. LC2012B26).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587. doi: 10.1155/2014/140587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840–848. doi: 10.1002/1097-0142(19850815)56:4<840::aid-cncr2820560423>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321–327. doi: 10.3748/wjg.v19.i3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. 2012;106:299–303. doi: 10.1002/jso.23073. [DOI] [PubMed] [Google Scholar]
- 6.Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. [Existence of alpha feto protein during gastric-origin secondary cancer of the liver] . Presse Med. 1970;78:1277–1278. [PubMed] [Google Scholar]
- 7.Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, Gotoda T, Kinjo F, Fujita J, Shimoda T. Histologic and immunohistochemical analyses of alpha-fetoprotein--producing cancer of the stomach. Am J Surg Pathol. 2012;36:56–65. doi: 10.1097/PAS.0b013e31823aafec. [DOI] [PubMed] [Google Scholar]
- 8.Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y, Yamagiwa H, Itoh T, Aizawa M. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58:119–126. doi: 10.1002/1097-0142(19860701)58:1<119::aid-cncr2820580121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827–1835. doi: 10.1002/1097-0142(19930915)72:6<1827::aid-cncr2820720606>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Yano T, Ishikura H, Wada T, Kishimoto T, Kondo S, Katoh H, Yoshiki T. Hepatoid adenocarcinoma of the pancreas. Histopathology. 1999;35:90–92. doi: 10.1046/j.1365-2559.1999.0728f.x. [DOI] [PubMed] [Google Scholar]
- 11.Bakir T, Aliyazicioglu Y, Bektas A, Siviloglu C, Ozgur O. Hepatoid adenocarcinoma of the stomach: report of five cases and review of the literature. Acta Gastroenterol Belg. 2006;69:330–337. [PubMed] [Google Scholar]
- 12.Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11:56. doi: 10.1186/1471-230X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J (Engl) 2011;124:1470–1476. [PubMed] [Google Scholar]
- 14.Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol. 2013;19:4437–4442. doi: 10.3748/wjg.v19.i27.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu CC, De-Chuan C, Lee HS, Chu HC. Pure hepatoid adenocarcinoma of the stomach with spleen and lymph-node metastases. Am J Surg. 2010;199:e42–44. doi: 10.1016/j.amjsurg.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Osada M, Aishima S, Hirahashi M, Takizawa N, Takahashi S, Nakamura K, Tanaka M, Maehara Y, Takayanagi R, Oda Y. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol. 2014;45:1243–1250. doi: 10.1016/j.humpath.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, Matsumoto Y. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359–365. doi: 10.1159/000065838. discussion 365. [DOI] [PubMed] [Google Scholar]
- 18.Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, Tornillo L. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27:1302–1312. doi: 10.1097/00000478-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Chu PG, Ishizawa S, Wu E, Weiss LM. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol. 2002;26:978–988. doi: 10.1097/00000478-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Yang CK, Zhao WJ, Dai QB, Zhang HH, Zheng W. [Clinical characteristics, diagnosis and treatment of hepatoid adenocarcinoma of the stomach] . Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:245–248. [PubMed] [Google Scholar]
- 21.Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 23.Kamoshida S, Suzuki M, Sakurai Y, Ochiai M, Kimura F, Kuwao S, Sakamoto K, Sugimoto Y, Fukushima M, Tsutsumi Y. Expression of chemoresistance-related proteins in alpha-fetoprotein-producing adenocarcinoma of the digestive organs. Oncol Rep. 2006;16:721–727. [PubMed] [Google Scholar]
- 24.Mahajan V, Gupta N, Gupta S, Sharma R. Hepatoid adenocarcinoma of stomach: case report of a rare histological variant. Indian J Pathol Microbiol. 2014;57:116–119. doi: 10.4103/0377-4929.130917. [DOI] [PubMed] [Google Scholar]
- 25.Giustozzi G, Goracci G, Bufalari A, Lauro A, Cirocchi R, Boselli C, Bartoli A, Monacelli M, Giansanti M, Moggi L. Hepatoid carcinoma of the stomach: is it still an unusual anatomo-clinical entity? Six cases-report. J Exp Clin Cancer Res. 1999;18:571–573. [PubMed] [Google Scholar]


