Abstract
Object: In order to provide an updated quantification of the association between alcohol intake and colorectal cancer, we conducted a meta-analysis of published observational studies. Method: Two cohort and 22 case-control studies presenting results for at least three categories of alcohol intake were identified from a PubMed search of articles published before July 2014. Data were extracted independently by two reviewers. Random effects meta-analyses, subgroup analyses, and meta regression were performed for modeling the dose-response relation. Result: The pooled relative risk (RR) for any alcohol intake compared with non/occasional drinking was 1.13 [95% confidence interval (CI), 1.09-1.17]. The RRs were 1.07 (95% CI, 1.02-1.13), 1.23 (95% CI, 1.15-1.32) and 1.37 (95% CI, 1.26-1.49) for light (≤12.5 g/day), moderate (12.6 to 49.9 g/day) and heavy drinking (≥50 g/day), respectively. The risks were consistent in the subgroup analyses of sex and tumor site. Conclusion: This meta-analysis provides strong evidence for an association between alcohol intake and colorectal cancer risk.
Keywords: Alcohol intake, colorectal neoplasms, meta-analysis
Introduction
Alcohol is widely consumed throughout the world and is thought to be related to more than 60 different medical conditions [1], and alcohol intake is a potentially modifiable behavior that may be related to risk for colorectal cancer [2]. The evidence that alcohol is a cause of bowel cancer is convincing in men and probable in women [3]. The National Institutes of Health [4], the National Cancer Institute [5], Cancer Research [6], the American Cancer Society [7], the Mayo Clinic [8], and the Colorectal Cancer Coalition [9], American Society of Clinical Oncology and the Memorial Sloan-Kettering Cancer Center list alcohol as a risk factor.
Moreover, epidemiologic studies suggest that increased alcohol is a risk factor for colorectal cancer. Previous reviews [10-13] and meta-analyses [14-16] of case-control and cohort studies suggested that high alcohol intake might be associated with an increased risk of colorectal cancer [17]. The epidemiological evidence has been complemented by recent molecular evidence on mechanisms that could explain the association [17]. However, several issues remained unresolved. First, the dose-response of alcohol intake with colorectal cancer risk has not yet been investigated in detail. Second, it is still uncertain whether the effect of alcohol varies across tumor site.
With the aim of investigating the risk of colorectal cancer at different levels of alcohol consumption, we conducted a meta-analysis of studies published before July 2014.
Methods for meta-analysis
Search strategy
A thorough search of the MEDLINE, EMBASE, and Cochrane Controlled Trials Register databases was performed using MESH terms “colorectal carcinoma”, “alcohol drinking”, “alcoholic beverages”, “colorectal neoplasms”. When necessary, manual searches of references from relevant articles were performed. Also, reference lists of the identified articles and previous literature reviews and meta-analyses were carefully examined for additional studies. The search was limited to studies published in English. Two researchers independently screened the list of references and excluded inappropriate papers. Disagreements were discussed with another reviewer and resolved by consensus.
Inclusion criteria
Two authors (Yue Wang and Helen Yang) independently evaluated the titles and abstracts of potentially eligible studies with the inclusion criteria as follows: (i) observational epidemiological studies (case-control, case-cohort, or cohort) on total alcohol intake and colorectal cancer incidence or mortality in general population, (ii) reporting the odds ratio (OR) or relative risk (RR) estimates with the corresponding 95% confidence intervals (CI) or sufficient information to calculate them for each alcohol exposure level, and (iii) reporting an association for at least three categories of alcohol consumption. When several reports were published on the same study, only the most recent and informative one was included.
Data extraction
Two reviewers (Hong Duan and Boshi Duan) independently assessed articles for inclusion, extracted data, and assessed quality. Quality assessment included assessment of randomization, allocation concealment, blinding, and description of withdrawals and dropouts and was used to give an overall rating of the risk of bias. The following information was sought form each paper: trial’s name, first author, year of publication, journal, number of patients in both groups, sex, tumor site, geographic region, country and follow-up duration (Tables 1 and 2).
Table 1.
Characteristics of case-control studies
| First author | Country | Sex | Site | Year | Cases | Controls | Duration | Variables adjusted | |
|---|---|---|---|---|---|---|---|---|---|
| Cope47 | United Kingdom | Male, Female | Colon, Rectum | 1991 | 66 | 83 | unknown | Age, sex | |
| Riboli48 | France | Male, Female | Colon, Rectum | 1991 | 252 | 641 | 1979-1985 | Age, calories without alcohol, intake of fiber from vegetables and fruit | |
| Honjo49 | Japan | Male | Colon | 1992 | 116 | 930 | 1989-1990 | Smoking, Self-Defense Forces Rank, BMI | |
| Martinez50 | United States | Male, Female | Colon, Rectum | 1995 | 157 | 480 | 1991-1993 | Age, sex, race, dietary fiber, dietary vitamin C, smoking BMI, family history, physical activity, NSAIDs | |
| Todoroki51 | Japan | Male | Colon | 1995 | 228 | 1484 | 1991-1992 | Pank, BMI, physical activity, hospital, survey season, smoking | |
| Ulrich52 | United States | Male, Female | Colon, Rectum | 1999 | 527 | 645 | 1991-1994 | - | |
| Morimoto53 | United States | Male, Female | Colon, Rectum | 2002 | 437 | 708 | 1991-1994 | Age, sex, BMI, HRT, smoking | |
| Tiemersma54 | Netherlands | Male, Female | Colon, Rectum | 2003 | 433 | 436 | 1995-2000 | Sex, age, indication for endoscopy | |
| Boyapati55 | United States | Male, Female | Colon, Rectum | 2004 | 177 | 228 | 1995-1997 | Age, sex, energy | |
| Toyomura56 | Japan | Male | Colon, Rectum | 2004 | 754 | 1547 | 1995-2002 | Bank, hospital, body mass index, physical activity, smoking | |
| Diergaarde57 | Netherlands | Male, Female | Colon, Rectum | 2005 | 278 | 414 | 1997-2001 | Age, gender, total energy intake | |
| Stern58 | United States | Male, Female | Colon, Rectum | 2006 | 753 | 799 | 1991-1995 | Age at diagnosis, sex, race, clinic, and exam date, study phase status, smoking status | |
| Tabata59 | Japan | Male | Colon, Rectum | 2006 | 446 | 914 | 1997-2001 | unknown | |
| Hazra60 | United States | Female | Colon, Rectum | 2007 | 556 | 557 | 1989-1998 | unknown | |
| Jung61 | United States | Male, Female | Colon, Rectum | 2008 | 530 | 645 | 1991-1994 | unknown | |
| Lightfoot62 | United Kingdom | Male, Female | Colon, Rectum | 2008 | 317 | 296 | 1997-2000 | unknown | |
| Shrubsole19 | United States | Male, Female | Colon, Rectum | 2008 | 639 | 1773 | 2003-2005 | Age, sex, site, year, recruitment type, BMI, height, indication for colonoscopy, educational attainment, race, family history, NSAIDs, physical activity, menopausal status, daily intakes of fruits and vegetables, dairy foods, meat, smoking | |
| Yamaji63 | Japan | Male, Female | Colon, Rectum | 2009 | 782 | 738 | 2004-2005 | Smoking, drinking status, BMI, family history, NSAIDs | |
| Yamamoto64 | Japan | Male, Female | Colon, Rectum | 2010 | 86 | 258 | 2004-2007 | unknown | |
| Shin43 | Korea | Male, Female | Colon, Rectum | 2011 | 1242 | 3019 | 2007-2009 | Sex, age, waist circumference, family history, smoking | |
| Corral65 | United States | Male, Female | Colon, Rectum | 2013 | 721 | 736 | 1991-1995 | unknown | |
| Hamachi66 | Japan | Male | Colon, Rectum | 2013 | 455 | 1052 | 1997-2001 | unknown | |
Table 2.
Characteristics of cohort studies
| First author | Country | Sex | Sites | Year | Cases | Non cases | Followed | Adjusted variable | |
|---|---|---|---|---|---|---|---|---|---|
| Giovannucci67 | United States | Male, Female | Colon | 1993 | 895 | 25 474 | Male (1986-1990) | Age, sex, BMI, parental history of colorectal cancer, body fat and dietary fiber intake, indications of endoscopy, history of endoscopy | |
| Female (1980-1990) | |||||||||
| Cho31 | United states | Female | Colon, Rectum | 2007 | 2408 | 39 246 | 1984-2002 | Age, smoking, BMI, physical activity, family history of colon cancer, history of endoscopic screening, year of endoscopy, NSAIDs, HRT, energy, folate, total fiber and calcium | |
Categories of alcohol consumption
Different studies used different units to express alcohol intake. Therefore, alcohol consumption was converted into grams of ethanol per day using the following conversion factors: 1 drink =12.5 g; 1 ounce =28.35 g; and 1 ml=0.8 g. The dose associated with each RR estimate was computed as the midpoint of the corresponding exposure category. When the highest category was open ended, the midpoint was calculated as 1.2 times its lower bound [18]. Nondrinkers or occasional drinkers were the reference category. We defined light alcohol drinking consumption as ≤1 drink/day (≤12.5 g/day of ethanol), moderate as 2-3 drinks/day (12.6-49.9 g/day of ethanol), and heavy as consumption of ≥4 drinks/day (≥50 g/day of ethanol). When more than one study category fell in the range considered for light, moderate or heavy alcohol drinking, or when the same set of controls was used for CRA site (colon and rectum), we combined the corresponding risk estimates by using the method according to Hamling et al [19].
Statistical analysis
All statistical tests were two-sided, and all statistical analyses were carried out with SPSS 16.0 and Stata Statistical Software 13.0. A random effects model was used to estimate pooled RRs in order to take into account the heterogeneity of the risk estimates and to provide more conservative estimates compared with the fixed effects model [20]. Forest plots were done for any, light, moderate, and heavy versus non-consumption and occasional alcohol consumption. Statistical heterogeneity between studies was assessed with the chi-square statistic and quantified by I2, a statistic that represents the percentage of total variation contributed by between-study variation [20,21]. A significant heterogeneity was defined as a P value <0.10. To investigate potential sources of between study heterogeneity, subgroup analyses and meta-regression models were conducted. Also, sensitivity analyses were carried out to assess whether the summary estimates are robust to inclusion of studies. Publication bias was assessed using the tests by Egger [22], Begg and Mazumdar [23], and the contour enhanced funnel plots [24].
A dose-response analysis was carried out using both linear and nonlinear random effects models on the natural logarithm of the RR using the method by van Houwelingen [25], which was modified by our group [26]. This method accounts for correlation between reported risk estimate within the same study, heterogeneity between the studies, and nonlinear dose-risk relation. Thirty-six second-order fractional polynomial random effects models and linear random effect models were tested. The best-fitting model, defined as the one with the lowest Akaike’s information criterion, a model fit statistic, was selected as the final dose-risk relation model.
Results
Study detail
Figure 1 shows the number of studies assessed and excluded through the stages of the meta-analysis. A total of 24 studies on colorectal cancer incidence and alcohol intake published between 1991 and 2013 were identified, among which 8 studies were from Asia (Japan and Korea), 5 from Europe (United Kingdom, France and Netherlands), and 11 from United States.
Figure 1.
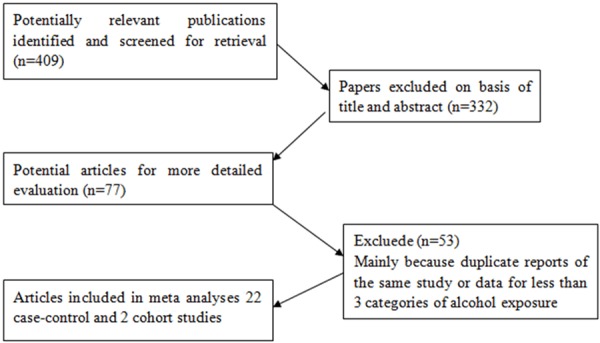
Flow diagram of assessment of studies identified in the systematic review.
As a whole, Figure 2 shows the study-specific and pooled RRs of colorectal cancer, along with 95% CIs, for any alcohol drinking versus none/occasional drinking. The overall pooled RR was 1.13 (95% CI, 1.09-1.17) and there was no significant between studies heterogeneity (I2=21.7%, p for heterogeneity =0.17). The corresponding estimates were 1.13 (95% CI, 1.09-1.17) for case–control studies (I2=26.6%, p for heterogeneity =0.12) and 1.15 (95% CI, 0.98-1.31) for cohort studies (I2=0.0%, p for heterogeneity =0.39). Data were available for light intake from 23 studies, for moderate intake from 20 studies and for heavy intake from 9 studies. The pooled RRs for light (≤1 drink/day), moderate (>1 to b3 drinks) and heavy drinking (≥3 drinks/day) were equal to 1.07 (95% CI, 1.02-1.13), 1.23 (95% CI, 1.15-1.32) and 1.37 (95% CI, 1.26-1.49) respectively (Table 3).
Figure 2.
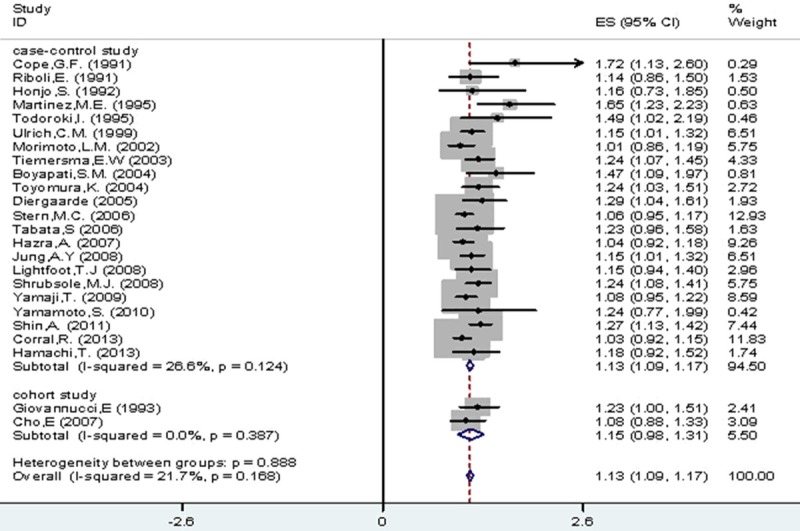
All drinkers vs. non-/occasional drinkers according to type of studies.
Table 3.
Stratified RR estimates for colorectal adenoma risk
| Factors stratified | Drinkers vs. non-/occasional drinkers | Light vs. non-/occasional drinkers | Moderate vs. non-/occasional drinkers | Heavy vs. non-/occasional drinkers | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||
| No. | RR | LCI | UCI | P value | I2 (%) | No. | RR | LCI | UCI | P value | I2 (%) | No. | RR | LCI | UCI | P value | I2 (%) | No. | RR | LCI | UCI | P value | I2 (%) | |
| All studies | 24 | 1.13 | 1.09 | 1.17 | 23 | 1.07 | 1.02 | 1.13 | 20 | 1.23 | 1.15 | 1.32 | 9 | 1.37 | 1.26 | 1.49 | ||||||||
| Study type | ||||||||||||||||||||||||
| Case-control | 22 | 1.13 | 1.09 | 1.17 | 0.17 | 21.70% | 21 | 1.08 | 1.02 | 1.14 | 0.06 | 33.00% | 18 | 1.24 | 1.15 | 1.33 | 0.01 | 52.10% | 9 | 1.37 | 1.26 | 1.49 | 0.69 | 0.00% |
| Cohort | 2 | 1.15 | 0.98 | 1.31 | 2 | 1.02 | 0.85 | 1.21 | 2 | 1.25 | 0.96 | 1.64 | 0 | unknown | unknown | unknown | ||||||||
| Sex | ||||||||||||||||||||||||
| Male | 8 | 1.19 | 1.07 | 1.32 | 0.21 | 24.00% | 7 | 0.97 | 0.86 | 1.10 | 0.71 | 0.00% | 7 | 1.28 | 1.15 | 1.44 | 0.15 | 30.90% | 7 | 1.38 | 1.22 | 1.57 | 0.47 | 0.00% |
| Female | 4 | 1.03 | 0.95 | 1.10 | 3 | 0.98 | 0.91 | 1.06 | 4 | 1.14 | 1.04 | 1.25 | 1 | 0.95 | 0.64 | 1.42 | ||||||||
| Geographical region | ||||||||||||||||||||||||
| Asia | 9 | 1.20 | 1.12 | 1.28 | 0.01 | 43.70% | 8 | 1.03 | 0.94 | 1.14 | 0.06 | 33.00% | 8 | 1.29 | 1.13 | 1.47 | 0.01 | 52.10% | 7 | 1.36 | 1.23 | 1.51 | 0.69 | 0.00% |
| Europe | 5 | 1.24 | 1.12 | 1.36 | 4 | 1.19 | 1.06 | 1.34 | 4 | 1.30 | 1.11 | 1.52 | 1 | 1.14 | 0.87 | 1.51 | ||||||||
| USA | 11 | 1.12 | 1.05 | 1.20 | 11 | 1.06 | 0.99 | 1.14 | 8 | 1.18 | 1.08 | 1.28 | 1 | 1.50 | 1.28 | 1.75 | ||||||||
| Tumor site | ||||||||||||||||||||||||
| Colon | 6 | 1.18 | 1.08 | 1.30 | 0.91 | 0.00% | 5 | 1.02 | 0.91 | 1.14 | 0.59 | 0.00% | 6 | 1.35 | 1.21 | 1.50 | 0.87 | 0.00% | 4 | 1.23 | 1.03 | 1.47 | 0.54 | 0.00% |
| Rectum | 3 | 1.42 | 1.03 | 1.96 | 2 | 1.28 | 0.83 | 1.98 | 3 | 1.41 | 0.95 | 2.08 | 2 | 1.77 | 1.09 | 2.88 | ||||||||
As for sex, Figure 3 showed RRs estimated for CRA incidence in male (1.11, 95% CI 1.00-1.23) and female (1.03, 95% CI 0.95-1.10) and individually, in the comparison between all drinkers and non-/occasional drinkers (I2=18.60%, P=0.27). And there was no significant difference in CRA risk between male and female among light (I2=0.00%, P=0.711), moderate (I2=30.90%, P=0.15) and heavy (I2=0.00%, P=0.474) drinkers, compared with non-/occasional drinkers (Table 3).
Figure 3.
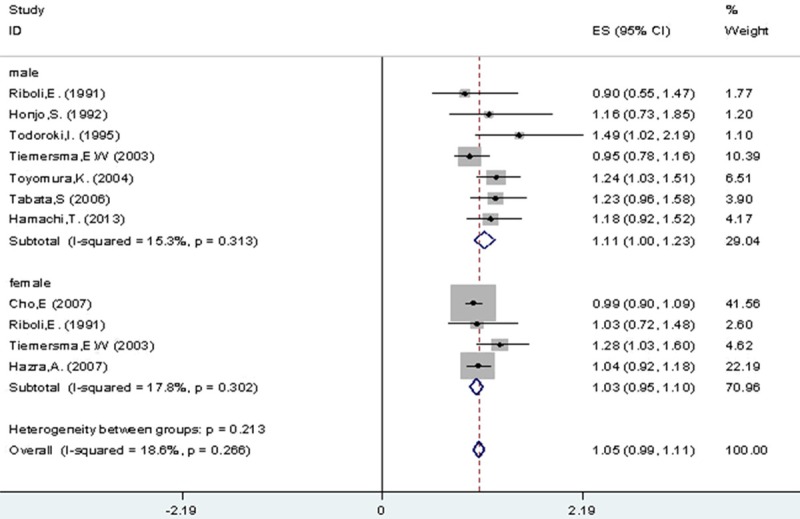
All drinkers vs. non-/occasional drinkers according to gender.
As for geographical region, we proposed RRs for CRA risk stratified by Asia, Europe and US. The result is (1.19, 95% CI 1.11-1.27), (1.22, 95% CI 1.10-1.34) and (1.10, 95% CI 1.05-1.15) respectively. Moreover, the risk in European studies was higher than them in the US and Asia. And there was difference in the pooled analysis of all drinkers (I2=21.70%, P=0.17), light drinkers (I2=33.00%, P=0.06) and moderate drinkers (I2=52.10%, P=0.00), compared with non-/occasional drinkers (Figure 4; Table 3).
Figure 4.
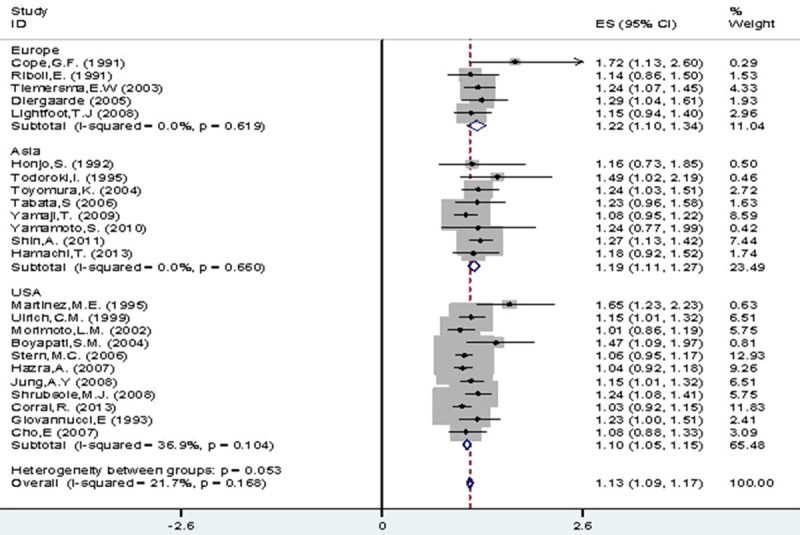
All drinkers vs. non-/occasional drinkers according to geographic region.
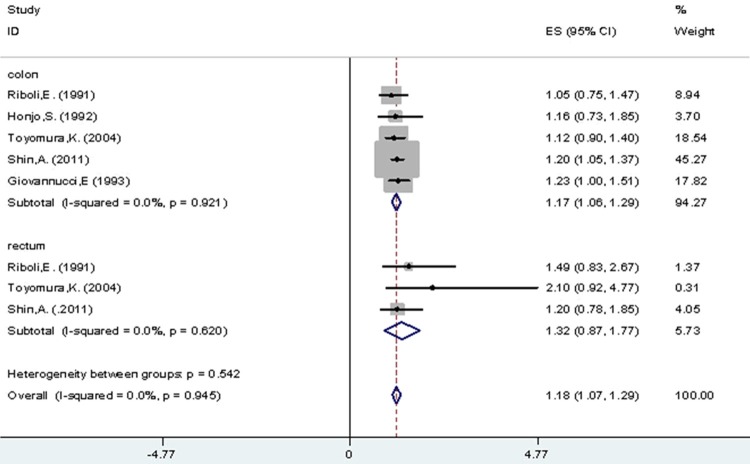
As for tumor site, we evaluated for CRA risk in colon and rectum were 1.17 (95% CI 1.06-1.29) and 1.32 (95% CI 0.87-1.77) respectively with no significant heterogeneity (I2=0.00%, P=0.911). In addition, there was no significant difference in CRA risk between colon and rectum among light (I2=0.00%, P=0.945), moderate (I2=0.00%, P=0.873) and heavy (I2=0.00%, P=0.535) drinkers, compared with non-/occasional drinkers (Figure 5; Table 3).
Figure 5.
All drinkers vs. non-/occasional drinkers according to tumor site.
Publication bias
Begg’s test was carried out to access the publication bias in our studies. In the analysis of all drinkers vs. non-/occasional drinkers, Begg’s test revealed a significant publication bias (Begg’s Test, P=0.03). However, the studies on light alcohol category and CRA risk showed no statistical evidence of publication bias (Begg’s Test, P=0.09). Moreover, the studies on moderate alcohol category and CRA risk also presented no statistical evidence of publication bias (Begg’s Test, P=0.167).
Sensitivity analyses
In the sensitivity analysis, when one study was removed and the rest were analyzed sequentially by meta-analysis. Any study in overweight or obesity group was omitted, the pooled RRs were not materially altered with the overall pooled RRs, indicating that our results were statistically robust.
Dose-response analysis
Our meta-regression analysis shows a significant dose-response relation between alcohol intake and colorectal cancer risk, the more alcohol intake, the higher risk of colorectal cancer. All drinkers were associated with 13% increased risk for CRA, the rational polynomial model estimates of RR were1.03 (95% CI 0.92-1.20), 1.08 (95% CI 1.02-1.19), 1.14 (95% CI 1.07-1.21) and 1.43 (95% CI 1.25-1.64) for 10, 25, 50 and 100 g/day of alcohol intake respectively, compared with nondrinkers or occasional alcohol drinkers (Figure 6).
Figure 6.

Dose-response association of alcohol intake and colorectal cancer risk.
Discussion
We have systematically reviewed published studies on the association between alcohol intake and the risk of colorectal cancer. In this meta-analysis, alcohol consumption was positively associated with risk for colorectal cancer.
In general, all drinkers were associated with 13% increased risk for CRA, compared with nondrinkers or occasional alcohol drinkers. The dose-response analysis demonstrated that for drinkers of 10, 25, 50 and 100 g/day alcohol consumption, the estimated RRs of CRA were 1.03 (95% CI 0.92-1.20), 1.08 (95% CI 1.02-1.19), 1.14 (95% CI 1.07-1.21) and 1.43 (95% CI 1.25-1.64) respectively, in comparison with non-/occasional drinkers. Our meta-regression analysis shows a significant dose-response relation between alcohol intake and colorectal cancer risk-that is, the more alcohol intake, the higher risk of colorectal cancer. Furthermore, it is acknowledged that the dose-response relation from meta-regression (that is, between study investigation) should be viewed as exploratory and could be prone to confounding. Meta-analysis with individual participant data would have an advantage both statistically and clinically [27,28] and, if available, should be used in the future to explore the dose-response relation further. Nevertheless, the dose-response relation found in our study is consistent with that observed from rigorously controlled trials with multiple levels of alcohol intake, which provided the most persuasive evidence.
In our study, the significant relationship between alcohol consumption and CRA risk was consistent for both female and male in the subgroup analyses of sex. Moreover, one research showed a stronger association in men compared to women, possibly because alcohol intake is higher and more popular in men than in women. As for tumor site, the association of alcohol drinking with colorectal cancer risk did not differ between colon and rectal anatomic subsites, which stands in line with previous meta-analysis [29-31] and pooled analysis [32,33]. Some previous observational studies and one pooled study [34,35-38] showed a stronger positive association of moderate and heavy alcohol drinking with cancer in the distal colon compared with cancer in the proximal colon, but the difference was not statistically significant. In terms of geographical region, a large number of researches enabled us to investigate whether there is a difference among Asian, European and USA populations. Our study has found the association was stronger in European studies, compared with the studies in the USA and Asia, except heavy and past drinkers. Potential explanations for these findings include (i) a high prevalence (up to 30%) of the slow-metabolizing variant of aldehyde dehydrogenase enzyme, which is associated with increased blood levels of acetaldehyde after alcohol ingestion [39], and (ii) other non-genetic factors, for instance, body composition [40]. The next step, further research about colorectal cancer-alcohol intake among South American and African populations should be done.
Furthermore, evidence suggests that alcohol can act as a prooxidant in tissues, including lung tissue [41-48], and on lipids, including lung membrane lipids [41,49]. Alcohol can induce the expression of enzymes that are related to carcinogen metabolism [50], and compounds other than ethanol that are contained in alcoholic beverages may have carcinogenic effects. Several mechanisms have been proposed for the effect of alcohol on risk for colorectal cancer. First, acetaldehyde, an oxidation product of alcohol, may be responsible for colorectal carcinogenesis [51,52]. A recent study reported that high levels of acetaldehyde in rat colon degrade folate, a nutrient that is hypothesized to reduce the risk for colorectal cancer [53]. Second, alcohol is an antagonist of methyl-group metabolism and may contribute to abnormal DNA methylation, an early step in colonic carcinogenesis [54,55]. Finally, greater alcohol intake may increase the risk for colorectal cancer indirectly through immune suppression, delay of DNA repair, activation of liver procarcinogens by induction of cytochrome P-450 enzymes, or changes in bile acid composition [56].
Moreover, acetaldehyde is produced by the liver as it breaks down ethanol. The liver then normally eliminates 99% of the acetaldehyde. An average liver can process 7 grams of ethanol per hour. For example, it takes 12 hours to eliminate the ethanol in a bottle of wine, giving 12 hours or more of acetaldehyde exposure. A study of 818 heavy drinkers found that those who are exposed to more acetaldehyde than normal through a defect in the gene for alcohol dehydrogenase are at greater risk of developing cancers of the upper gastrointestinal tract and liver [57]. There are many associations between alcohol drinking and different types of cancer. Data that is based from 2009, there were about 3.5 percent of cancer deaths in the U.S. alone because of alcohol drinking [58].
Our study had several strengths. First, our meta-analysis included a large number of studies published up to July 2014, and these cancer cases allowed to investigate the risk associated with three categories of alcohol consumption. Then Begg’s test was carried out to access the publication bias in our studies, and did not support the presence of major publication bias, providing further indication of the robustness of our findings. Finally, linear and nonlinear random effects models on the natural logarithm of the RR were used to investigate the association between colorectal cancer risk and alcohol consumption, which allowed us to conduct traditional meta-analysis by categories of alcohol drinking and dose-response analysis.
Limitations of our study, first, we noted that the majority of the data were derived from case-control studies, which may be subject to certain types of bias, for instance, recall and selection bias. But the findings got from case-control studies were in line with prospective cohort studies. Then, for non-drinkers of a specific alcoholic beverage might drink other type of beverage, so the type of alcoholic beverage together with lifetime exposure to alcohol, and drinking patterns, were not included in our study. As a result, considering certain type of beverage might induce to an underestimation of the risk associated with the true amount of alcohol consumed. Then, the type of alcoholic beverage, as well as lifetime exposure to alcohol, and drinking patterns, were not included in the analyses because nondrinkers of a specific alcoholic beverage might drink other beverages. Thus, considering specific beverages could lead the true amount of alcohol consumed to be underestimated. The next, we had only one measure of alcohol consumption at baseline and could not investigate a whole lifetime alcohol consumption, changes in alcohol consumption or alcohol consumption at younger ages. Finally, no attempt was made to identify unpublished work and grey literature, for example university theses or conference proceedings. As a result, publication bias may have influenced the results [59,60]. And only English literatures were included in this study, it is possible that our findings are biased for many non-English literatures are not included.
Conclusion
Our results have shown that alcohol consumption was associated with an increase in risk for colorectal cancer. Moreover, the risk was consistent in subgroup analyses of sex and tumor site, while it was stronger in European studies than the studies in the US and Asia. Thus, public health recommendations for colorectal cancer prevention should consider limiting intake of alcoholic beverages.
Disclosure of conflict of interest
None.
References
- 1.Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98:1209–28. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institutue for Cancer Research; 1997. [Google Scholar]
- 3. Types of cancer. World Cancer Research Fund. Archived from the original on 9 June 2009. Retrieved 29 June 2009. [Google Scholar]
- 4. Colorectal Cancer-Step 1: Find Out About Colorectal Cancer Risk. National Cancer Institute. Retrieved 29 June 2009. [Google Scholar]
- 5. “Colorectal Cancer Prevention”. National Cancer Institute. 7 May 2009. Retrieved 29 June 2009. [Google Scholar]
- 6. “Food types and bowel cancer”. Cancer Research. 19 September 2008. Retrieved 29 June 2009. [Google Scholar]
- 7. “What Are the Risk Factors for Colorectal Cancer?”. American Cancer Society. 18 May 2009. Archived from the original on 19 April 2008. Retrieved 26 June 2009. [Google Scholar]
- 8. “Colon Cancer: Risk factors”. Mayo Clinic. 2 May 2008. Retrieved 29 June 2009. [Google Scholar]
- 9. “Assessing Your Risk for Colorectal Cancer”. Colorectal Cancer Coalition. 9 January 2009. Retrieved 29 June 2009. [Google Scholar]
- 10.Alcohol drinking. IARC Working Group, Lyon, 13-20 October 1987. IARC Monogr Eval Carcinog Risks Hum. 1988;44:1–378. [PMC free article] [PubMed] [Google Scholar]
- 11.Kune GA, Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992;18:97–111. doi: 10.1080/01635589209514210. [DOI] [PubMed] [Google Scholar]
- 12.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993;15:499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 13.Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523–6. doi: 10.1016/s0899-9007(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 14.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–5. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–19. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Longnecker MP, Orza MJ, Adams ME, Vioque J, Chalmers TC. A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer. Cancer Causes Control. 1990;1:59–68. doi: 10.1007/BF00053184. [DOI] [PubMed] [Google Scholar]
- 17.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 18.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic doseresponse data. Epidemiology. 1993;4:218–28. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–70. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 24.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnelplots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 25.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 26.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functionsfor modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159:1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 27.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 28.Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient- versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med. 2002;21:371–87. doi: 10.1002/sim.1023. [DOI] [PubMed] [Google Scholar]
- 29.Corrao G, Bagnardi V, Zambon A, Arico S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction. 1999;94:1551–1573. doi: 10.1046/j.1360-0443.1999.9410155111.x. [DOI] [PubMed] [Google Scholar]
- 30.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 31.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2007;120:664–671. doi: 10.1002/ijc.22299. [DOI] [PubMed] [Google Scholar]
- 32.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Holmberg L, Kim DH, Malila N, Miller AB, Pietinen P, Rohan TE, Sellers TA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 33.Mizoue T, Inoue M, Wakai K, Nagata C, Shimazu T, Tsuji I, Otani T, Tanaka K, Matsuo K, Tamakoshi A, Sasazuki S, Tsugane S Research Group for Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol. 2008;167:1397–1406. doi: 10.1093/aje/kwn073. [DOI] [PubMed] [Google Scholar]
- 34.Akhter M, Kuriyama S, Nakaya N, Shimazu T, Ohmori K, Nishino Y, Tsubono Y, Fukao A, Tsuji I. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: the Miyagi Cohort Study. Eur J Cancer. 2007;43:383–390. doi: 10.1016/j.ejca.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Bongaerts BW, van den Brandt PA, Goldbohm RA, de Goeij AF, Weijenberg MP. Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer. 2008;123:2411–2417. doi: 10.1002/ijc.23774. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari P, Jenab M, Norat T, Moskal A, Slimani N, Olsen A, Tjønneland A, Overvad K, Jensen MK, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Rohrmann S, Linseisen J, Boeing H, Bergmann M, Kontopoulou D, Trichopoulou A, Kassapa C, Masala G, Krogh V, Vineis P, Panico S, Tumino R, van Gils CH, Peeters P, Bueno-de-Mesquita HB, Ocké MC, Skeie G, Lund E, Agudo A, Ardanaz E, López DC, Sanchez MJ, Quirós JR, Amiano P, Berglund G, Manjer J, Palmqvist R, Van Guelpen B, Allen N, Key T, Bingham S, Mazuir M, Boffetta P, Kaaks R, Riboli E. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2007;121:2065–2072. doi: 10.1002/ijc.22966. [DOI] [PubMed] [Google Scholar]
- 37.Hu J, Morrison H, Mery L, DesMeules M, Macleod M Canadian Cancer Registries Epidemiology Research Group. Diet and vitamin or mineral supplementation and risk of colon cancer by subsite in Canada. Eur J Cancer Prev. 2007;16:275–291. doi: 10.1097/01.cej.0000228411.21719.25. [DOI] [PubMed] [Google Scholar]
- 38.Sharpe CR, Siemiatycki J, Rachet B. Effects of alcohol consumption on the risk of colorectal cancer among men by anatomical subsite (Canada) Cancer Causes Control. 2002;13:483–491. doi: 10.1023/a:1015700415808. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000) Alcohol Clin Exp Res. 2001;25:15S–32S. doi: 10.1097/00000374-200105051-00005. [DOI] [PubMed] [Google Scholar]
- 40.Mizoue T, Inoue M, Wakai K, Nagata C, Shimazu T, Tsuji I, Otani T, Tanaka K, Matsuo K, Tamakoshi A, Sasazuki S, Tsugane S Research Group for Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol. 2008;167:1397–1406. doi: 10.1093/aje/kwn073. [DOI] [PubMed] [Google Scholar]
- 41.Nachiappan V, Mufti SI, Chakravarti A, Eskelson CD, Rajasekharan R. Lipid peroxidation and ethanol-related tumor promotion in Fischer-344 rats treated with tobacco-specific nitrosamines. Alcohol Alcohol. 1994;29:565–74. [PubMed] [Google Scholar]
- 42.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–63. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 43.Knecht KT, Bradford BU, Mason RP, Thurman RG. In vivo formation of a free radical metabolite of ethanol. Mol Pharmacol. 1990;38:26–30. [PubMed] [Google Scholar]
- 44.Kukielka E, Cederbaum AI. The effect of chronic ethanol consumption on NADH- and NADPH-dependent generation of reactive oxygen intermediates by isolated rat liver nuclei. Alcohol Alcohol. 1992;27:233–9. [PubMed] [Google Scholar]
- 45.Ishii H, Kurose I, Kato S. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J Gastroenterol Hepatol. 1997;12:S272–82. doi: 10.1111/j.1440-1746.1997.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 46.Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20:138A–46A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 47.Dupont I, Lucas D, Clot P, Menez C, Albano E. Cytochrome P4502E1 inducibility and hydroxyethyl radical formation among alcoholics. J Hepatol. 1998;28:564–71. doi: 10.1016/s0168-8278(98)80279-1. [DOI] [PubMed] [Google Scholar]
- 48.Yang M, Coles BF, Delongchanmp R, Lang NP, Kadlubar FF. Effects of the ADH3, CYP2E1, and GSTP1 genetic polymorphisms on their expressions in Caucasian lung tissue. Lung Cancer. 2002;38:15–21. doi: 10.1016/s0169-5002(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 49.Manautou JE, Carlson GP. Ethanol-induced fatty acid ethyl ester formation in vivo and in vitro in rat lung. Toxicology. 1991;70:303–12. doi: 10.1016/0300-483x(91)90005-l. [DOI] [PubMed] [Google Scholar]
- 50.Seitz HK, Garro AJ, Lieber CS. Enhanced pulmonary and intestinal activation of procarcinogens and mutagens after chronic ethanol consumption in the rat. Eur J Clin Invest. 1981;11:33–8. doi: 10.1111/j.1365-2362.1981.tb01762.x. [DOI] [PubMed] [Google Scholar]
- 51.Seitz HK, Simanowski UA, Garzon FT, Rideout JM, Peters TJ, Koch A, Berger MR, Einecke H, Maiwald M. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology. 1990;98:406–13. doi: 10.1016/0016-5085(90)90832-l. [DOI] [PubMed] [Google Scholar]
- 52.Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann Med. 1996;28:195–200. doi: 10.3109/07853899609033120. [DOI] [PubMed] [Google Scholar]
- 53.Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer. 2000;86:169–73. doi: 10.1002/(sici)1097-0215(20000415)86:2<169::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 55.Choi SW, Stickel F, Baik HW, Kim YI, Seitz HK, Mason JB. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J Nutr. 1999;129:1945–50. doi: 10.1093/jn/129.11.1945. [DOI] [PubMed] [Google Scholar]
- 56.Kune GA, Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992;18:97–111. doi: 10.1080/01635589209514210. [DOI] [PubMed] [Google Scholar]
- 57.Homann N, Stickel F, König IR, Jacobs A, Junghanns K, Benesova M, Schuppan D, Himsel S, Zuber-Jerger I, Hellerbrand C, Ludwig D, Caselmann WH, Seitz HK. Alcohol dehydrogenase 1C*1 allele is a genetic marker for alcohol-associated cancer in heavy drinkers. Int J Cancer. 2006;118:1998–2002. doi: 10.1002/ijc.21583. [DOI] [PubMed] [Google Scholar]
- 58.What is the evidence that alcohol drinking is a cause of cancer? National Cancer Institute [1] 2 [Google Scholar]
- 59.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and Related baises. Health Technol Assess. 2000;4:1–115. [PubMed] [Google Scholar]
- 60.Steenbrink F, de Groot JH, Veeger HE, Meskers CG, van de Sande MA, Rozing PM. Pathological muscle activation patterns in patients with massive rotator cuff tears, with and without subacromial anaesthetics. Man Ther. 2006;11:231–237. doi: 10.1016/j.math.2006.07.004. [DOI] [PubMed] [Google Scholar]