Abstract
Anaplastic lymphoma kinase (ALK)-positive diffuse large B-cell lymphoma (ALK + DLBCL) is a rare and poorly characterized subtype of lymphoma. Reports suggest that this type of tumor responds poorly to standard regimens for non-Hodgkin’s lymphoma, with rituximab playing no therapeutic role due to the absence of CD20 expression. In view of the expression of ALK in this disease, it is plausible that the ALK inhibitor crizotinib may be an effective treatment. We report a case of a 21-year-old male ALK + DLBCL patient. He initially received five cycles of CHOP-21 (vincristine, pirarubicin, cyclophosphamide and prednisone) and achieved a partial remission (PR) but soon deteriorated. He was subsequently treated with five courses of the salvage chemotherapy regimen ICE (ifosfamide, carboplatin and etoposide) and achieved PR again. He refused to accept an autologous stem-cell transplantation, after which the disease progressed rapidly. We administered two courses of an alternative salvage chemotherapy regimen containing GEMOX and dexamethasone with the addition of the ALK inhibitor crizotinib. His symptoms alleviated for a short time but soon worsened and the patient died of massive progressive disease.
Keywords: Anaplastic lymphoma kinase, non-hodgkin lymphoma, spleen, crizotinib
Introduction
Anaplastic lymphoma kinase- (ALK-) positive diffuse large B-cell lymphoma (ALK + DLBCL) is a rare neoplasm recognized as a separate entity by the latest WHO classification of hematological malignancies [1]. It usually affects young adults and has a male predominance. The chromosomal translocation t (2; 17) (p23; q23) is the most commonly reported cytogenetic abnormality in this disease, which leads to fusion of the genes CLTC (clathrin) and ALK [5]. Most patients present at an advanced stage, follow an aggressive clinical course and have poor outcome. Although characteristically a nodal disease, extranodal involvement has been reported [2-4]. We present a case in which the patient presented with extranodal involvement of the spleen in keeping with previously reported cases [3,8]. Histologically, the tumor cells had a plasmablastic or immunoblastic appearance, with a sinusoidal pattern of infiltration, and a unique immunophenotypic profile largely characterized by lack of expression of CD20, and positivity for plasma cell markers such as CD138, epithelial membrane antigen (EMA), and multiple myeloma oncogene 1 (MUM1).
In reporting this case we would like to highlight the aggressive nature and high recurrence rate of this subset of DLBCL as well as its poor response to conventional chemotherapy. The recent introduction of the small molecule ALK inhibitor crizotinib provides a potential new therapeutic option for patients with this disease that warrants further investigation.
Case report
In December 2012, a 21-year-old male was admitted with abdominal pain of the left quadrant for 3 days. He had no fever, night sweats or weight loss and reported no history of other diseases. Abdominal computed tomography (CT) revealed splenomegaly with retroperitoneal lymphadenopathy and a low-density mass in the body and tail of the pancreas (Figure 1). Splenectomy was performed on January 15th 2013, histopathological analysis revealed a diffuse infiltration of tumor cells that were rich in plasma with vesicular chromatin and prominent nucleoli. The tumor cells lacked of expression EMA and CD20, but were highly positive for CD138, MUM1, CD4 and cytoplasmic ALK, whereas only single cells showed expression of CD30 and CD38 (Figure 2). Additional immunohistochemistry results are provided in Table 1. Clonal rearrangements of IGH-FR1 and IGκ-VJ were detected by PCR (Figure 3), and EBER (Epstein-Barr virus) immunohistochemical staining was negative. An ALK gene rearrangement was detected by FISH (Figure 4), while RT-PCR detected a CLTC-ALK fusion transcript which was confirmed by Sanger sequencing (Figure 5). Relevant clinical and cytogenetic data of the patient is summarized in Table 1. Positron emission tomography–computed tomography (PET-CT) revealed enlarged hyper-metabolic lymph nodes in the patient’s left neck, left adrenal area, hepatic portal, lesser omentum and retroperitoneal space with some of them fused. Thus the patient was diagnosed with “splenic ALK + DLBCL, Stage III, Group A” with an IPI score of 3. The patient was assigned into the moderate-high risk group and prescribed five cycles of CHOP-21 (vincristine 1.4 mg/m2 iv d1; pirarubicin 60 mg/m2 iv d1; cyclophosphamide 750 mg/m2 iv d1; and prednisone 50 mg po bid d1-5) were administered with abdominal pain relieved after one cycle of the regimen. Reassessment by PET-CT in May 2013 showed that multiple lymph nodes in the patient’s abdomen had shrunk with average standardized uptake (SUV) values returning to normal while retroperitoneal lymph nodes had increased both in number and 18F-FDG metabolism rate with a maximum SUV value of 10.2. We subsequently changed the protocol from CHOP to ICE (etoposide 0.1 g/m2 iv d1-3; carboplatin 600mg iv d2; and IFO 5 g/m2 iv d2), but after one course the patient discontinued treatment. In October 2013 the patient was admitted in hospital again with mild fever and lumbodorsal pain. Multiple lymph nodes in the left neck and abdomen were enlarged with markedly increased SUV values. After another four cycles of ICE with an interval of 21 days, PET-CT performed on March 15th 2014 revealed decreased SUV values and shrinking of lymph nodes in the neck and abdomen but irregular soft tissue shadows were observed in the anterior mediastinum and pelvic cavity. With symptoms relieved, the patient refused to accept therapy up to October 7th 2014, by which time the disease was rapidly progressing. He presented with left cervical lymphadenopathy and persistent backache. Lactate dehydrogenase (LDH) levels were exorbitantly increased (858u/l). After two courses of GEMOX plus Dexamethasone with the addition of ALK inhibitor crizotinib (Gemcitabine 1 g/m2 iv d1; 8 Oxaliplatin 100 mg/m2 iv d1; Dexamethasone 10 mg/m2 iv d1-8; and Crizotinib 250mg po bid), his symptoms were relieved with CT revealing decreased lymphadenopathy. The LDH level also fell to 568 u/l for a short time. Unfortunately the disease progressed rapidly with increased LDH and platelets (Figure 6). Abdominal CT showed a polyserositis and the patient died two months later.
Figure 1.
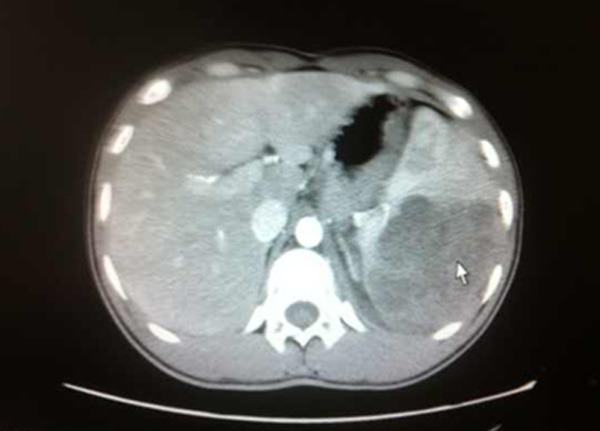
Mass in the spleen by CT scan (shown by the arrow).
Figure 2.
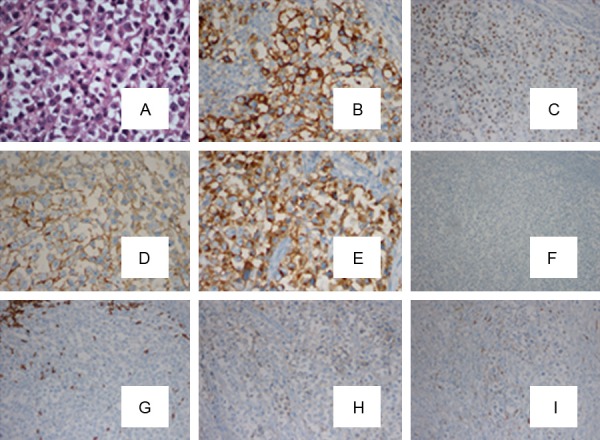
HE×400 times (A) and IHC staining of the spleen mass. The tumor tissue strongly expressed CD138 (B), MUM1 (C), CD4 (D) and Cytoplasmic granular staining of ALK (E), but negative for EMA (F) and CD20 (G). Weak expression of CD30 (H) and CD38 (I) was observed.
Table 1.
Relevant clinical and cytogenetic data of the patient
| Case | Sex/ age | Stage | Treatment | Response/survival | ALK staining | Morphology | Immunophenotype | PCR analysis | Karyotype | FISH identification | RT-PCR | EBV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/24 | IIIA | CHOP-21×5 | Dead after 24 months of diagnosis | Cytoplasmic granular | Large cell with plasmatic differentiation | CD138+ | IGH-FR1 | 46, XY [11] | ALK rearrangement | CLTC-ALK | Negative |
| ICE×5 | CD4+ | IGK-VJ | ||||||||||
| Gemox+Dex+crizotinib×2 | MUM1+ | |||||||||||
| ALK+ | ||||||||||||
| λ+ | ||||||||||||
| CD30+weak | ||||||||||||
| CD38+weak | ||||||||||||
| CD43+weak | ||||||||||||
| CD57+weak | ||||||||||||
| CD20- | ||||||||||||
| CD15- | ||||||||||||
| CD79a- | ||||||||||||
| CD3- | ||||||||||||
| CD7- | ||||||||||||
| S100- | ||||||||||||
| LCA- | ||||||||||||
| K- | ||||||||||||
| CK- | ||||||||||||
| EBER- |
Figure 3.
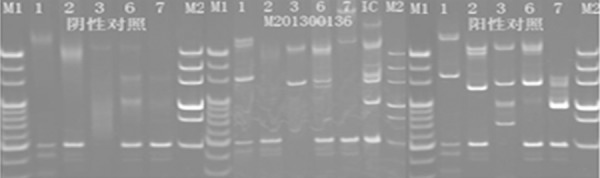
Clonal rearrangements of IGH-FR1, IG K-VJ were detected by PCR. M1 and M2 representative markers, IC within the control sample, showing tube one and six amplified in line with the target size of the amplified bands.
Figure 4.
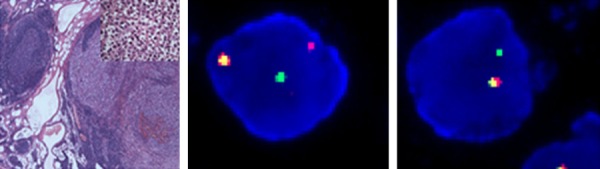
HE staining of spleen tumor tissue (40×; left panel) and Fluorescence in-situ hybridization (FISH) revealed a genomic break in the ALK1 locus (middle panel) while in some tumor cells there was also evidence of partial loss of the rearranged ALK gene (right panel).
Figure 5.
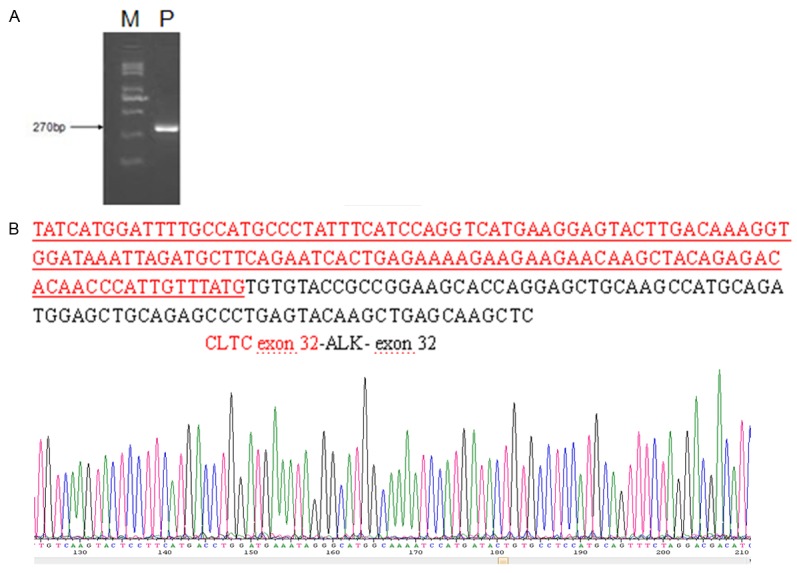
Molecular analysis. A. RT-PCR revealed a chimeric CLTC-ALK fusion transcript of 270 bp (P, patient; M, 250 bp size marker). B. Direct sequencing analysis of RT-PCR product showed that the rearrangement occurred at exon 32 of CLTC and exon 32 of ALK. Upper panel: CLTC-ALK fusion cDNA sequence. Red, CLTC exon 32; BLACK, ALK exon 32.
Figure 6.
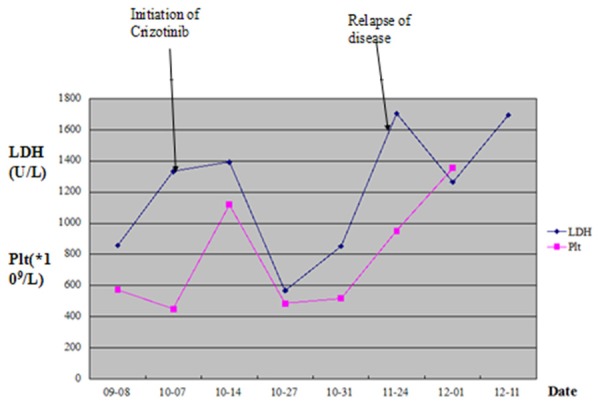
LDH level and platelet counts of this patient after undergoing a second relapse.
Discussion
ALK + DLBCL was first reported by Delsol et al in 1997 based on a series of seven cases and was officially defined as a new subtype of lymphoma by the WHO in 2008 [6]. To date, less than 100 cases have been reported. The disease spans all age groups (9 to 72 years), with a median of 38 years and occurs with a male: female ratio of 3:1 [5]. It most frequently presents with painless enlarged lymph nodes, predominantly located in the neck and mediastinum, and extra nodal involvement of nasopharynx, tongue, bone, gastrointestinal tract, liver, spleen and ovary [2-4]. Involvement of the spleen, as observed in the present case, is rarely seen. In contrast, the morphological features and IHC staining of the neoplastic cells was similar to those of previously reported cases (see Table 1), being intermediate to large sized and immunoblastic or plasmablastic in appearance with a single prominent central nucleus and moderate amounts of eosinophilic to amphophilic cytoplasm. Cells were generally positive for CD138, ALK, MUM1 and IgA, weakly positive for CD30 and CD38, and negative for CD20. We identified an ALK rearrangement by FISH and detected the CLTC-ALK transcript by RT-PCR, thus further confirming the diagnosis.
Interestingly, EMA was found to be negative in our patient. To our knowledge this is only the second reported case of EMA-negative ALK + DLBCL. EMA is aberrantly glycosylated and overexpressed in a variety of epithelial cancers, and plays a crucial role in progression of the disease. Tumor-associated EMA differs from the EMA expressed in normal cells with regard to its biochemical features, cellular distribution, and function. In cancer cells, EMA participates in intracellular signal transduction pathways and regulates the expression of its target genes at both the transcriptional and post-transcriptional level, and thus may provide a possible therapeutic target [7].
The ALK gene encodes a tyrosine kinase receptor belonging to the insulin receptor superfamily, which is normally silent in lymphoid cells [9]. Indeed, ALK protein is rarely expressed in normal tissues and its expression in malignancy is often companied by causal chromosomal aberrations. ALK protein localization is determined by the specific chromosomal translocation, of which t (2; 17) (p23; q23) is most commonly seen, resulting in a CLTC-ALK fusion gene and ALK protein localized to the cytoplasm. A nucleophosmin (NPM)-ALK fusion gene formed by t (2;5) (p23;q35) translocation is also recurrent in this disease, and is associated with ALK protein localized to both the cytoplasm and nucleus [10,11]. However, exceptions to this pattern have been reported; Onciu et al described a case of NPM-ALK + DLBCL case with granular expression of ALK protein only [12]. Recently, Seung et al found a rare case of RANBP2-ALK + DLBCL in which IHC demonstrated that ALK protein was located on the nuclear membrane [13]. There have been further reports of atypical localization of ALK protein that implicate additional genetic abnormalities [14-16]. In the present case we observed granular stains of ALK in the cytoplasm, suggestive of the t (2; 17) which was confirmed by RT-PCR for the CLTC-ALK fusion gene (Figure 5).
Effective treatment strategies for ALK+ DLBCL have not been investigated in detail due to the low incidence of this disease. Standard treatment regimens include combined chemotherapy, chemotherapy and radiotherapy, or radiotherapy alone. Our case was in accordance 90% of the reported cases in showing evidence of progression [3]. Case reports are suggestive of a poor prognosis associated with ALK + DLBCL, with outcome depending largely on clinical stage. The average DFS is 41 months when diagnosed at early stage while the median OS drops to 11 months when diagnosed at progression. There has been no effective protocol for the disease and combined chemotherapy is currently the main treatment despite the poor response observed in some patients. Table 2 provides a summary of treatment programs and the outcome of all ALK + DLBCL cases reported to date. CHOP or similar protocols are the main choice and the 5-year OS was only 25%. In the present case after five courses of CHOP the patient achieved PR but soon relapsed, after which another five cycles of ICE regimen had little effect, possibly highlighting an underlying poor sensitivity to chemotherapy generally. HSCT is recommended for patients with poor prognosis, but an analysis by Beltran et al showed that seven of eight patients receiving auto-HSCT died, with OS after transplant ranging from 3 to 44 months [3]. Recently Zanelli et al reported a stage III, group B patient who survived 35 months after undergoing chemotherapy, radiotherapy and auto-HSCT [17]. Rituximab was not considered due to the absent expression of CD20 on tumor cells and an absence of supportive evidence. Amor et al detected activation of the STAT3 pathway in a SQSTM1-ALK + patient and suggested STAT3 pathway inhibitors could be targets for some cases of ALK+DLBCL with rare cytogenetic abnormalities [18]. Crizotinib is an inhibitor of tyrosine kinase receptors including ALK and CD117 and has been found to be effective against inflammatory myofibroblastic tumor or non-small cell lung cancer with ALK rearrangement [19,20]. The FDA has approved crizotinib as a molecularly targeted agent for NSCLC with ALK rearrangement. Preclinical studies of ALK-positive DLBCL found that inhibition of ALK-activity resulted in sustained tumor regression in the xenotransplant tumor model and cell lines. These findings support a role of CLTC-ALK in the maintenance of the malignant phenotype thereby providing a rational therapeutic target for these otherwise refractory tumors [21]. Interestingly, Armstrong et al observed that different types of ALK fusion proteins produced differential effects on proliferation, transformation and invasion in vitro and in vivo. There is also evidence that different ALK fusion genes exhibit differential sensitivity to ALK inhibition [22,23]. These trials have provided solid evidence for the application of ALK-inhibitors. Recently Wass et al reported a relapsed ALK+ DLBCL case after crizotinib treatment, in which the patient achieved PR but relapsed quickly due to crizotinib resistance [24]. Despite the short remission time in the patient, crizotinib showed some efficacy in treating ALK + DLBCL and provided evidence for further clinical research. Although our patient demonstrated clinical improvement as a result of chemotherapy, the disease was unfortunately rapidly progressive. He was subsequently treated with two courses of GEMOX + Dexamethasone with additional ALK inhibitor crizotinib and the symptoms improved. Computer tomography revealed an improvement of lymphadenopathy and LDH levels declined transiently but symptoms deteriorated rapidly with increased LDH and platelets. The patient died two months later due to massive progressive general disease. Nevertheless, we show a partial response to this new agent. It is plausible to speculate that the sooner we use this new molecular targeted drug, the more effective it may be; it will be important to determine this in future trials.
Table 2.
Summary of treatment and outcome of previously reported ALK + DLBCL cases
| Author | Case no. | Sex | Age | Stage | First therapy | Slvage therapy | Present clinical status |
|---|---|---|---|---|---|---|---|
| Delsol1 [1] | 1 | M | 53 | IVA | CTX plus intrathecal MTX | BMT | Died of disease after 26 months |
| 2 | M | 15 | I | COPAD+Ara-C | Alive without disease after 156 months | ||
| 3 | M | 37 | II | M-BACOD×2 | Case 3, 4, 7 died of disease after 9~33 months | ||
| 4 | M | 44 | III~IV | B-CHOP | See case 3 | ||
| 5 | F | 67 | MOPP+XRT×1 | Lost to follow up after 11 months | |||
| 6 | M | 51 | ACVBP×2 | Alive without disease after 14 months | |||
| 7 | M | 60 | See case 3 | ||||
| Gascoyne [2] | 1 | M | 46 | III | CTX | CTX+XRT | Alive without disease after 27 months |
| 2 | F | 45 | NA | NA | N/A | ||
| 3 | M | 49 | IV | CHOP+XRT | PR | Alive with progressive disease after 9 months | |
| 4 | M | 48 | IA | CTX | Alive without disease after 27 months | ||
| 5 (Delsol case 1) | M | 53 | |||||
| 6 | M | 58 | IV | CHOP+Rtiuximab | Died of disease after 6 months | ||
| De Paepe [3] | 1 | M | 10 | II | ALCL-99 | SFOP-LMB96 | Alive without disease after 6 months |
| 2 | F | 13 | III | NHL-BFM ALCL99 | BMT | Died of disease after 3 months | |
| 3 | M | 26 | II | CHOP×4VIM×1/DHAP×1 | Hper-CVAD+BMT | Alive without disease after 44 months | |
| Chikatsu [4] | 1 | F | 36 | IV | Combination of CTX | Died of disease after 11 months | |
| Onciu [5] | 1 | M | 16 | IV | LMB 89, weekly vinblastine, intrathecal CTX and palliative XRT | Died of disease after 24 months | |
| 2 | M | 10 | II | POG8719,XRT | DHAP×3 | Alive without disease after 156 months | |
| Adam [6] | 1 | M | 35 | IIA | CHOEP-21×5 | Auto-BMT, CTX | Died of disease after 14 months |
| McMan [7] | 1 | M | 21 | IIE | CHOP×6 | Alive without disease after 24 months | |
| Colomo [8] | 1 | M | 34 | NR | Died of disease after 6 months | ||
| Ishii [9] | 1 | M | 33 | NR | CHOP | Allo-pbsct | Died of disease after 31 months |
| Rudzski1 [10] | 1 | M | 48 | IIIB | CHOP×3 | Died of disease after 3 months | |
| 2 | M | 49 | IVB | CTX | Still alive | ||
| Gesk [11] | 1 | M | 13 | II | ALCL-99 | CTX | Partial remission |
| 2 | F | 12 | II | CTX | Alive without disease after 4 years | ||
| 3 | M | 16 | IV | CTX | BMT | Died of disease after 12 months | |
| Isimbald [12] | 1 | F | 9 | I | AIEOP | ICE, PVDA | Died of disease after 9 months |
| Bubala [13] | 1 | M | 9 | III | Used lymphoblastic lymphoma protocal then LMB89 | Died of disease after 5 months | |
| Reichard [14] | 1 | F | 41 | I | CHOP+XRT | Alive without disease after 58 months | |
| 2 | F | 49 | I | CHOP+XRT | Alive without disease after 22 months | ||
| 3 | M | 71 | IV | CHOP+XRT | Died of disease after 22 months | ||
| 4 | M | 53 | I | NR | NR | ||
| Stachurski [15] | 1 | M | 33 | IVB | CHOP×5 + intrathecal MTX | Relapse 10 months after diagnosis | |
| Hyou Wook Lee [16] | 1 | F | 26 | IV | CHOP×6 | Lost to follow up after 6 months | |
| 2 | M | 35 | IV | CHOP×8 | Died of disease after 18 months | ||
| 3 | M | 24 | IV | CHOP×6 | IMVP-16×2 | Died of disease after 17 months | |
| Shuji Monose [17] | 1 | M | 53 | IV | High-dose Ara-C + auto-HSCT | Developed recurrent disease 4 months after CR | |
| 2 | M | 41 | IIE | CHOP | Achieved CR after 5 months follow up | ||
| Beltran [18] | 1 | M | 27 | IVB | EPOCH×6 | Hper-CVAD | Alive without disease after 11 months |
| 2 | F | 41 | IA | XRT | Alive without disease after 13 months | ||
| 3 | F | 13 | IIB | LNH96-2002 | Alive without disease after 62 months | ||
| 4 | M | 70 | IIIB | CHOP-21×6 | Alive without disease after 72 months | ||
| Roosbroeck [19] | 1 | M | 27 | IA | CHOP×4 +XRT | Alive without disease after 27 months | |
| 2 | F | 33 | IVA | CHOP×7 | Alive without disease after 19 months | ||
| Bodwel [20] | 1 | M | 66 | IVB | CTX | Died 3 weeks after diagnosis | |
| Takeuchi [21] | 1 | M | 67 | IIA | CHOP×6 | Developed recurrent disease 6 months after CR | |
| Ke Li [22] | 1 | M | 44 | IIE | CTX | N/R | |
| Cerchieltil [23] | 1 | F | 13 | IIA | Intensive chemotherapy + auto-hHSCT + XRT | Relapsed 53 days post-transplant | |
| Shi [24] | 1 | M | 49 | IIB | CHOP×6 | ICE×2 + auto-HSCT + XRT | Developed recurrent disease 6 weeks post-transplant |
| Yin [25] | 1 | F | 17 | IIIA | CHOP×5 | Alive without disease after 6 months | |
| Francisco [26] | 1 | M | 31 | IIIAS | CHOP,Hper-CVAD | ICE×3 | Died 238 days after diagnosis |
| d’Amore [27] | 1 | M | 39 | IIIB | CHOP | DHAP, dexa-BEAM | Died 11 months after diagnosis |
| Zanelli [28] | 1 | M | 53 | IIIB | CODOX-M×2, IVAC×2, high-dose BEAM, auto-HSCT + intrathecal chemotherapy with MTX and Ara-C | Alive without disease after 35 months | |
| Wass [29] | 1 | F | 27 | IV | CHOP×6, XRT | DHAP×2, ICE×2, Auto-HSCT, crizotinb | Died 30 days after crizotinib treatment |
| Chapman [30] | 1 | M | 39 | IIIA | R-CHOP | Died 6 weeks after diagnosis | |
| This study | 1 | M | 24 | IIIA | CHOP×5 | ICE×5,GEMOX+dexamethasone r+ crizotinb | Died 60 days after crizotinib treatment |
Commentary: CTX: chemotherapy; MTX: methotrexate; NR: not reported; NA: not available XRT: radiation therapy; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; PBSCT: peripheral blood stem cell transplant; ICE: ifosfamide, carboplatin, etoposide; PVDA, prednisone, vincristine, doxorubicin, asparaginase; DAHP: dexamethasone, cytarabine, cisplatin; CHOEP-21: cyclophosphamide, adriamycin, vincristine, etoposide, prednisone; BMT: bone marrow transplantation; hyperCVAD: hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with cytarabine and methotrexate; EPOCH: cyclophosphamide, vincristine, doxorubicin, etoposide and prednisone; DHAP: cisplatin, cytarabine, and dexamethasone; BEAM: carmustine, etoposide, cytarabine, and melphalan; GEMOX: gemcitabine, cisplatin.
To conclude, we diagnosed a rare case of ALK + DLBCL with spleen involvement and explored its pathology, cytogenetics, diagnosis and treatment. Due to its low incidence and non-specific morphology, ALK + DLBCL is easily misdiagnosed, with accurate identification requiring particular vigilance from physicians and pathologists. Treating this disease also remains a considerable challenge therefore the small molecule ALK inhibitor crizotinib in combination with other agents may provide a potential new therapeutic option. Further investigation in clinical trials is now warranted.
Acknowledgements
We gratefully acknowledge the pathologists in our hospital for their expertise and generous provision of data. Our study is supported by Medical Science and technology development Foundation of Nanjing Department of Health (YKK14056).
Disclosure of conflict of interest
None.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th editon. Swerdlow SH, editor. Lyon: IARC; 2008. pp. 253–254. [Google Scholar]
- 2.Morgan EA, Nascimento AF. Anaplastic lymphoma kinase-positive large B-cell lymphoma: an underrecognized aggressive lymphoma. Adv Hematol. 2012;2012:529572. doi: 10.1155/2012/529572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltran B, Castillo J, Salas R. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. J Hematol Oncol. 2009;2:11. doi: 10.1186/1756-8722-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishida M, Yoshida K. Anaplastic lymphoma kinase-positive large B-cell lymphoma: a case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Choung HS, Kim HJ, Kim WS. Cytomorphology and molecular characterization of CLTC-ALK rearrangement in 2 cases of ALK-positive diffuse large B-cell lymphoma with extensive bone marrow involvement. Korean J Lab Med. 2008;28:89–94. doi: 10.3343/kjlm.2008.28.2.89. [DOI] [PubMed] [Google Scholar]
- 6.Delsol G, Lamant L, Mariamr B. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood. 1997;89:1483–90. [PubMed] [Google Scholar]
- 7.Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–42. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stachurski D, Miron PM, Al-Homsi S. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma with a complex karyotype and cryptic 3’ ALK gene insertion to chromosome 4q22-24. Hum Pathol. 2007;38:940–45. doi: 10.1016/j.humpath.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 10.Gascoyne RD, Lamant L, Martin-Subero JI. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102:2568–73. doi: 10.1182/blood-2003-03-0786. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–32. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onciu M, Behm FG, Downing JR. ALK-positive plasmablastic B-cell lymphoma with expression of the NPM-ALK fusion transcript: report of 2 cases. Blood. 2003;102:2642–4. doi: 10.1182/blood-2003-04-1095. [DOI] [PubMed] [Google Scholar]
- 13.Lee SE, Kang SY, Takeuchi K. Identification of RANBP2-ALK fusion in ALK positive diffuse large B-cell lymphoma. Hematol Oncol. 2014;32:221–4. doi: 10.1002/hon.2125. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi K, Soda M, Togashi Y. Identification of a novel fusion, SQSTM1-ALK, in ALK-positive large B-cell lymphoma. Haematologica. 2011;96:464–7. doi: 10.3324/haematol.2010.033514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedwell C, Rowe D, Moulton D. Cytogenetically complex SEC31A-ALK fusions are recurrent in ALK-positive large B-cell lymphomas. Haematologica. 2011;96:343–6. doi: 10.3324/haematol.2010.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Roosbroeck K, Cools J, Dierick D, Thomas J. ALK-positive large B-cell lymphomas with cryptic SEC31A-ALK and NPM1-ALK fusions. Haematologica. 2010;95:509–11. doi: 10.3324/haematol.2009.014761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanelli M, Valli R, Capodanno I. Lymphoma Kinase-Positive Large B-Cell Lymphoma. Description of a case with an unexpected clinical outcome. Int J Surg Pathol. 2014;23:78–83. doi: 10.1177/1066896914536223. [DOI] [PubMed] [Google Scholar]
- 18.d’Amore ES, Visco C, Menin A. STAT3 pathway is activated in ALK-positive large B-cell lymphoma carrying SQSTM1-ALK rearrangement and provides a possible therapeutic target. Am J Surg Pathol. 2013;37:780–6. doi: 10.1097/PAS.0b013e318287791f. [DOI] [PubMed] [Google Scholar]
- 19.Butrynski JE, D’Adamo DR, Hornick JL. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–33. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak EL, Bang YJ, Camidge DR. Anaplastic lymphoma Kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerchietti L, Damm-Welk C, Vater I. Inhibition of anaplastic lymphoma kinase (ALK) activity provides a therapeutic approach for CLTC-ALK-positive human diffuse large B cell lymphomas. PLoS One. 2011;6:e18436. doi: 10.1371/journal.pone.0018436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong F, Duplantier MM, Trempat P, Hieblot C, Lamant L, Espinos E, Racaud-Sultan C, Allouche M, Campo E, Delsol G, Touriol C. Differential effects of X-ALK fusion proteins on proliferation, transformation, and invasion properties of NIH3T3 cells. Oncogene. 2004;23:6071–82. doi: 10.1038/sj.onc.1207813. [DOI] [PubMed] [Google Scholar]
- 23.Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, Meder L, Lovly CM, Heukamp LC, Pao W, Küppers R, Thomas RK. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18:4682–90. doi: 10.1158/1078-0432.CCR-11-3260. [DOI] [PubMed] [Google Scholar]
- 24.Wass M, Behlendorf T, Schädlich B. Crizotinib in refractory ALK-positive diffuse large B-cell lymphoma: a case report with a short-term response. Eur Haematol. 2014;92:268–70. doi: 10.1111/ejh.12240. [DOI] [PubMed] [Google Scholar]


