Abstract
Objective: To observe the changes of intestinal inflammation on PDIA3 gene knockout IBS rats and its effect on immune function. Methods: 36 SD rats were randomly divided into four groups: the control group (n = 8); IBS- empty virus group (IBS-GFP, which); IBS-PDIA3 knockout group (n = 12); IBS- the control group (n = 12). After modeling, colon and ileocecal tissue pathology in each group were observed separately. Changes of immune and inflammatory markers were measured. At the same time, ultrastructural changes in each group were observed by electron microscopy. Results: Compared with the IBS control group, inflammation was reduced significantly in IBS-PDIA3 knockout group. IgE, IL-4 and IL-9 and the level of intestinal trypsin type were decreased significantly. Furthermore, mast cell degranulation and PAR 2 receptor reduced significantly. Conclusion: PDIA3 may play an important role in the development of IBS by mediating through immune responses of mucosal abnormalities. However, the mechanism needs to be confirmed in further study.
Keywords: PDIA3, knockout, irritable bowel syndrome, immune response, colon
Introduction
Irritable bowel syndrome (IBS) is a frequently-occurring disease worldwide. The incidence of IBS is 10-20% in European and American population [1], which is 6.5-10.1% in Asian countries; and in recent years, the incidence has gradually increased [2]. Due to unclear etiology and pathogenesis of IBS and the lack of effective means of diagnosis and treatment in clinical, the symptoms of IBS patients are long-standing, seriously impacting the patient’s learning, work and life. Compared with normal persons, IBS patients seek medical help more easily, cost high medical expenses, and suffer indirect economic losses caused by absenteeism due to illness [3]. Thus, IBS is not only a clinical problem, but also a noteworthy social problem.
At present, the etiology and pathogenesis of IBS is not very clear. Existing research suggested that the incidence of IBS may be related to the following factors [4]: 1) intestinal motility disorders: diarrhea-predominant IBS manifestates as shortened gastrointestinal transit time and intestinal power hyperthyroidism such as increased colon contraction, while there is underpowered intestinal in constipation-predominant IBS; 2) increased visceral sensitivity: rectal balloon dilation tests showed that pain threshold of patients with IBS decreased, and the sensitivity to mechanical stimulation such as rectal dilatation increased; 3) abnormal perception of the central nervous system: functional MRI studies showed that the brain-reacting zone of IBS patients to rectal balloon distension is different from that of normal persons, and the reaction zone is differed between diarrhea and constipation-predominant IBS; 4) dysregulation of brain-gut axis: the gut-incoming signal treatment and dysregulation of the enteric nervous system in the central nervous system are related with IBS symptoms; 5) psychological abnormalities: Some IBS patients have anxiety, stress, depression, insomnia and other psychological abnormalities; furthermore, mental and psychological stress can trigger or aggravate IBS symptoms , indicating that psychological factors are closely related with IBS; 6) intestinal mucosal immune dysfunction.
Recent studies have found that mucosal immune dysfunction played an important role in the pathogenesis of IBS. Colonic mucosa of patients with IBS presented increasing number immune cells (CD3 +, CD4 + and CD8 + T cells), and accompanied increasing expression of cytokines (IL-5, IL-13, IL-6, TNF-α, IL-1β, etc.) [5-7]. However, the intestinal mucosal immune dysfunction role in the pathogenesis of IBS should be further elucidated.
When PDIA3 was in (major histocompatibility complex, MHC) class I molecules antigenic peptide, it can catalyze the MHC Iα2 domain disulfide bond rupture and reconstruct, which made the antigen binding groove was more suitable for antigen peptide and played an important role in endogenous antigen presentation [8]. Preliminary findings found that, comared with the control group, PDIA3 protein expression was significantly higher in IBS rat colon mucosa [9], indicating that PDIA3 may play an important role in the development of IBS.
PDIA3, which was also known as ERp57, is a member of protein disulfide isomerase (Protein disulfide isomerase, PDI) family. It can catalysis the redox isomerization reactions of protein or polypeptide disulfide bonds. Recent studies have found that PDIA3 played an important role in presentation of endogenous antigen. Antoniou et al reported that in the process of MHC molecules antigen-presenting, PDIA3 molecules directly entered into the peptide binding groove of MHC class I molecules and binded with them, which made the antigen binding groove was more suitable for antigenic peptides [10]. Santos et al further confirmed that PDIA3, MHC-I and tapasin were combined together in the process of antigen presentation and helped MHC class I molecules binding to antigenic peptides [11]. Stepensky et al found that PDIA3 enhanced the stability of MHC- I molecule binding to antigen peptide [12]. So far there was no report about the effect of PDIA3-/-on MHC-I.
It has been confirmed that mast cells (MC) is directly related to the pathogenesis of IBS. Colon MC Infiltration and media releasing are the reasons for IBS patients with symptoms, which is also closely related to visceral hypersensitivity in patients with IBS [13]. Histamine, serotonin, fibrinolytic enzymes media were released when MC were activated. It activated sensory neurons including the gastrointestinal tract, and caused hyperalgesia or allodynia. Plasmin can activate nociceptors-protease activated receptor 2 (PAR-2), which was located on the root of the colon behind spinal cord neuronal membrane, and caused the nociceptors lasted high excitability [14]. Present study confirms MC can be activated in a variety of ways. The most classic one is the IgE recognized and bridged with its receptor FcεRI, leading to degranulation [15]. IL-4 is specific cytokine which promotes the production of IgE. Upregulation expression of IL-4 can cause the production of antigen-specific IgE, leading to activation of MC [16]. IL-9 can increase the expression of FceRIα which located on the surface of mast cell, leading MC to be more easily be activated [17]. Previous studies showed that IL-9 belonged to the Th2 cytokines. However, under certain circumstances, more T cell (Th9 cells, Th17 cells, Treg cells) also secrete IL-9 [18]. It has been reported that intestinal IL-4 was upregulated in acute intestinal infection with post infection irritable bowel syndrome (PI-IBS) mice. But after a successful modeling, IL-4 expression level restored to normal or even reduced [19]. Therefore in the early days of IBS, IL-4 may play a role in the activation of the MC. Although the relationship between IL-9 and IBS has not been reported, IL-9 may also be benefit for activation of MC. These above data suggested that, in the development of IBS, both IL-4 and IL-9 were involved in the activation of MC, leading to the degranulation of MC.
In summary, we speculate that PDIA3 may play an important role in the development of IBS by mediating through immune responses of mucosal abnormalities. PDIA3 promote the presentation of endogenous antigen, mediating DC to increase the endogenous antigen sensitivity and reactivity, inducing excessive immune of T cells. Cytokines IL-4, IL-9 were produced in this process and MC was in a high sensitive state even degranulated and therefore resulting in the pathogenesis of IBS.
Materials and methods
Experimental animals
36 SD rats without deformity, trauma and skin infections were prepared (Purchased from the Animal Center of Third Military Medical University, Chongqing). The body weight was (250 ± 10) g. They were caged and fed with pellet (free diet). They were given 12 h of light per day for adaptive feeding a week in order to perform the experiment. All animals were fed a standard pellet feed by the Third Military Medical University Experimental Animal Center. Animal experiments were carried out more than seven days of adaptive feeding after purchase. Experimental groups were divided randomly. They were divided into four groups: 8 rats in the control group. 4 rats were in the IBS1 empty virus group. 12 rats were in IBS1 knockout group and 12 rats were in IBS2 group.
IBS animal modeling method
(1) The control group: normal rats without any treatment.
(2) IBS empty virus group: Normal rats were treated with intravenous injection of empty virus. 24 h before the experiment, rats with empty virus were fasted but water. After ether anesthesia, silicone tube which connected to a syringe was inserted through the anus (from the anus 8 cm). 40 mL/L acetic acid 1 mL was poured into the colon. Slowly pulled out the silicone tube, and oppress anus by hand. Elevate the rat’s tail for 30 s, and then flush the colon with 0.01 mol/L PBS 1 mL. Return the rats with freely water consumption, and begin restraint stress on the 7 days. The rats were placed in a special transparent cylindrical barrel (which can be replaced by a Nongfushangquan mineral water bottle without bottleneck) limiting limbs but did not affecting their breathing, and 2 h later the rats were placed in cages. This was performd for three days, 2 h each day.
(3) IBS knockout group: The gene-knockout rats which were purchased from Shanghai Biomodel Organism Science & Technology Development Co. Ltd (Shanghai, China) were the objective, and they were fasted but water for 24 h before the experiment. The modeling method was the same as empty virus group.
(4) IBS group: Normal rats were fasted but water for 24 h before the experiment. The IBS modeling method was the same as the previous.
Collection of colon specimen and hematoxylin-eosin (hematoxylin-eosin, HE) staining
On the second day of the experiment, we randomly selected 2 mice from model group and control group separately. 3% pentobarbital (40 mg/kg) were used to anesthetize rat and 1 cm bowel of colon were taken (from the anal about 6 cm). On the 7th day of the experiment, 1 cm bowel of colon (from the anal about 6 cm) was taken from all the rats before colorectal distension validation experiment. The HE staining of tissue was carried out separately. Observation was taken under optical microscope: whether there was edema, flaky bleeding and ulceration in mucosa and submucosa. Whether the structural of colonic mucosa and crypt structure was intact. Whether the epithelium, layers of tissue and other pathological changes were complete.
Detection of total IgE levels in peripheral blood serum, IL-4, IL-9 levels and tryptase levels in gut and intestinal tissue by ELISA
According to the method described in the literature, peripheral blood of rats were taken and stored at -20°C. For the detection of pancreatic tissue type, we took two biopsy specimens, first weighed and then rinsed 0.9% NaCI solution. Placed in 1 ml HBSS balanced salt solution and incubated in 37°C for 1 h. The supernatant was then separated. Total serum IgE, IL-4 and IL-9 levels were determined by ELISA. The concentrations of tryptase and specific steps refered to kit instruction. Standard calibration curve were drawn according to the standard samples. Each set of standards and samples repeated twice and the average values were used.
Real-time PCR was used to detect the expression level of IL-4, IL-9 mRNA colonic mucosa of rats
Rreferring to the relevant literatures, the expression levels of IL-4, IL-9 mRNA of colonic mucosa in each group were detected using Real-time PCR method. Primers’ sequences were shown in Table 1.
Table 1.
Primer design and detection of IL-4 and IL-9 mRNA by Real-time PCR
| Gene | Primer sequence | |
|---|---|---|
| IL-4 (ID: 287287) | Upstream | 5’TTGCTGTCACCCTGTTCTGC3’ |
| Downstream | 5’CCGTGGTGTTCCTTGTTGC3’ | |
| IL-9 (ID: 116558) | Upstream | 5’GTCCTTGCCTCTGCTTTGC3’ |
| Downstream | 5’GGGTCGTCCTTCAGGTTTTC3’ | |
| Actin | Upstream | 5’CCCATCTATGAGGGTTACGC3’ |
| Downstream | 5’TTTAATGTCACGCACGATTTC3’ |
Observation of DC distribution and the number of intestinal mucosa label with immunohistochemical CD103 in IBS rats
CD103 were used in immunohistochemistry. Distribution of colorectal DC were observed at low magnification (×100). Combined with morphology, the number of DC were counted under high magnification (×400). 10 non-overlapping field of view 400 times were selected randomly in each slice. The number of DC and the total number of cells in each field were counted. Take the average, and we can get the each DC‰ by the formula. DC‰ = DC total number/total number of cells ×1000‰. Results were represented with the median (interquartile range)‰. The different number of DC in colonic mucosa among the groups were compared.
Toluidine blue staining method were used to count intestinal mucosa MC of IBS rat
We took colon tissue of rats in each group, and then fixed the slice conventionally. Dewaxing to water, and we distilled and then washed. Dipped in toluidine blue liquid for 30 mins, 0.2% acetic acid was used to differentiate until they were got the clearly purple granules. Finally, dehydration, transparent, and neutral balata fixation were performed.
Observation of morphology of intestinal mucosal mast cell and ultrastructure of degranulation mast cell by TEM
Three rats in each group were randomly selected for the observation of morphology of intestinal mucosal mast cell and degranulation by TEM. Material collection and observation methods were performed according to previous literatures.
Detection of intestinal expression level of PAR-2 by western blot
The rats’ colon tissue were placed in a mortar, and then rubbed into powder in liquid nitrogen. Then the right amount of icy saline were added to make colonic homogenate. Take equal amounts of protein samples in each group (40 µg) and separate by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). And then they were transferred to wet nitrocellulose (NC film, Sigma, USA). They were enclosed by 3% bovine serum albumin (BSA) and shaked for 3 h, and then were incubated with 4% PAR-2 antibody (1:200) overnight. The membrane was washed with washing buffer 5 mins for 3 times. Then 1: 2000 dilution of alkaline phosphatase-labeled secondary antibody were added, and they were shaken at room temperature for 2 h. Washing the membrane with washing buffer 3 min for 5 times and DAB was added as a chromogen and stained (Nanjing KGI). Net absorbance values of target zone were analyzed by image processing apparatus (USA Gene Company).
Results
Comparsion on morphology of rats’ colon and ileocecal
Nuclei were stained to bright blue by hematoxylin. Cartilage matrix and calcium particles presented dark blue. Cytoplasm was stained to different shades of pink by eosin. Eosinophilic granular in cytoplasm represented bright red. Collagen fibers were pale pink. Elastic fibers were bright pink. RBC was orange. Protein liquids were pink. From Figure 1 we can conclude that the lesions in colon were significant than that in ileocecal.
Figure 1.
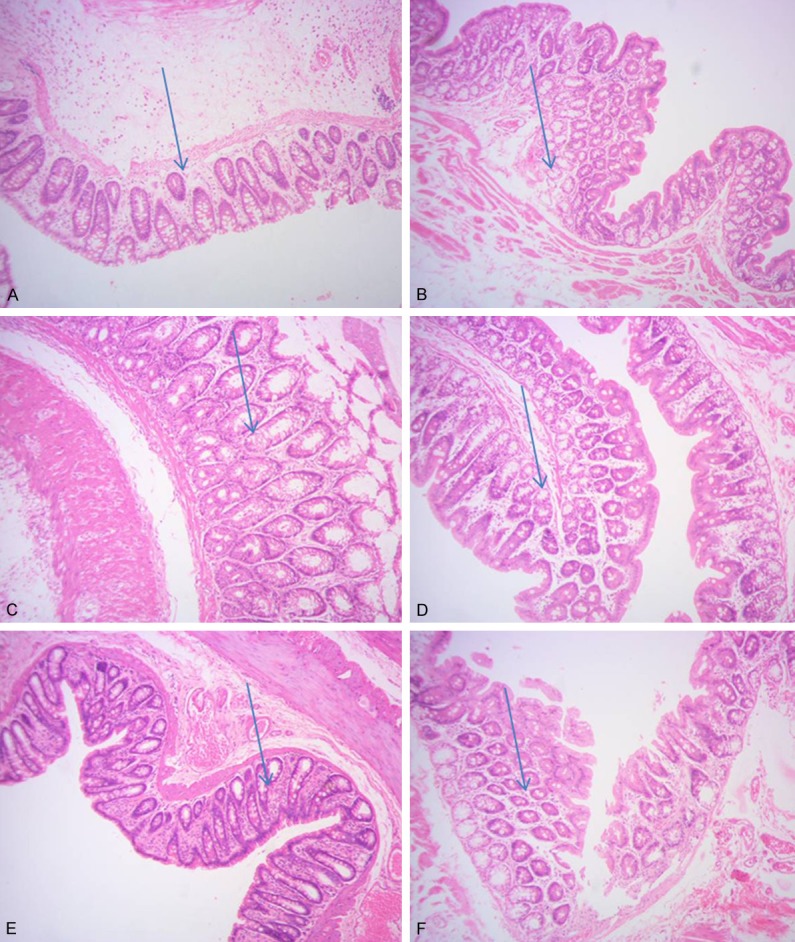
Comparisos on morphological of rats’ colon and ileocecal. A: IBS model of the second day, colon tissue 100×; B: IBS model of the second day, ileocecal 100×; C: IBS model of the seventh day, colon tissue 100×; D: IBS model of the seventh day, the ileocecal 100×; E: Normal SD rat’s colon tissue 100×; F: Normal SD rat’s ileocecal 100×.
Comparison of peripheral blood serum total IgE, IL-4, IL-9 and the level of intestinal trypsin
As we can see from Table 2, compared with IBS- control group, IgE, IL-4, IL-9 and the level of intestinal trypsin significantly decreased in IBS- knockout group with significant difference (P<0.05).
Table 2.
Comparisons on Total level of IgE, IL-4, IL-9 and intestinal trypsin in each group
| A | B | C | D | |
|---|---|---|---|---|
| IgE (µg/L) | ||||
| 0.10 ± 0.00 | 0.20 ± 0.00* | 0.10 ± 0.00 | 0.28 ± 0.05*,# | |
| IL-4 (µg/L) | ||||
| 0.04 ± 0.01 | 0.14 ± 0.01* | 0.05 ± 0.01 | 0.15 ± 0.02*,# | |
| IL-9 (µg/L) | ||||
| 0.08 ± 0.01 | 0.10 ± 0.01* | 0.08 ± 0.01 | 0.21 ± 0.01*,# | |
| Intestinal trypsin (ng/mL) | 0.08 ± 0.00 | 0.17 ± 0.01* | 0.11 ± 0.01* | 0.23 ± 0.02*,# |
Compared with the blank control group (A),
P<0.05;
compared with group C,
P<0.05.
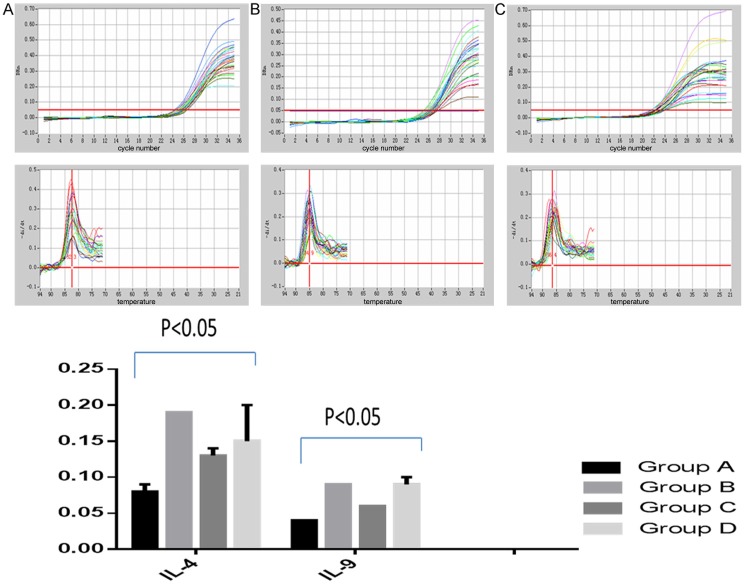
Comparisons of IL-4 and IL-9 mRNA in each group
As was shown in Table 1, primer sequences content of IL-4 and IL-9 mRNA were detected by real-time quantitative PCR. As was shown in Figure 2, compared with IBS- control group, expression level of IL-4 and IL-9 mRNA were significantly decreased in IBS- knockout group with significant difference (P<0.05).
Figure 2.
Quantitative PCR for detection the content of IL-4 and IL-9 mRNA.
Observations of distribution of ileocecal mucosa DC in IBS rats by immunohistochemical CD103 labeled
As was shown in Figure 3, after labeled with the CD103, we observed the distribution of ileocecal colon mucosa DC in each group. We found that, compared with D group, the distribution of DC in Group C increased significantly. There was no significant difference between group A and group B.
Figure 3.
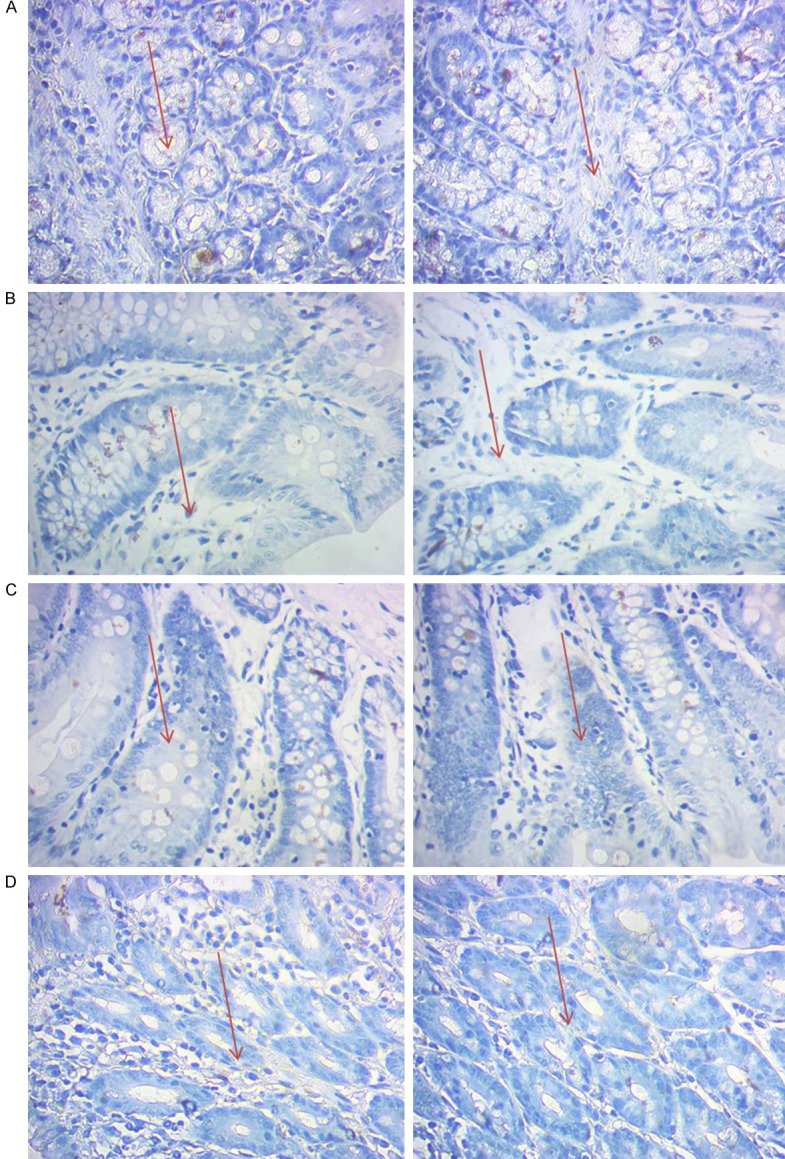
Observation of distribution of ileocecal mucosa DC in IBS rats labeled with immunohistochemical CD103 (×400).
Toluidine blue staining method for the enumeration of intestinal mucosal MC
Toluidine blue staining methods were used to observe the changes in the distribution of the number of intestinal MC among groups. We can see that the distribution of MC was the most in the IBS-control group (Figure 4). The number of MC decreased significantly after knockout of PDIA3.
Figure 4.
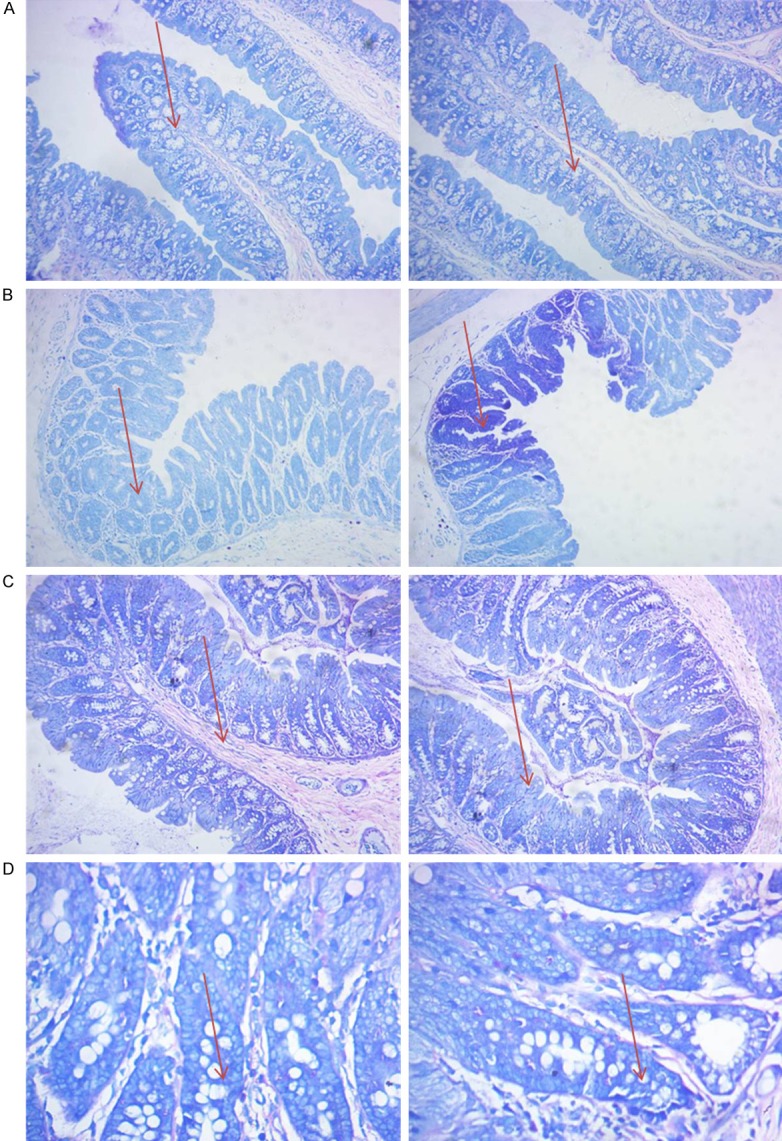
Toluidine blue staining method for counting the intestinal mucosa MC (×100).
Observation of morphology of intestinal mucosal mast cell and ultrastructure of degranulation mast cell by TEM
Electron microscope ultrastructure in each group showed that mast cell cytoplasm were filled with coarse secretory granules, including heparin, histamine, eosinophil chemotactic factor and so on. It can be found that in the IBS control group mast cells released granules matter (degranulation of mast cells). But after knockout of IBS-PDIA3, degranulation was reduced obviously (Figure 5).
Figure 5.
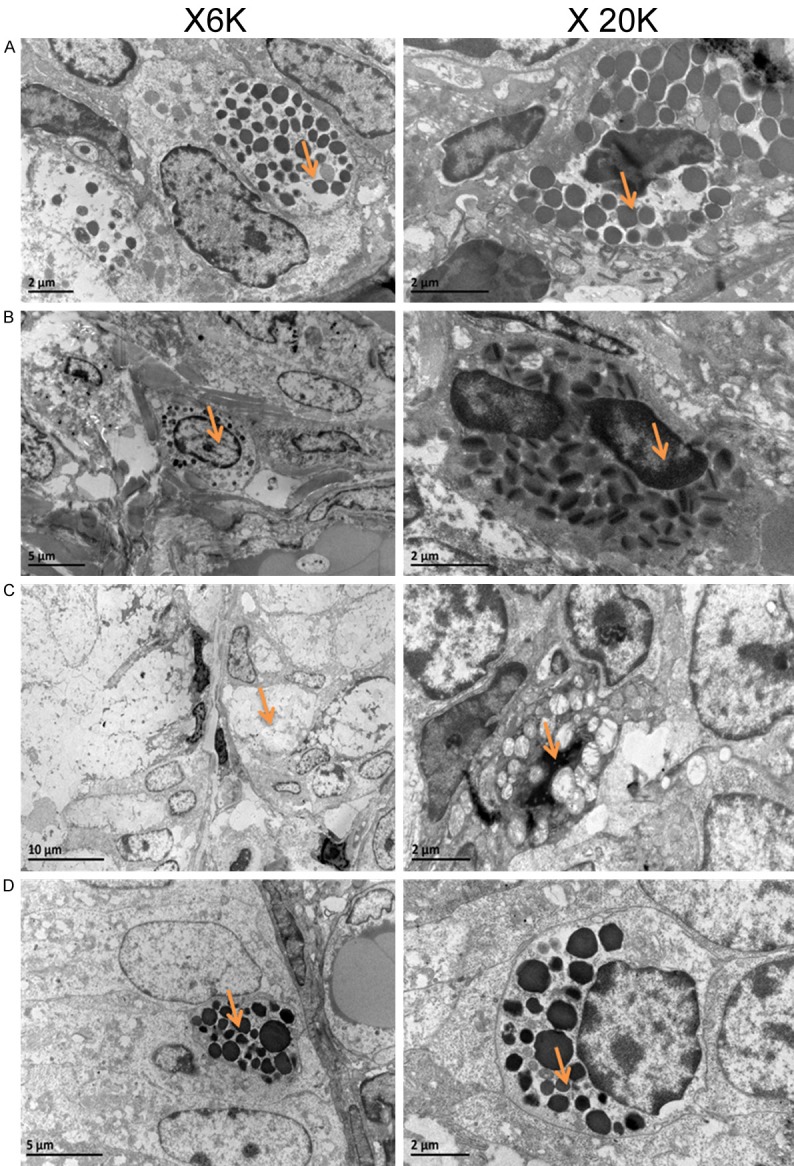
Observation of morphology of intestinal mucosal mast cell and ultrastructure of degranulation mast cell by TEM.
Detection of intestinal expression level of PAR-2 and PAID3 by western blot
In PAID knockout group, the expression level of PDIA was significantly reduced. Also, expression level of PAR-2 receptor was significantly decreased. While in IBS control group PAR-2 receptors was significantly up-regulated (Figure 6).
Figure 6.
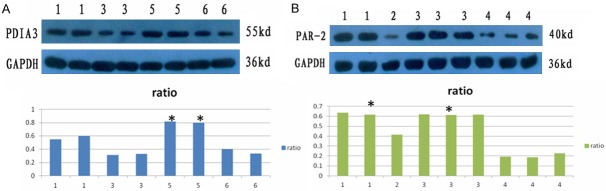
Expression of PAR-2, PAID3 protein. 1: showed the ileocecal intestinal mucosa in the control group; 2: showed the ileocecal intestinal mucosa in IBS1-GFP group; 3: showed the ileocecal intestinal mucosa in IBS-PAID knockout group; 4: showed ileocecal intestinal mucosa in IBS-control group; 5: showed the colon tissue of the blank control group; 6 showed the colon tissue in IBS1-PAID group. A: *P<0.05, compared to IBS-control group; B: *P<0.05, compared to IBS1-PAID group.
Discussion
Irritable Bowel Syndrome was a functional bowel disorder which was based on abdominal pain or discomfort and accompanied by abnormal defecation character. Currently the pathogenesis of IBS was not fully understood. The possible pathophysiological mechanisms include intestinal motility disorders, visceral hypersensitivity, brain-gut axis interactions, inflammation, and intestinal nerve-immune-endocrine network regulation disorders. Increased sensitivity in visceral was the main pathological feature of its physiological basis. Protein disulfide isomerase (protein disulfide isomerase, PDI) mainly existed in multifunctional protein of mammalian and yeast endoplasmic reticulum lumen.
PDI family is a sulfhydryl-disulfide oxidoreductase which played an important role in the endoplasmic reticulumd and belonged to folding enzymes. Its main function was catalyzed oxidative folding of the endoplasmic reticulum nascent peptide chain and play an important role in degradation pathway of endoplasmic reticulum associated proteins, protein transport calcium homeostasis, antigen-presenting viruses and other aspects. PDIA3 is an important component of sulfhydryl protein oxidoreductases of the family. Studies have shown that PDIA3 protein was upregulated in visceral hypersensitivity rat model of colon tissue indicating that overexpression of PT3IA3 correlated with abnormal immune response of colonic drilling membrane. PD1A3 may be an important protein molecule in the reaction of leading to intracellular immune response signal transduction cascade amplification reaction. In this study, we use PD1A3 knockout rats as subjects. We found that immunization targets and levels of inflammatory cytokines were significantly decreased after PDIA3 knockout and inflammation were reduced significantly, confirming that PDIA3 gene played an important role in the pathogenesis of IBS.
In addition, intestinal mast cells located nearby mucosal blood glance, lymphatic and nerves. It both had immunological activity and secreted a variety of media. It was major intestinal antigen receptor and involved in immune regulation of intestinal mucosa. Mast cells played an important role in the pathogenesis of IBS. Several studies have demonstrated that cell numbers of ileocecal mast in IBS patients were significantly higher than that in the controls. When a specific antigen stimulated the body immune system, sensory nerve endings excitement and signal transmission can be caused. At the same time, the production of IgE antibodies were induced. The combination of antigen and antibody can cause the activation of mast cell, degranulation, and release a variety of biologically active media such as tryptase, histamine, serotonin, prostaglandins. Tryptase was a specific neutral protease of secretory granules in almost all mast cells, which was a specific marker of mast cell activation and degranulation. Tryptase was a strong activator of protease activated receptor-2 (PAR-2), while the activation of PAR-2 were closely related to high sensitivity of visceral. Clinical studies have found the expression level of colon trypsin and tryptase as well proteolytic activity were increased in IBS patients. In this study, pancreatic and intestinal IgE levels were significantly decreased after PDIA3 knockout. At the same time the number of mast cells was significantly reduced, and the expression level of PAR-2 was significantly downregulated. This further confirmed that PDIA3 gene played an important role in the pathogenesis of IBS.
In short, PDIA3 may play an important role in the development of IBS by mediating through immune responses of mucosal abnormalities. However, the mechanism needs to be confirmed in further study.
Acknowledgements
This study was supported by National Natural Science Foundation of China (H0307).
Disclosure of conflict of interest
None.
References
- 1.Spinelli A. Irritable bowel syndrome. Clin Drug Investig. 2007;27:15–33. doi: 10.2165/00044011-200727010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Chang FY, Lu CL, Chen TS. The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil. 2010;16:389–400. doi: 10.5056/jnm.2010.16.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265–269. doi: 10.1007/s11894-009-0039-x. [DOI] [PubMed] [Google Scholar]
- 4.Ghaith O, El-Halabi M, Hashash JG, Sharara AI. Investigational agents for the irritable bowel syndrome. Expert Opin Investig Drugs. 2010;19:1161–1178. doi: 10.1517/13543784.2010.513380. [DOI] [PubMed] [Google Scholar]
- 5.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 6.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389–398. doi: 10.1111/j.1365-2982.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 8.Aerssens J, Camilleri M, Talloen W, Thielemans L, Göhlmann HW, Van Den Wyngaert I, Thielemans T, De Hoogt R, Andrews CN, Bharucha AE, Carlson PJ, Busciglio I, Burton DD, Smyrk T, Urrutia R, Coulie B. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou AN, Santos SG, Campbell EC, Lynch S, Arosa FA, Powis SJ. ERp57 interacts with conserved cysteine residues in the MHC class I peptide-binding groove. FEBS Lett. 2007;581:1988–1992. doi: 10.1016/j.febslet.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Lu B, Chen D, Meng L, Shen Y, Chen S. Proteomic analysis of colonic mucosa in a rat model of irritable bowel syndrome. Proteomics. 2010;10:2620–30. doi: 10.1002/pmic.200900572. [DOI] [PubMed] [Google Scholar]
- 11.Santos SG, Campbell EC, Lynch S, Wong V, Antoniou AN, Powis SJ. Major histocompatibility complex class I-ERp57-tapasin interactions within the peptide-loading complex. J Biol Chem. 2007;282:17587–17593. doi: 10.1074/jbc.M702212200. [DOI] [PubMed] [Google Scholar]
- 12.Stepensky D, Bangia N, Cresswell P. Aggregate formation by Erp57-deficient MHC class I peptide-loading complexes. Traffic. 2007;8:1530–1542. doi: 10.1111/j.1600-0854.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 14.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugajev V, Bambousková M, Dráberová L, Dráber P. What precedes the initial tyrosine phosphorylation of the high affinity IgE receptor in antigen-activated mast cell? FEBS Lett. 2010;584:4949–4955. doi: 10.1016/j.febslet.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwada M, Levy DM, McKeag L, Murray K, Schröder AJ, Canfield SM, Traver G, Rothman PB. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci U S A. 2010;107:821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louahed J, Kermouni A, Van Snick J, Renauld JC. IL-9 induces expression of granzymes and high-affinity IgE receptor in murine T helper clones. J Immunol. 1995;154:5061–5070. [PubMed] [Google Scholar]
- 18.Li H, Rostami A. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol. 2010;5:198–209. doi: 10.1007/s11481-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 19.Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of post infective gut dysfunction. Gastroenterology. 2005;129:131–141. doi: 10.1053/j.gastro.2005.03.049. [DOI] [PubMed] [Google Scholar]