Abstract
Airway remodeling can lead to irreversible airflow obstruction and persistent airway hyper-responsiveness, which is the pathological basis of refractory asthma. To investigate the preventive effect of protocatechuic aldehyde on airway remodeling in asthmatic mice by lung morphometry methods. BALB/c mice were used to establish model of airway remodeling by ovalbumin (OVA) inhalation. Bronchoalveolar lavage fluid (BALF) were collected for eosinophils (EOS) count and detection of interleukin 4 (IL-4), interleukin-13 (IL-13) and interferon (IFN-γ) content. The left lung pathological sections were performed HE, AB-PAS and Masson staining. The epithelial lamina thickness of the left main bronchus (Re), the smooth muscle layer thickness (Rm), the number of goblet cells and goblet cell area percentage (%Ac) and gas side of the road and vascular collagen deposition (%Aco, %Avc) situation were measured. Protocatechuic aldehyde gavage made the reduction of BALF EOS count. IL-4 and IL-13 levels also decreased, while the IFN-γ level increased. The left main bronchus Re, Rm, goblet cell count, Ac% and Aco% and Avc% reduced. Protocatechuic aldehyde can significantly control airway inflammation and prevent airway remodeling.
Keywords: Protocatechuic aldehyde, asthma, airway remodeling, lung morphometry
Introduction
Asthma is a common respiratory and occurring disease. With the development of new drugs and the promotion of standardized treatment of asthma, many of the symptoms are well controlled, and the quality of life has been significant improved. But we found that asthma attack is a still inevitable disease in practice even under the strict implementation of standardized treatment regimens, the overall progress continues to show a chronic course [1-3]. Now it is believed that the airway remodeling in asthma may occur in early [4]. In airway remodeling, reversibility of airflow obstruction reduced, the ventilation dysfunction with main expiratory phase aggravated, and lung residual volume increased, which leads to varying degrees of emphysema, and even pulmonary heart disease. The asthma attack symptoms can be temporarily controlled through appropriate means, but airway remodeling gradually increased due to persistent and progressive development. Therefore, if the treatment of asthma did not started from the treatment of airway remodeling, asthma cannot be relieved from the root.
Salvia are common herbs in traditional Chinese medicine for the treatment of cardiovascular and cerebrovascular diseases, which was used in clinical treatment of pulmonary heart disease [5] and asthma. With chemical name of 3, 4-hydroxybenzaldehyde, protocatechuic aldehyde is one of major water-soluble components in the traditional Chinese medicine Salvia [6]. Study on the extraction process for the preparation of protocatechuic aldehyde from Salvia is relatively mature. Currently, whether the protocatechuic aldehyde impacts on asthma has not been reported. Recent studies have shown that protocatechuic aldehyde can inhibit the phosphorylation of phosphatidylinositol 3-kinase (PI-3K) and mitogen-activated protein kinase (MAPK), thus inhibiting the migration and proliferation of vascular smooth muscle [7]. And in airway remodeling, PI-3K and MAPK are main pathways involved in the regulation [8-10], airway smooth muscle is the heart of the pathological changes in airway remodeling, and involves in airway remodeling of extracellular matrix through cell proliferation, hypertrophy, migration, and secretory cells. Therefore, we hypothesizes that protocatechuic aldehyde inhibits airway remodeling.
In this study, ovalbumin inhalation was used to establish a mouse model of airway remodeling, in order to explore the pharmacological effects and mechanism of protocatechuic aldehyde on airway remodeling in asthma mice, which provided experimental evidence for clinical application of protocatechuic aldehyde in the prevention airway remodeling.
Materials and methods
Animals
50 female SPF grade BALB/c mice with age of 6-8 weeks (provided by the Experimental Animal Center of Guangdong Medical College, Animal Certificate of Conformity: SCXK (Guangdong) 2004-2008 2007A034), weight of 20±2 g were housed separately in a clean environment with room temperature (24-28°C) and humidity of 70%. The bedding was changed daily, water and food intake were free. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Guangdong Medical College.
Preparation of protocatechuic aldehyde
5 kg of Salvia (Zhanjiang Hang Seng Pharmaceutical Co., Ltd., China) was used, impurities were removed and cut into sections with 3-5 cm, rinsed with potable water and distilled water. Drained and dried, crushed into powder with 10-18 meshes. Salvia powder was put into the extract pot, added 10 times the amount of distilled water, boiled for 2 hours, filtered, and retained the first decoction. The dregs were boiled in 8 times the amount of distilled water for 1.5 hours, filtered, and retained a second frying liquid. The dregs were boiled in 8 times the amount of distilled water for 1.5 hours, filtered, and retained the third decoction. Removed the dregs, and then merged the three decoctions. The decoction was neutralized with hydrochloric acid, adsorbed with adsorption column charged by weakly basic macroporous resin D301M. After sorbent saturated, eluted with 50% ethanol solution, then with 0.4% of sodium hydroxide aqueous solution, the eluent was collected and concentrated to about 1 L, the PH value was adjusted to be 2 with hydrochloric acid, adsorbed with adsorption column charged by weak polar sorbent AB-8 under acidic conditions, eluted with pure 40 L, then switched to 5 % ethanol solution, collected eluate, and performed vacuum filtration, and the filtrate was concentrated to a final of 100 ml. Then performed stilling to get approximately 6.15 g of protocatechuic aldehyde crystals. After HPLC, the purity reached to 95%. Dissolved in distilled water prior to use to prepare a 2 mg/ml (high dose) and 1 mg/mL (low dose) suspension, mice were administered orally in an amount of 0.1 mL/10 g/d.
Preparation of PA suspension
8 PA tablets (Guangdong Bang Min Pharmaceutical Co., Zhunzi H44021838, China) were ground together with 1 g of Tween 80 in a mortar, then diluted with 100 ml distilled water, 0.4 mg/ml suspension was prepared. Mouse was performed gavage with a dosage of 0.1 ml/10 g/d.
Grouping and treatment
Female BALB/c mice with age of 6-8 weeks were randomly divided into five groups, 10 for each group. The control group (CON) was intraperitoneally injected 0.5 ml saline containing 2.5 mg of aluminum hydroxide gel (Nanning Baihui Pharmaceutical Group Co., Zhunzi H45020626, China) and multi subcutaneous sensitization on the 0, 7th, 14th day. On the 21th day, aerosol inhalation of saline was performed once a day, 30 min for each time. On the 28th day, daily oral administration of 0.1 ml distilled water for 10 g body weight was carried out, and 30 minutes after oral administration of distilled water, aerosol inhalation of saline was performed on every other day, three times a week for eight weeks. The model group (OVA) was intraperitoneally injected 0.5 ml sensitization liquid and multi subcutaneous sensitization on the 0, 7th, 14th day. The sensitization liquid was prepared by ovalbumin 1 mg OVA (V grade, Sigma, USA) and 6.25 ml aluminum hydroxide gel dissolving in 50 ml of normal saline, and began to challenge on 21th day with 1% OVA normal saline solution, once a day, 30 min for each time. On the 28th day, daily oral administration of 0.1 ml distilled water for 10 g body weight was carried out, and 30 minutes after oral administration of distilled water, aerosol inhalation of saline was performed on every other day, three times a week for eight weeks. The sensitization and challenge of the prednisone acetate (OVA + PA) group, protocatechuic aldehyde high dose group (OVA + PCAH), protocatechuic aldehyde low-dose group (OVA + PCAL) were the same with the OVA group. On the 28th day, suspension of OVA + PA or OVA + PCAH or OVA + PCAL was daily orally administrated, 0.1 ml for 10 g body weight, 30 minutes after oral administering on every other day, challenged by inhalation, the inhalation time and method were the same with the OVA group (Figure 1).
Figure 1.
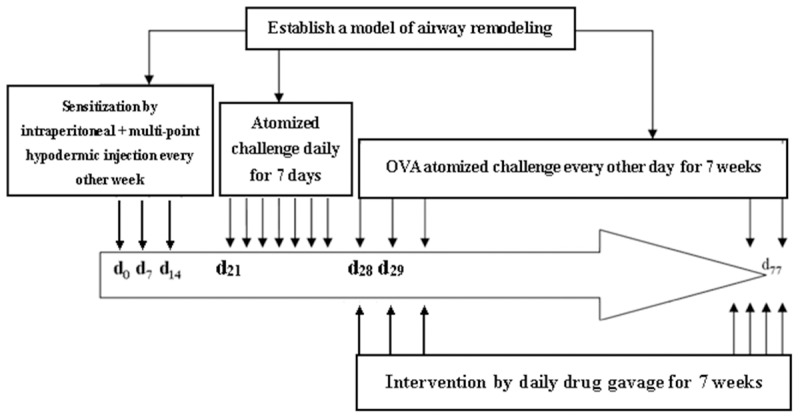
Experimental design.
Bronchoalveolar lavage
According to our previous method [11], 48 h after the last challenge, mice were sacrificed. The chest door of left lung was opened and ligated, the left lung was cut and fixed with 10% formalin, the remaining lung was performed bronchoalveolar lavage lung perfusion. Bronchoalveolar lavage method was bluntly dissected to expose trachea before skin tracheotomy, rapidly made a “T” shaped incision in the lower trachea with 22G intravenous catheter tubes for tracheal intubation, carefully pulled out the needle with the needle into the core, then pushed the indwelling needle forward about 0.5 cm, fixed by a thread, slowly injected 1 ml PBS through the plastic tube with a syringe, right lung sufficient expansion was visible, retained for one minute, then slowly retraced, and repeated for three times, a total of 3 ml PBS solution was injected. The fluid recoveries were more than 85%. Bronchoalveolar lavage fluid (BALF) was collected in a centrifuge tube and stored temporarily at 4°C refrigerator. 0.1 ml BALF used for eosinophil count. The remaining BALF was centrifuged with 12000 rpm for 5 minutes, the supernatant was stored at -20°C refrigerator to be measured cytokine interleukin-4 (IL-4), interleukin-13 (IL-13) and interferon (IFN-γ) levels. Murine IL-4, IL-13 and IFN-γ ELISA kits were purchased from the Haixi Tang Biotechnology Limited. Other reagents were domestic or imported analytical reagents.
Preparation of lung tissue sections
The left lung was performed conventional fixation, dehydration, transparent, wax dipping and embedding after cutting right lung in accordance with the red line in Figure 2, the tissues embedded below the red line were lung tissues. Try best to place all of the lung tissue in one direction when embedded. Adjusted the block holder for fixing paraffin in the slicing, so that each specimen was from the cross-section vertical to the longitudinal axis of the cut for the left lung down to the lungs, started recording the number of Σμm when cut to the lung tissues, at least three paraffin sections with thick of 5 μm was successfully cut between Σμm 1000 μm and 1500 μm (shown in Figure 2, the left main bronchus could be seen in each slice). Three sections of each specimen were obtained for HE, AB-PAS and Masson staining.
Figure 2.
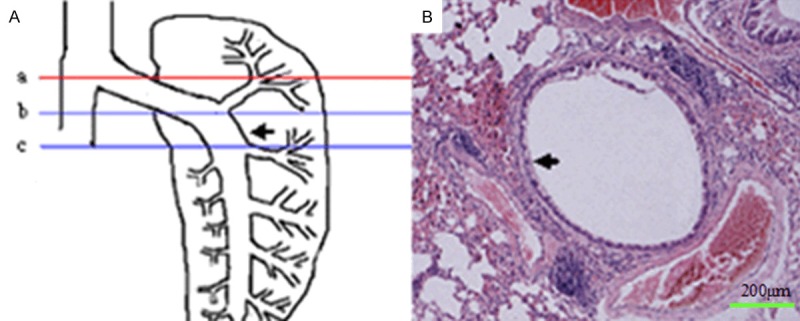
Schematic representation of sample collection and parameter measuring. A. “a” line indicates the site where the porta pulmonis was cut open. The region between “b” line and “c” line was the sites where lung sections were collected. This region was derived by extension of porta pulmonis. The distance between “a” line and “b” line was approximately 1000 μm. The distance between “a” line and “c” line was approximately 1500 μm. At least 3 paraffin sections with a thickness of 5 μm/section were collected from “b” line and “c” line. The arrow in the figure indicates left main bronchus. B. One large bronchus (arrow) and three associated large vessels (one pulmonary artery and two pulmonary veins) were observed in the transaction. This bronchus was selected for the morphometrical analysis.
HE staining
Paraffin sections were deparaffinized to washing. In routine HE staining, hematoxylin treated for 8 minutes, 1% ethanol eosin for 3 minutes, mounted by neutral gum. HE staining semi-quantitative assessment was performed for assessing the degree of bronchial inflammatory cell infiltration in surroundings, each slice were assessed the degree of inflammatory cell infiltration of the left main bronchus and the surrounding three large vessels, the mean values were obtained. The criteria were no inflammatory cells (0), a few inflammatory cells (1 point), more uneven distribution of inflammatory cells (2 points), a large number of inflammatory cells in relatively uniform distribution and rare gathered into a group (3 points), a large number of inflammatory cells clump (4 points).
AB-PAS staining
Paraffin sections were deparaffinized to washing, stained by AB dye for 30 minutes, treated with 3% acetic acid for 3 minutes, then distilled water for three times. Treated by 0.5% periodic acid oxidation for 10 minutes, washed by tap water, then immersed in distilled water for twice. Stained by Schiff liquid for 15 minutes. Rinsed by fluid water for 3 min, washed by distilled water for twice. Then performed dehydration, transparent, mounting and HE staining. AB-PAS positive stained mucilage showed purple. After capturing images by microscopic photograph, Irregular AOI and Segmentation function of Image-Pro Plus 6.0 image analysis software were used to test the left main bronchus epithelial lamina thickness (Re), smooth muscle thickness (Rm), AB-PAS-positive goblet cells stained goblet cell count and area (AG, except for the area of positive staining mucus) on each slice to calculate the relative percentage of goblet cell area and epithelial lamina area (%AG). Of which, airways epithelial lamina and smooth muscle layer thickness were the average thickness conversed from the average radius by measuring epithelial lamina area and the area of smooth muscle layer.
Masson staining
Paraffin sections were deparaffinized to washing. Treated by ponceau acid fuchsin solution for 5 minutes, then slightly differentiated by 0.2% acetic acid aqueous solution, rinsed with distilled water, and treated by 5% phosphomolybdic acid solution for 8 minutes, directly immersed into aniline blue dye for 5 minutes, then treated by 0.2% acetic acid aqueous solution for 2 minutes. After dehydration, transparent, mounting and HE staining, the collagen fibers with Masson staining were blue, muscle fibers, cellulose, red blood cells showed red. After capturing images by microscopic photograph, IrregularAOI and Segmentation function of Image-Pro Plus 6.0 image analysis software were used to measure the collagen deposition area in the selected range (selected a measurement range along the left main bronchus and the outer diameter of the collagen fibers around blood vessels) and the total area of outer diameter range. The relative percentage of target airway, perivascular collagen deposition area (AACO and Avc) and the total area within sedimentary diameter range (AT and At) of target airway collagen fibers was calculated, namely ACO/AT%.
Statistical analysis
Experimental data were indicated by x̅ ± s. SPSS13.0 analysis software was used. Normal distribution of data among the groups were compared using ANOVA, homogeneity of variance using the Bonferroni test, while heterogeneity of variance by Tamhane’s T2 test. P < 0.05, P < 0.01, P < 0.001 indicated statistically significant.
Results
EOS count and content of IL-4, IL-13 and IFN-γ
Compared with the control group, EOS counts and IL-13 and IL-4 content in BALF of the OVA group increased, while the content of IFN-γ reduced (P < 0.01 or P < 0.001). Compared with the OVA group, EOS counts and IL-13 and IL-4 content in BALF of the OVA + PA and OVA + PCAH groups in BALF decreased (P < 0.01 or P < 0.05), while the content of IFN-γ increased (P < 0.01). EOS counts and IL-13 and IL-4 content in BALF of the OVA + PCAL group (P < 0.01) decreased, the content of IL-13 had no significant change (P > 0.05), and the content of IFN-γ increased (P < 0.05; Figure 3).
Figure 3.
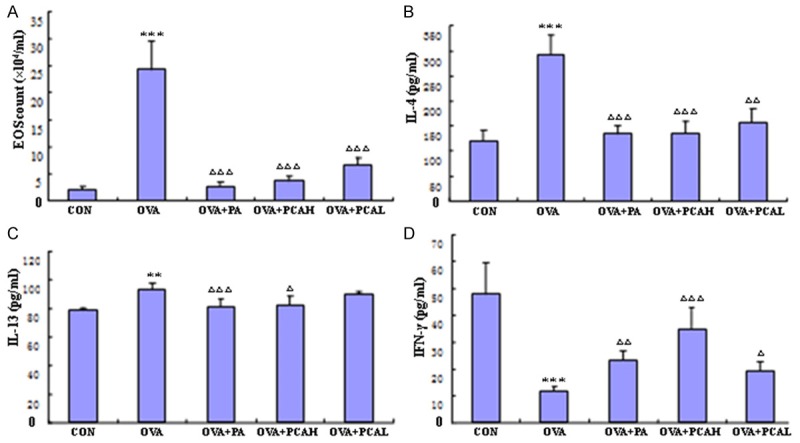
The effects of drugs on the count of Eos, the level of IL-4, IFN-γ and IL-13 in BALF of asthmatic mice. A. EOS counting. B. The level of IL-4. C. The level of IL-13. D. The level of IFN-γ. N = 10, x̅ ± s. **P < 0.01, ***P < 0.001 compared to COM groups. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 compared to OVA groups.
HE staining
Pathological changes of lung tissue for mice in each group after HE staining was observed under light microscope. Significant airway change of model mice was visible, the airway epithelial lamina and smooth muscle layer thickened, goblet cells were hyperplasia, submucosal glands were hypertrophy, airway wall was congestion, intraluminal exudate and inflammatory cells infiltration were significant, alveolar ducts, alveolar sacs and alveolar septa were damaged, a large number of inflammatory cells were seen to aggregate in lung field. The lung tissue of mice in the control group showed no inflammation. The mice lung tissue inflammation in each treatment groups significantly reduced compared with the OVA group, the OVA + PA had the most obvious alleviation, followed by OVA + PCAH (Figure 4A-E).
Figure 4.
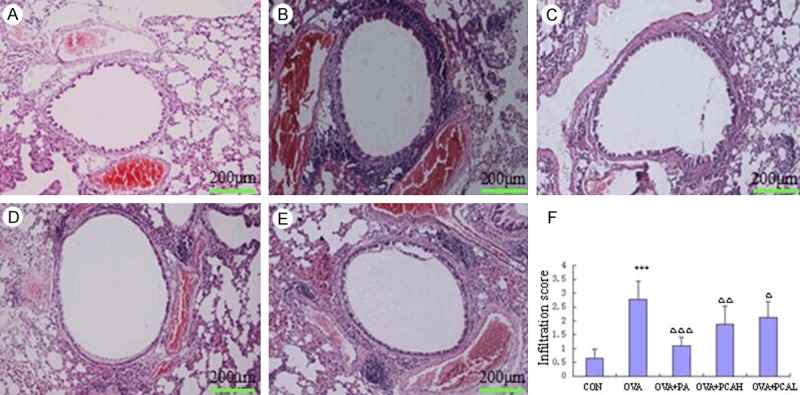
Comparisons of HE staining for left main bronchus (100×) and lung inflammatory cell infiltration score in BABL/c mice. A. Control group: no or rarely seen inflammatory cell infiltration around the airway. B. OVA group: inflammatory cell infiltration around the airway and three large vessels, distributed into pieces or groups, tracheal epithelium and smooth muscle layer significantly thickened compared with the control group, lung interval widened. C. OVA + PA group: compared with the OVA group, airway and perivascular inflammatory cell infiltration significantly reduced, no distribution into groups, no obvious wide alveolar septa compared with the control group. D. OVA + PCAH group: compared with the control group, the airway epithelial lamina and smooth muscle layer had no significant thickening, not obvious widened alveolar septa, but the airway and perivascular inflammatory cell infiltration still increased significantly, occasionally a small amount into the group distribution. E. OVA + PCAL group: showing the airway and perivascular infiltration of inflammatory cells into the group distribution, still widened alveolar septa, the thickness of airway epithelial lamina and smooth muscle layer reduced no obvious than the OVA group. F. comparison of infiltration score of lung inflammatory cells. N = 10, x̅ ± s. ***P < 0.001 compared to COM groups. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 compared to OVA groups.
HE staining semi-quantitative assessment was used to assess the infiltration extent of the left main bronchus and the adjacent perivascular inflammatory cells to obtain the scores of the inflammatory cells in the lungs (Figure 4F). The scores of lung inflammatory cells in the OVA group was higher than that of the control group (P < 0.001). The scores of the OVA + PA, OVA + PCAH, OVA + PCAL groups were lower than that of the OVA group (P < 0.001, P < 0.01 or P < 0.05).
AB-PAS staining
After AB-PAS staining for lung tissues, the mucin granule of goblet cells were stained to be deep purple (Figure 5A-E). The left main bronchus morphometry measurement results showed that the index of model mice increased compared with the control group (P < 0.01 or P < 0.001). Compared with the OVA group, the smooth muscle layer thickness of the OVA + PA group was slightly lower, but the difference was not statistically significant (P > 0.05), the percentage of epithelial lamina thickness, goblet cell count and goblet cell area decreased (P < 0.01 or P < 0.001). The indexes of the OVA + PCAH and OVA + PCAL groups reduced (P < 0.05, P < 0.01 or P < 0.001), especially the decrease of OVA + PCAH group was significant (Figure 5F-I).
Figure 5.
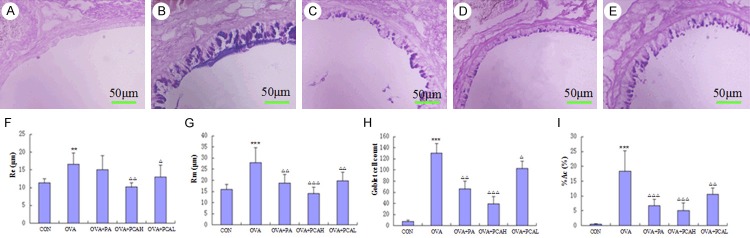
AB-PAS staining (400×) and morphometric measurements of target airway in BABL/c mice. (A) Control group: the airway epithelial lamina and smooth muscle layer was thinner, no positive staining of the epithelial goblet cells. (B) OVA group: compared with the control group, the airway epithelial lamina and smooth muscle layer thickened, positive staining of epithelial goblet cells significantly increased, and goblet cells were hypertrophy, mucus secretion increased in dark purple. (C) OVA + PA group: the thickness of the airway epithelial lamina and positive stained goblet cells significantly reduced compared with the OVA group. (D) OVA + PCAH group: the airway epithelial lamina and smooth muscle layer had no significant thickening, the stained positive epithelial goblet cells were rare, and were close to the level of the control group. (E) OVA + PCAL group: the thickness of airways epithelial lamina and smooth muscle layer decreased compared with the OVA group, the positive stained epithelial goblet cells was still more, but no significant hypertrophy of goblet cells. Quantitative and positioning study of morphometry of lung in AB-PAS staining is shown in (F) (the thickness of smooth muscle layer, Rm), (G) (the thickness of epithelial lamina, Re), (H) (goblet cell count), (I) (the percentage of goblet cell area, % Ac). N = 10, ***P < 0.001 compared to COM groups. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 compared to OVA groups.
Manson staining
After Manson staining of lung tissue, smooth muscle and red blood cells were red, collagen fibers were blue (Figure 6A-E). The left main bronchus morphometry measurement results showed that the index of model mice increased compared with the control group (P < 0.001). Compared with the OVA group, the indexes of the OVA + PA group were lower (P < 0.01 or P < 0.05), the decrease of OVA + PCAH was significantly lower than that of the OVA + PA group (P < 0.01 or P < 0.001), surrounding bronchial collagen deposition of the OVA + PCAL group was lower (P < 0.05), and perivascular collagen deposition had no difference compared with the OVA group (P > 0.05; Figure 6F, 6G).
Figure 6.
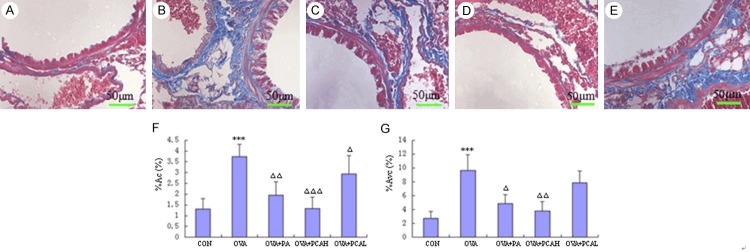
Manson staining (400×) and morphometric measurements of target airway in BABL/c mice. (A) Control group: less airway and perivascular collagen deposition, no or rare collagen deposition in the basement under the plexiform layer. (B) OVA group: a lot of collagen deposition in the airway and vascular smooth muscle surroundings, a lot of collagen deposition in the reticular layer under the airway basal membrane and interstitial lung, the entire field of vision was blue and purple. (C) OVA + PA group: airway and perivascular collagen deposition was significantly reduced compared with OVA group, and only a small amount of collagen fibers in the reticular layer under basement membrane and interstitial lung. (D) OVA + PCAH group: only a few collagen deposition in the entire field of vision, and no difference with the control group. (E) OVA + PCAL group: more airways and perivascular collagen deposition, more obvious interstitial lung collagen deposition. Quantitative and positioning study of morphometry of lung in Manson staining is shown in (F) (the collagen coagulation around bronchial, %ACO) and (G) (the collagen coagulation around vascular, % Avc). N = 10, ***P < 0.001 compared to COM groups. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 compared to OVA groups.
Discussion
In this study, mice airway remodeling model was prepared by ovalbumin inhalation method, lung morphometry methods were used to explore the effect of protocatechuic aldehyde on the prevention and treatment of airway remodeling in asthmatic mice. The results suggested that all OVA-sensitized BALB/c mice had varying degrees of asthma attacks after inhalation of the same allergens. The airway epithelial lamina of model mice lung tissue was swelling, smooth muscle thickened, the airway wall and the surroundings had a lot of EOS and lymphocyte predominant inflammatory cell infiltration. These changes were consistent with the pathological changes of airway remodeling reported in the literature [12-14]. This experiment showed that induction of airway remodeling model in BALB/c mice was successful.
Currently, domestic and international evaluation for airway remodeling model focused on histomorphometry. But histomorphometric method choosing by different researchers differed widely. For example, in the different choosing of fixed-embedded lung lobes, and some selected the right, the mid or lower lobes for embedding sections, some selected the left lung, and some researchers directly chose the entire right lung for embedding, even the left and right lung together were embedded, prepared into sections, and observed. Morphometry measurement methods for lung tissue sections also varied, without fixed comparison sites, the comparability between the models will be greatly reduced. On the measurement methods, the specific morphological differences were more obvious. For example, Ford et al [15] collected five complete bronchial cross-sectional images under light microscope with 15 to 200 times were analyzed. Francisco et al [16] chose five horizons (up, down, left, right and mid) of each slice for 16 specimens to acquire bronchial cross-section images under 200 times light microscope and analyzed. Chen et al [17] randomly selected each slice of five bronchi with diameter of 300-1000 μm, long and short diameters ratio ≥ 0.6 to observe. Ma et al [18] selected at least 10 bronchi per slice to perform observation. In the above researches, because of the lack of comparability among samples researchers in the selection of the sites in slices, and big differences among the structures of different hierarchical bronchial epithelial lamina and smooth muscles, such as the progressively reduction of goblet cells, usually no goblet cells in small bronchi, while the progressively increase of the amount of smooth muscles, so under the premise of no guarantee for the same number and type in each slice cross-section of the bronchi, even multiple bronchial cross-sections were selected, the error brought by nature differences cannot be avoided.
The innovation of this study was the use of external signs of lung tissue (hilar) to effectively locate, then pathological sections were prepared within a fixed range. The iconic site of selected morphometric measurements was only one, that is, the left main bronchus. The operation was simple with strong repeatability and comparability. And in the specific morphological measurement methods, the present study combined the IrregularAOI and Segmentation function of Image-Pro Plus 610 image analysis software, the automatic tracking function in Irregular was used to precisely define the boundary of the epithelial lamina, calculated the respective average thickness by measuring the area of the epithelial lamina and smooth muscle layer, and reduced the artificial error in defining boundary for skin layer. Moreover, taking the characteristics such as crease bronchial epithelial lamina edge and smooth muscle layers into account, in the calculation of specific indicators, perimeter was not chosen as the conversion indicator, while a unified definition of an area was used as a conversion indicator, the experiment results were more scientific and persuasive. In this study, the morphological metrology results under HE, AB-PAS and Masson staining for the iconic sites showed that the indexes of the OVA group were significantly higher than those in the control group, the differences were significant, which can reflect pathological features of airway remodeling in asthmatic mice.
Glucocorticoids were the most effective anti-inflammatory drugs, which were the most commonly used in clinical treatment of asthma medication, can effectively improve lung function and airway obstruction [19]. In this study, glucocorticoids were used as a positive control drug to observe the effect of PA on each index of airway remodeling. The results showed that oral administration of PA produced a more significant intervention effect on airway remodeling of asthmatic mice, which not only significantly inhibited the infiltration of inflammatory cells in asthmatic airways, but also inhibit subsequent epithelial lamina thickness of the airway wall in chronic inflammation and brought back to normal levels. Although the increased thickness of smooth muscle layer and collagen deposition in airway played a certain degree of inhibition, but not completely, this was consistent with the reports of Johnson et al [20] and Trifilieff et al [21]. In other words, even though PA intervention in early asthma can well suppress airway inflammation, only partially inhibited airway remodeling process.
Protocatechuic aldehyde with high doses significantly inhibited asthmatic airway inflammation and inflammatory mediators. Compared with the OVA group, EOS count in BALF was significantly lower, which was close to the level of PA group, and the content of IL-13 and IL-4 was significantly lower, while a significant increase in IFN-γ levels (reaching the level of the control group). The inflammatory cell infiltration score of lung tissue in OVA + PCAH group was still higher than the control group, but significantly lower than the OVA group. The effect of OVA + PCAL on asthmatic airway inflammation and inflammatory mediators was weaker than the high dose group. After the administration of OVA + PCAH, although the role of reducing inflammation in asthma airway was slightly worse compared with PA, remained significant effect compared with the OVA group. And the role of OVA + PCAH administration in reducing IL-13 and IL-4 content in BALF had no difference compared with the OVA + PA group, and the increased IFN-γ content was superior to OVA + PA group. Although the effect in OVA + PCAL group was not more significant than high dose group, but still had some improvement on airway inflammation and inflammatory mediators. Test results in the measurement of lung tissue morphometry showed that the airway smooth muscle layer thickness, the thickness of the epithelial lamina and goblet cell count, percentage of goblet cell area, bronchi and perivascular collagen deposition of the OVA + PCAH group decreased significantly compared with the OVA group, including airway smooth muscle layer thickness and the thickness of the epithelial lamina, bronchial and perivascular collagen deposition basically reached the normal control group. In other words, the OVA + PCAH group was better in the inhibition of airway remodeling than PA. OVA + PCAL group had no significant effect as the OVA + PCAH group, but the index was significantly reduced compared with the OVA group.
The mechanisms of protocatechuic aldehyde in anti-asthmatic airway remodeling may be related to the anti-inflammatory effects. Airway remodeling in asthma was airway wall damage based on airway inflammation, not complete repair involved in a variety of cells, inflammatory mediators and growth factors [22-25]. This process was very complex mechanism involving in the effect of TGF-β family and Th1, Th2 cytokines [26-29]. TGF-β produced by asthma epithelial cells, fibroblasts, eosinophils and airway macrophages stimulated fibroblasts synthesis and secretion of extracellular matrix (ECM) proteins including collagen I, collagen III, fibronectin, transparent connexin, tenascin and proteoglycans, while a significant increase in airway mucosal TGF-β expression, and related with the basement membrane thickening, the number of fibroblasts and the severity of disease [30]. This directly or indirectly induced airway hyperresponsiveness caused by the degranulation of mast cells and EOS, as well as inflammatory cytokines IL-4. IL-4 stimulated cultured human fibroblasts, synthesis of extracellular matrix [31], also promoted the proliferation of human fibroblasts [32]. IL-13 was a major factor causing fibrosis in a lot of chronic infections and autoimmune diseases. The interactions of IL-13 and TGF-β1 were the main factor leading to chronic inflammation and tissue fibrosis [33]. IL-13 can independently involve in bronchial asthma and airway inflammation remodeling [34]. The IFN-γ was a Th1 cells release cytokine with inhibition of airway remodeling. The imbalance of Thl/Th2 directly or indirectly played a very important role not only in the pathogenesis of asthma and its respective release of inflammatory cytokines in the whole process, as well as airway remodeling process. Therefore, in this study, the IL-4, IL-13 and IFN-γ levels in BALF were detected, and the molecular mechanisms of protocatechuic aldehyde in the prevention of airway remodeling were explored. The decreased IL-4 and IL-13 content in BALF of the OVA + PCAH group had no difference compared with OVA + PA group, while the increasing IFN-γ content was superior to the OVA + PA group,which can more fully correct Th1/Th2 imbalance. This was one of the probable reasons for the better effect of protocatechuic aldehyde inhibiting airway remodeling than PA.
Currently, researches about the prevention treatment of protocatechuic aldehyde on asthma and airway remodeling were still in its infancy. Further researches will help us to find effective drugs to inhibit airway remodeling, and make efforts to solve the treatment problem of asthma.
Acknowledgements
This study was supported by Guangdong Science and Technology Program project funded projects (No. 2011B031600007).
Disclosure of conflcit of interest
None.
References
- 1.Rowe BH, Villa-Roel C, Majumdar SR, Abu-Laban RB, Aaron SD, Stiell IG, Johnson J, Senthilselvan A AIR Investigators and AIR Investigators. Rates and correlates of relapse following emergency department discharge for acute asthma: a canadian 20-site prospective cohort study. Chest. 2015;147:140–149. doi: 10.1378/chest.14-0843. [DOI] [PubMed] [Google Scholar]
- 2.Sładek K. Remodelling in obturative diseases and treatment. Pol Merkur Lekarski. 2010;29:227–230. [PubMed] [Google Scholar]
- 3.Haahtela T. Lung function decline in asthma and early intervention with inhaled corticosteroids. Chest. 2006;129:1405–1406. doi: 10.1378/chest.129.6.1405. [DOI] [PubMed] [Google Scholar]
- 4.Yang MS, Lee HS, Kim MH, Song WJ, Kim TW, Kwon JW, Kim SH, Park HW, Chang YS, Cho SH, Min KU. Rhinitis patients with sputum eosinophilia show decreased lung function in the absence of airway hyperresponsiveness. Allergy Asthma Immunol Res. 2013;5:232–238. doi: 10.4168/aair.2013.5.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Huang Y, Zhao C, Qin X, Zhu Q, Chen S, Qu J. Salvia miltiorrhiza Injection on Pulmonary Heart Disease: A Systematic Review and Meta-Analysis. Am J Chin Med. 2014;42:1315–1331. doi: 10.1142/S0192415X14500827. [DOI] [PubMed] [Google Scholar]
- 6.Gu M, Su ZG, Janson JC. One-step purification of 3,4-dihydroxyphenyllactic acid, salvianolic acid B, and protocatechualdehyde from Salvia miltiorrhiza Bunge by isocratic stepwise hydrogen bond adsorption chromatography on cross-linked 12% agarose. J Chromatogr Sci. 2008;46:165–168. doi: 10.1093/chromsci/46.2.165. [DOI] [PubMed] [Google Scholar]
- 7.Moon CY, Ku CR, Cho YH, Lee EJ. Protocatechuic aldehyde inhibits migration and proliferation of vascular smooth muscle cells and intravascular thrombosis. Biochem Biophys Res Commun. 2012;423:116–121. doi: 10.1016/j.bbrc.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 8.Hu R, Pan W, Fedulov AV, Jester W, Jones MR, Weiss ST, Panettieri RA Jr, Tantisira K, Lu Q. MicroRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway. FASEB J. 2014;28:2347–2357. doi: 10.1096/fj.13-247247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velden JL, Hoffman SM, Alcorn JF, Tully JE, Chapman DG, Lahue KG, Guala AS, Lundblad LK, Aliyeva M, Daphtary N, Irvin CG, Janssen-Heininger YM. Absence of c-Jun NH2-terminal kinase 1 protects against house dust mite-induced pulmonary remodeling but not airway hyperresponsiveness and inflammation. Am J Physiol Lung Cell Mol Physiol. 2014;306:L866–875. doi: 10.1152/ajplung.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang L, Li F, Bao A, Zhang M, Chung KF, Zhou X. Activation of p38 mitogen-activated protein kinase in ovalbumin and ozone-induced mouse model of asthma. Respirology. 2013;18:20–29. doi: 10.1111/resp.12189. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Fu H, Yang H, Wang H, Zhang H, Qin D. Smooth muscle progenitor cells involved in the development of airway remodeling in a murine model of asthma. Asian Pac J Allergy Immunol. 2014;32:203–210. doi: 10.12932/AP0395.32.3.2014. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Barbón D, Ludwig MS, Martin JG. Airway remodeling: lessons from animal models. Clin Rev Allergy Immunol. 2004;27:3–21. doi: 10.1385/CRIAI:27:1:003. [DOI] [PubMed] [Google Scholar]
- 13.Royce SG, Patel KP, Samuel CS. Characterization of a novel model incorporating airway epithelial damage and related fibrosis to the pathogenesis of asthma. Lab Invest. 2014;94:1326–1339. doi: 10.1038/labinvest.2014.119. [DOI] [PubMed] [Google Scholar]
- 14.Berair R, Saunders R, Brightling CE. Origins of increased airway smooth muscle mass in asthma. BMC Med. 2013;11:145. doi: 10.1186/1741-7015-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grünig G. IL-13 and IFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–1777. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 16.Francisco JS, Moraes HP, Dias EP. Evaluation of the Image-Pro Plus4.5 software for automatic counting of labeled nuclei by PCNA immunohistochemistry. Braz Oral Res. 2004;18:100–104. doi: 10.1590/s1806-83242004000200002. [DOI] [PubMed] [Google Scholar]
- 17.Chen PF, Luo YL, Wang W, Wang JX, Lai WY, Hu SM, Cheng KF, Al-Abed Y. ISO-1, a macrophage migration inhibitory factor antagonist, inhibits airway remodeling in a murine model of chronic asthma. Mol Med. 2010;16:400–408. doi: 10.2119/molmed.2009.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Ma X, Ma Z, Wang J, Sun Z, Yu W, Li F, Ding J. Effect of Hyssopus officinalis L. on inhibiting airway inflammation and immune regulation in a chronic asthmatic mouse model. Exp Ther Med. 2014;8:1371–1374. doi: 10.3892/etm.2014.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leemans J, Kirschvink N, Clercx C, Snaps F, Gustin P. Effect of short-term oral and inhaled corticosteroids on airway inflammation and responsiveness in a feline acute asthma model. Vet J. 2012;192:41–48. doi: 10.1016/j.tvjl.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Johnson PR, Black JL, Carlin S, Ge Q, Underwood PA. The Produetion of extraeellular matrix proteins by human passively sensitized airway smooth musele cells in culture: the effect of beclomethasone. Am J Respir Crit Care Med. 2000;162:2145–2151. doi: 10.1164/ajrccm.162.6.9909111. [DOI] [PubMed] [Google Scholar]
- 21.Trifilieff A, EI-Hashim A, Bertrand C. Time course of inflamrnatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1120–1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- 22.Hsu CH, Hu CM, Lu KH, Yang SF, Tsai CH, Ko CL, Sun HL, Lue KH. Effect of selective cysteinyl leukotriene receptor antagonists on airway inflammation and matrix metalloproteinase expression in a mouse asthma model. Pediatr Neonatol. 2012;53:235–244. doi: 10.1016/j.pedneo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Henderson WR Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 24.Chen WJ, Liaw SF, Lin CC, Lin MW, Chang FT. Effects of zileuton on airway smooth muscle remodeling after repeated allergen challenge in brown Norway rats. Respiration. 2013;86:421–429. doi: 10.1159/000353427. [DOI] [PubMed] [Google Scholar]
- 25.Palmans E, Kips JC, Pauwels RA. Prolonged allergen exposure induces struetural airway changes in sensitized rats. Am J Respir Crit Care Med. 2000;161:627–635. doi: 10.1164/ajrccm.161.2.9902094. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Hwang HJ, Kim Y. Modeling the role of TGF-β in regulation of the Th17 phenotype in the LPS-driven immune system. Bull Math Biol. 2014;76:1045–1080. doi: 10.1007/s11538-014-9946-6. [DOI] [PubMed] [Google Scholar]
- 27.Loubaki L, Hadj-Salem I, Fakhfakh R, Jacques E, Plante S, Boisvert M, Aoudjit F, Chakir J. Co-culture of human bronchial fibroblasts and CD4+ T cells increases Th17 cytokine signature. PLoS One. 2013;8:e81983. doi: 10.1371/journal.pone.0081983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JR, Nishioka M, Chakir J, Risse PA, Almaghlouth I, Bazarbashi AN, Plante S, Martin JG, Eidelman D, Hamid Q. IL-22 contributes to TGF-β1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res. 2013;14:118–130. doi: 10.1186/1465-9921-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin XJ, Zhang GS, Zhang X, Qiu ZW, Wang PL, Li YW, Li W, Xie QM, Ke YH, Lee JJ, Shen HH. Protein tyrosine phosphatase SHP2 regulates TGF-β1 production in airway epithelia and asthmatic airway remodeling in mice. Allergy. 2012;67:1547–1556. doi: 10.1111/all.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodeling. Clin Exp Allergy. 2004;34:497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin-4. J Clin Invest. 1992;90:1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trautmann A, Krohne G, Bröcker EB, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1998;160:5053–5057. [PubMed] [Google Scholar]
- 33.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13 alpha2 receptor is involved in nduction of TGF-betal production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]


