Abstract
Background: Propofol is the most widely used drug in the induction of general anesthesia, however its disadvantages of injection pain has always been a problem for clinical anesthetists. Many strategies have been proposed and magnesium sulfate is one of them. This is the first meta-analysis studies evaluating effects of magnesium sulfate pretreatment for preventing propofol-induced injection pain. Methods: We searched MEDLINE, EMBASE, Google scholar and the Cochrane Database of Systematic Review databases for randomized controlled trials (RCTs) that evaluated the prophylactic effect of magnesium sulfate on propofol injection pain. Meta-analyses were performed using RevMan 5.3 software. Results: Five RCTs involving 545 participants were included. Magnesium sulfate allows more patients experiencing no pain or mild pain during propofol injection ([RR] 2.70, 95% [CI] 1.10-6.64, P=0.03, 2.12,95% CI 1.46-3.08, P < 0.0001, respectively). And the number of patients with severe pain (RR 0.12, 95% CI 0.06-0.25, P < 0.00001) on injecting propofol were significantly decreased. However, no statistical significance was found between magnesium sulfate group and placebo group in moderate pain (RR 0.22, 95% CI 0.05-0.97, P=0.05). Conclusion: Our meta-analysis suggested that pretreatment with magnesium sulfate intravenously before injecting propofol allow more patients to experiencing no pain during propofol injection and can reduce the intensity of injection pain effectively without causing any adverse effect.
Keywords: Pain, injection, propofol, magnesium sulfate
Introduction
Propofol is the most widely used intravenous anesthetic drug in induction and maintenance of anesthesia for its rapid onset, short duration and prompt recovery. However, its disadvantage of pain at injection site is unpleasant experience for most patients, especially when a small vein on the dorsum of hand was selected. The pain can be highly sharp, aching, or burning which may decrease the patients’ satisfaction with anesthetic care. It is reported that incidence of propofol injection pain varies between 28% and 90% in adults and 28% and 85% in children in the absence of other pretreatments [1]. It has been ranked as the seventh among the top 33 clinical problems of anesthesia in current clinical setting by American anesthesiologists [2].
Mechanism of the propofol induced pain has been unclear. Yet as reported, one of the potential reasons may be that propofol is insoluble in water and prepared in oil emulsion which consists of long-chain triglyceride solution [3]. This lipid solvents can directly irritate the skin, mucous membranes, and venous intima, and thus stimulate nociceptors and free nerve endings [4]. What’s more, propofol can also lead to a delayed pain at about 15 seconds after injection of propofol due to the activation of kallikrein and bradykinin [5]. Various factors, including the site of injection, speed of injection, vein size, aqueous phase propofol concentration, propofol temperature, blood buffering, and the concomitant use of various drugs, appear to influence this pain [6-8]. Many strategies has been proposed to reduce propofol-induced pain with variable results including both pharmacological (e.g. pre-treatment with lidocaine, ondansetron, magnesium sulfate, nafamostat, ketamine or topical nitroglycerine application with propofol, diluting propofol with 5% dextrose or 10% intralipid and using medium and small-chain triglycerides) and non-pharmacological methods have been used [9,10,4,11-15]. Despite many studies have been conducted on this issue, there is still much controversy. Lidocaine is the most popular method for reducing this pain. However, lidocaine can not entirely control propofol induced pain [10]. Consequently the ideal method of prevention of propofol injection pain is still unclear, there is a need to investigate other effective and convenient methods.
Magnesium has been used for many years as an antidysrhythmic for the treatment of eclampsia and for intraoperative and postoperative analgesia [16-19]. And it has also been used in many trials to provide analgesia for pain caused by propofol injection [20]. Thus we performed this study to assess the effect and safety of magnesium sulfate for reducing incidence and intensity of propofol injection pain. It could provide the basis of future clinic application. We critically analyzed the published data of the effects of magnesium sulfate on decreasing propofol injection pain.
Materials and methods
The current meta-analysis was performed following the guidelines recommended in the Cochrane Handbook for Systematic Reviews of Interventions [21] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22].
Search strategy
Online databases of MEDLINE (from 1966 to November 2014), EMBASE (from 1982 to November 2014), Google scholar and the Cochrane Database of Systematic Review were searched for randomized controlled trials (RCTs) that studied the effect and safety of magnesium sulfate on propofol-induced injection pain, two reviewers in duplicate. The following MeSH terms and keywords were used for our research: magnesium sulfate AND propofol. Language of publication was not restricted. The most recent search was conducted on November 10, 2014. A secondary reference review was also conducted by hand searching to ensure that no relevant studies were missed. Reviews, abstracts, correspondence, and letters were excluded. No search was performed for unpublished studies. The search strategy is summarized in Figure 1.
Figure 1.
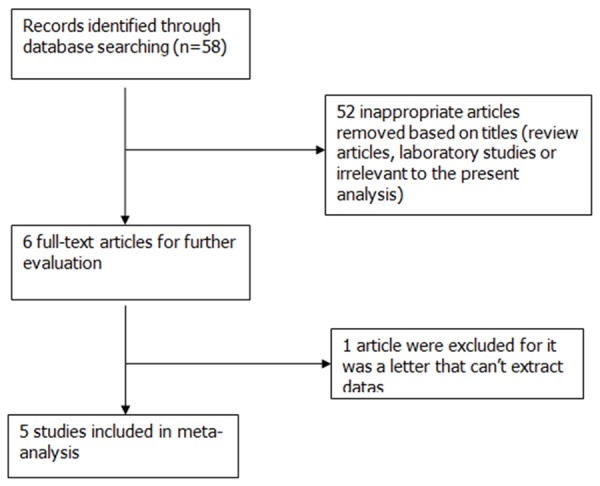
Flow chart outlining retrieved, excluded, and evaluated randomized controlled trials.
Inclusion and exclusion criteria
Trials meeting the following criteria were included: (1) randomized controlled trials (RCTs); (2) studies included magnesium sulfate group and placebo group (saline); (3) full-text articles available; (4) presence of detailed clinical data. Two investigators (Mengzhu Li and Xiang Zhao) read the full-text articles and decided whether the study met the inclusion criteria independently. All controversies were resolved via consensus. The primary endpoint of the present review was the pain score of propofol injection pain.
Data extraction
Two investigators (Mengzhu Li and Xiang Zhao) reviewed the studies and extracted the data independently using a standard data collection form. Any discrepancy in the process of data extracting was resolved by consensus. The following information was extracted: author’s name, year of publication, country in which the study was conducted, interventions between magnesium sulfate and placebo group, number of subjects in treatment and placebo groups, injection site and size of indwelling needle, assess method of pain.
Quality assessment
We used the Cochrane Collaboration’s tool to evaluate the quality of all included trials for assessing risk of bias [23]. Two reviewers (Mengzhu Li, Xiang Zhao) used the tool to assess the following types of biases respectively: allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Any disagreement was resolved by a third author.
Statistical analysis
Statistical analysis was performed using Rev Man 5.3 software from the Cochrane Collaboration. The heterogeneity across each effect size was evaluated with chi-square test and I2 statistic, which is useful for assessing consistency between trials. I2 > 50% suggested that there was significant heterogeneity. A fixed-effect model was used if the P value of the chi-square test was > 0.10 or I2 < 50%, otherwise, the random-effects model was adopted. Sensitivity analysis was performed to explore the impact of an individual study by omitting each study in turn and investigating the influence of each study on the overall pooled estimate. Due to the limited number of trials, subgroup analysis was not conducted.
Results
Search results
Our electronic searches identified 58 articles through the following databases: MEDLINE, EMBASE, Google scholar and the Cochrane Database of Systematic Review. We screened titles and abstracts, find 6 studies were potentially eligible and the other 52 were excluded for they were not RCTs that investigated the effect and safety of magnesium sulfate on propofol-induced injection pain. Among the 6 potentially eligible articles, 1 was excluded for it was a letter that cannot extract data.
Therefore, 5 studies [24-28] with a total of 545 patients were ultimately found to fulfill the inclusion criteria and contained the required data for the planned comparison. The process used to identify eligible studies is illustrated in Figure 1.
Study characteristics and quality
The characteristics of each included trial are described in Table 1. Among the five trials, one was conducted in America [28], four in Asia [24-27]. All trials were published between 2002 and 2014 and all of them were in English. In the five trials, three of them compared the effect of magnesium sulfate and lidocaine on propofol-induced injection pain [25,27,28]. All subjects in the selected trials received general anesthesia and propofol was used in the induction. Magnesium sulfate was given before administering propofol to the treatment groups according to the protocol used by each trial. Two trials established i.v. access with 18-G cannula [25,26], the other three trials used 20-G cannula [24,27,28]. The indwelling needle was inserted into brachial vein of both arms in one trial [24], while the other four trials selected a vein on dorsum of hand [25-28]. Pain on injection was assessed using a four-point scale in all five studies. But Galgon RE etc. only showed the proportions of subjects experiencing propofol injection pain and mean (95% CI) group pain response scale scores of each group in their study [28], however the number of patients at each level of pain scores were listed in other four studies [24-27]. So we only extracted the number of patients without injection pain in the study of Galgon RE etc. Among the five selected articles, four [24-27] reported the positive effect of magnesium sulfate on reducing propofol-induced injection pain, one study [28] did not find any positive results.
Table 1.
Basic characteristics of the trials included in the meta-analysis
| Articles | Country | Journal | Pretreatment (magnesium group vs placebo group) | Size of indwelling needle and injection site | Numbers of subjects (magnesium group vs placebo group) | Assesment method of pain |
|---|---|---|---|---|---|---|
| Galgon, R. E. 2014 | USA | J Anesth | Magnesium sulfate 0.25 mg IV. vs 0.9% sodium chloride solution IV | A 20-gauge needle was inserted into a vein on the dorsum of the hand | 44 vs 39 | A four-point scale |
| Alipour, M. 2014 | Iran | Iran Red Crescent Med J | Magnesium Sulfate 2 mmol IV. vs saline solution IV | A 20-gauge needle was inserted into a vein on the dorsum of the hand | 56 vs 56 | A four-point scale |
| Singh, D. K. 2011 | India | Saudi J Anaesth | Magnesium sulfate 2.48 mmol IV. vs normal saline IV | A 18-gauge needle was inserted into a vein on the dorsum of the hand | 25 vs 25 | A four-point scale |
| Agarwal, A. 2004 | India | Can J Anaesth | Magnesium sulfate 1 g IV. vs normal saline IV | A 18-gauge needle was inserted into a vein on the dorsum of the hand | 100 vs 100 | A four-point scale |
| Memis, D. 2002 | Turkey | Anesth Analg | Magnesium sulfate 2.48 mmol IV. vs saline IV | A 20-gauge needle was inserted into the brachial vein of both arms | 50 vs 50 | A four-point scale |
Risk of bias of included studies
Among all selected trials, each study was described as a randomized trial. In four studies the randomized sequence and allocation sequence concealment were adequately conducted [25-28]. Four studies used the allocation concealment method [25-28], and the other one were unclear [24]. All studies were blinded to participants and personnel. Incomplete outcome data bias and selective reporting bias were all considered as low, as we could not obtain each study’s original protocol. The included and excluded criterions were reported in details in all trials. Risk of bias assessment of each study is described in Figures 2 and 3, showing that most of the studies had high quality. The risk of bias tool, Rev Man 5.3 was used.
Figure 2.
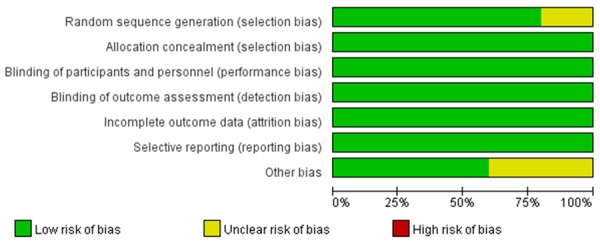
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Figure 3.

Risk of bias summary: review authors’ judgments about each risk of bias item for each included studies.
Sensitivity analysis
Sensitivity analyses were performed to assess whether the pooled estimate of injection pain will alter by removing each study sequentially and reanalyzing. Finally, the corresponding pooled RRs were all consistent with the original outcomes.
Clinical outcomes
Meta-analysis outcomes show that magnesium sulfate allows more patients experiencing no pain (risk ratio [RR] 2.70, 95% confidence interval [CI] 1.10-6.64, P=0.03, Figure 4) or mild pain (RR 2.12, 95% CI 1.46-3.08, P < 0.0001, Figure 5) during propofol injection. And the cumulative number of patients with severe pain (RR 0.12, 95% CI 0.06-0.25, P < 0.00001, Figure 6) during propofol injection were also significantly decreased with the pretreatment of magnesium sulfate. However, no statistical significance was found between magnesium sulfate group and placebo group in moderate pain (RR 0.22, 95% CI 0.05-0.97, P=0.05, Figure 7). In the analysis of none pain, moderate pain and severe pain, a random-effects model was used for significant heterogeneity exist between trials detected (I2=89%; I2=55%, I2=89%, respectively, Figures 4, 6 and 7).
Figure 4.
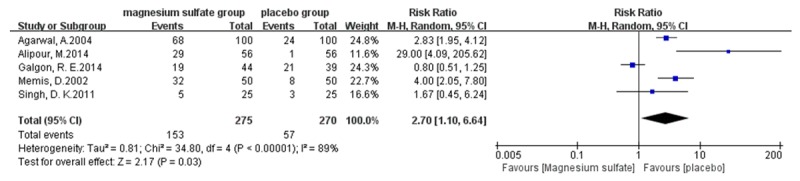
Forest plot of RR. Magnesium sulfate group and placebo group on preventing propofol injection pain with 95% CI.
Figure 5.
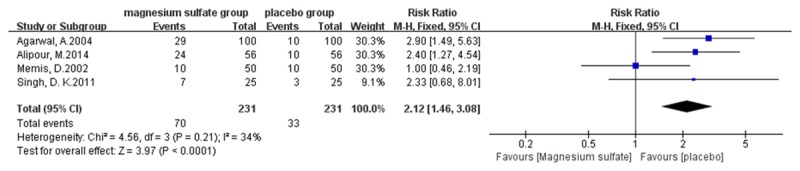
Forest plot of RR. Magnesium sulfate group and placebo group on reducing the mild propofol injection pain with 95% CI.
Figure 6.
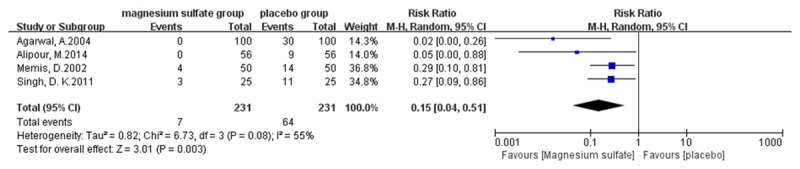
Forest plot of RR . Magnesium sulfate group and placebo group on reducing the severe propofol injection pain with 95% CI.
Figure 7.
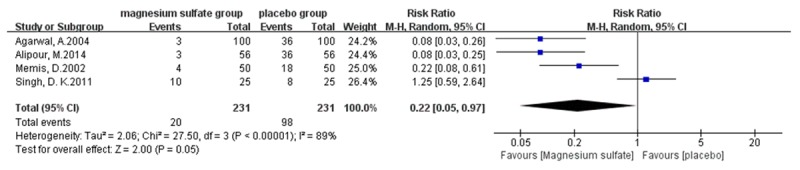
Forest plot of RR. Magnesium sulfate group and placebo group on reducing the moderate propofol injection pain with 95% CI.
Adverse effects
Two trials reported pain on injection site of magnesium sulfate. No complications such as pain, edema, or allergic reactions were observed at the injection site within the first 24 h after the operation.
Discussion
The propofol injection pain has always been identified as a troubling concern for anesthesiologists. However the exact mechanism of pain on injection remains unclear. Many drugs such as magnesium sulfate, flurbiprofen, axetil paracetamol, ondansetron, granisetron, dexmedetomidine, alfentanyl, fentanyl, lidocaine, ketamine [27-31] have been used to alleviate this pain. Yet, despite lots of trials attempting to discover an intervention to alleviate the propofol injection pain, no intervention has consistently affected its complete relief. Current evidence suggests that propofol injection in an antecubital vein is most effective; however, this is not always practical in clinical practice. In addition to lidocaine pretreatment with and without venous occlusion, as well as a mixture of lidocaine with propofol also consistently provide a relative risk reduction [6,7]. They were all failed to gain popularity.
Magnesium sulfate is called the nature physiological calcium channel blocker and it probably interferes with calcium channel and NMDA receptors [20,24]. Its analgesic mechanisms may as follows: (1) Magnesium is an antagonist of the N-methyl-D-aspartate (NMDA) receptor ion channel. The NMDA receptor is coupled to an ion channel permeable to K+ and Ca+. Magnesium sulfate blocks NMDA receptor currents in a voltage-dependent manner by blocking the receptor channel [32]. This may partially explain its analgesic activity [20]; (2) Magnesium sulfate is a kind of calcium channel blocker, many calcium channel blockers have antinociceptive effects and potentiate the analgesic effects of morphine in patients with chronic pain [20,33]; (3) Magnesium sulfate also has a vasodilatory effect mediated by endothelium-derived nitric oxide, nitric oxide donors protect vascular endothelium from ischemia and reperfusion-mediated endothelial dysfunction [14]. On the basis of the above mechanisms, magnesium sulfate may be another alternative to provide analgesia for pain caused by propofol injection. So we conducted this meta-analysis.
A total of 545 patients were included in the RCTs of this meta-analysis, of whom 275 were allocated to magnesium sulfate therapy, and 270 to the placebo group (saline). This meta-analysis aimed to evaluate the effects of magnesium sulfate on propofol injection pain. To our best knowledge, this article is the first meta-analysis to study effects of magnesium sulfate pretreatment on preventing propofol-induced injection pain. Our analysis outcomes suggest that magnesium sulfate significantly relieve the propofol injection pain. Pretreatment with magnesium sulfate allow more patients experience no pain or mild pain during propofol injection and decrease the number of patients with severe pain induced by propofol injection. Although two trials reported the pain on injection site of magnesium, the intensity was faint so it may do not require treatment and subsided in a few seconds. However, the Figures 4 and 5 may appear that placebo group had more no pain or mild pain. Indeed, in a research when the upper limit and floor level of 95% CI of the RR were all > 1 and the transverse line of 95% CI is on the right of the invalid line, then we can say that the incidence of event in experimental group is larger than control group. If the event which is investigated is adverse, the experimental factor can accelerate the adverse event, and this experimental factor may be harmful. Nevertheless, if it’s a good event, the experimental factor may promote this event, and the experimental factor can be beneficial. Therefore, in our research the Figures 4 and 5 indicated that pretreatment with magnesium sulfate was beneficial factor which can allow more patients to have no pain or mild pain.
No complications such as pain, edema, or allergic reactions were observed at the injection site within the first 24 h after the operation.
Heterogeneity is an unavoidable disadvantage in interpreting meta-analysis results.
We adopted a random-effect model if I2 > 50% which indicate that heterogeneity exists. As it is reported that the site of injection, indwelling needle, speed of injection, vein size, aqueous phase propofol concentration, propofol temperature, blood buffering, and the concomitant use of various drugs may influence the incidence and intensity of propofol injection pain, the heterogeneity observed in our analysis of overall incidence of propofol injection pain may be explained by the above factors.
We also performed sensitivity analysis, which did not change the final results either.
This review has several limitations. First, the number of RCTs regarding the outcomes was limited. Second, all individual studies used in our analyses had small sample sizes. Third, researchers were based on different study protocols (including administration time of test drugs before propofol induction , the difference in gauge of catheter needle, flow rate of drug injection, assessed pain scores in different methods) that may have lead to significant data heterogeneity.
Due to considerable heterogeneity as well as a limited number of RCTs regarding the outcomes, caution should be given when interpreting the results and additional well controlled, randomized trials are still needed to confirm our results.
In summary, our meta-analysis suggested that pretreatment with magnesium sulfate intravenously before injecting propofol can reduce the intensity of injection pain effectively without causing any adverse effect.
Acknowledgements
We thank all authors of the publications included in this study for contributing information as required.
Disclosure of conflict of interest
None.
References
- 1.Stark R, Binks S, Dutka V, O’Connor K, Arnstein M, Glen J. A review of the safety and tolerance of propofol (‘Diprivan’) Postgrad Med J. 1984;61:152–6. [PubMed] [Google Scholar]
- 2.Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999;88:1085–91. doi: 10.1097/00000539-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Devlin JW, Lau AK, Tanios MA. Propofol-Associated Hypertriglyceridemia and Pancreatitis in the Intensive Care Unit: An Analysis of Frequency and Risk Factors. Pharmacotherapy. 2005;25:1348–52. doi: 10.1592/phco.2005.25.10.1348. [DOI] [PubMed] [Google Scholar]
- 4.Ambesh SP, Dubey PK, Sinha PK. Ondansetron pretreatment to alleviate pain on propofol injection: a randomized, controlled, double-blinded study. Anesth Analg. 1999;89:197–9. doi: 10.1097/00000539-199907000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Scott R, Saunders D, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43:492–4. doi: 10.1111/j.1365-2044.1988.tb06641.x. [DOI] [PubMed] [Google Scholar]
- 6.Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, Pace NL, Apfel CC. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110. doi: 10.1136/bmj.d1110. [DOI] [PubMed] [Google Scholar]
- 7.Picard P, Tramer MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90:963–9. doi: 10.1097/00000539-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Tan C, Onsiong M. Pain on injection of propofol. Anaesthesia. 1998;53:468–76. doi: 10.1046/j.1365-2044.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattila M, Koski E. Venous sequelae after intravenous propofol (‘Diprivan’)--a comparison with methohexitone in short anaesthesia. Postgrad Med J. 1984;61:162–4. [PubMed] [Google Scholar]
- 10.King SY, Davis MF, Wells EJ, Murchison DJ, Pryor PJ. Lidocaine for the prevention of pain due to injection of propofol. Anesth Analg. 1992;74:246–9. doi: 10.1213/00000539-199202000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R, Harper N, Chadwick S, Vohra A. Pain on injection of propofol Methods of alleviation. Anaesthesia. 1990;45:439–42. doi: 10.1111/j.1365-2044.1990.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 12.McCulloch M, Lees N. Assessment and modification of pain on induction with propofol (Diprivan) Anaesthesia. 1985;40:1117–20. doi: 10.1111/j.1365-2044.1985.tb10615.x. [DOI] [PubMed] [Google Scholar]
- 13.McCrirrick A, Hunter S. Pain on injection of propofol: the effect of injectate temperature. Anaesthesia. 1990;45:443–4. doi: 10.1111/j.1365-2044.1990.tb14329.x. [DOI] [PubMed] [Google Scholar]
- 14.Barker P, Langton J, Murphy P, Rowbotham D. Effect of prior administration of cold saline on pain during propofol injection. Anaesthesia. 1991;46:1069–70. doi: 10.1111/j.1365-2044.1991.tb09927.x. [DOI] [PubMed] [Google Scholar]
- 15.Klement W, Arndt J. Pain on injection of propofol: effects of concentration and diluent. Br J Anaesth. 1991;67:281–4. doi: 10.1093/bja/67.3.281. [DOI] [PubMed] [Google Scholar]
- 16.Begum R, Begum A, Bullough CH, Johanson RB. Reducing maternal mortality from eclampsia, using magnesium sulphate. Eur J Obstet Gynecol Reprod Biol. 2000;92:223–4. doi: 10.1016/s0301-2115(99)00274-2. [DOI] [PubMed] [Google Scholar]
- 17.Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology. 1996;84:340–347. doi: 10.1097/00000542-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Iseri LT, French JH. Magnesium: nature’s physiologic calcium blocker. Am Heart J. 1984;108:188–93. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 19.Koinig H, Wallner T, Marhofer P, Andel H, Horauf K, Mayer N. Magnesium sulfate reduces intra-and postoperative analgesic requirements. Anesth Analg. 1998;87:206–210. doi: 10.1097/00000539-199807000-00042. [DOI] [PubMed] [Google Scholar]
- 20.Wong CH, Dey P, Yarmush J, Wu WH, Zbuzek VK. Nifedipine-induced analgesia after epidural injection in rats. Anesth Analg. 1994;79:303–6. doi: 10.1213/00000539-199408000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.0. 2 (updated September, 2009) The Cochrane Collaboration. 2009. www.cochrane-handbook.org/(accessed 18 May 2009) 2010.
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint-preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89:873–80. [PubMed] [Google Scholar]
- 23.Higgins J, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memis D, Turan A, Karamanloglu B, Süt N, Pamukçu Z. The use of magnesium sulfate to prevent pain on injection of propofol. Anesth Analg. 2002;95:606–8. doi: 10.1097/00000539-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A, Dhiraj S, Raza M, Pandey R, Pandey CK, Singh PK, Singh U, Gupta D. Vein pretreatment with magnesium sulfate to prevent pain on injection of propofol is not justified. Can J Anesth. 2004;51:130–33. doi: 10.1007/BF03018771. [DOI] [PubMed] [Google Scholar]
- 26.Singh DK, Jindal P, Singh G. Comparative study of attenuation of the pain caused by propofol intravenous injection, by granisetron, magnesium sulfate and nitroglycerine. Saudi J Anaesth. 2011;5:50–4. doi: 10.4103/1658-354X.76511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alipour M, Tabari M, Alipour M. Paracetamol, Ondansetron, Granisetron, Magnesium Sulfate and Lidocaine and Reduced Propofol Injection Pain. Iran Red Crescent Med J. 2014;16:e16086. doi: 10.5812/ircmj.16086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galgon RE, Strube P, Heier J, Groth J, Wang S, Schroeder KM. Magnesium sulfate with lidocaine for preventing propofol injection pain: a randomized, double-blind, placebo-controlled trial. J Anesth. 2014:206–11. doi: 10.1007/s00540-014-1892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Zhu J, Xu L, Zhang X, Wang H, Luo Z, Zhao Y, Yu Y, Zhang Y, Shi H. Efficacy and safety of flurbiprofen axetil in the prevention of pain on propofol injection: A systematic review and meta-analysis. Med Sci Monit. 2014;20:995–1002. doi: 10.12659/MSM.890102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Zhang Y, Lu Y, Dong C. Preemptive dexmedetomidine to prevent propofol injection pain in children. Ir J Med Sci. 2014:375–8. doi: 10.1007/s11845-014-1122-3. [DOI] [PubMed] [Google Scholar]
- 31.Atashkhoyi S, Negargar S, Hatami-Marandi P. Effects of the addition of low-dose ketamine to propofol-fentanyl anaesthesia during diagnostic gynaecological laparoscopy. Eur J Obstet Gynecol Reprod Biol. 2013;170:247–50. doi: 10.1016/j.ejogrb.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Altura BT, Altura BM. Endothelium-dependent relaxation in coronary arteries requires magnesium ions. Br J Pharmacol. 1987;91:449–51. doi: 10.1111/j.1476-5381.1987.tb11235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda HF, Bustamante D, Kramer V, Pelissier T, Saavedra H, Paeile C, Fernandez E, Pinardi G. Antinociceptive effects of Ca2+ channel blockers. Eur J Pharmacol. 1992;217:137–41. doi: 10.1016/0014-2999(92)90833-p. [DOI] [PubMed] [Google Scholar]


