Abstract
Objective: This study is to determine the regulatory role of nitric oxide in mouse blastocyst hatching. Methods: Kunming female mice were superovulated and then mated with mature male mice. On day 2.5 of their pregnancy, the pregnant mice were killed and morulae were flushed from their uterine horns with culture media. Morulae were cultured in media with different concentrations of N-nitro-L arginine methyl ester (L-NAME), sodium nitroprusside (SNP), 8-Br-3’-5’-cyclic guanosine monophosphate (8-Br-cGMP) or the combination of L-NAME with SNP or 8-Br-cGMP for 48 h. The hatched blastocysts were examined on day 5 and the expressions of epithelial nitric oxide synthase (eNOS) and active cysteinyl aspartate specific proteinase 3 (caspase 3) were observed under confocal laser scanning microscope. Results: L-NAME significantly reduced the expression of eNOS in blastocyst cells. With the increase of the concentrations of L-NAME, SNP or 8-Br-cGMP, blastocyst hatching rate was significantly lowered. In addition, 5 mM L-NAME, 2 μM SNP and 2 μM 8-Br-cGMP completely inhibited blastocyst hatching. Low concentrations of SNP or 8-Br-cGMP in culture media containing 5 mM L-NAME significantly reversed the inhibition of blastocyst hatching and promoted hatching development. Moreover, 5 mM L-NAME and 2 μM 8-Br-cGMP had no significant influence on the expression of active caspase 3 in blastocyst cells. SNP (> 500 nM) significantly increased the expression of active caspase 3 in blastocyst cells. Conclusions: NO/cGMP pathway plays an important role in mouse blastocyst hatching. Excessive or depleted NO can interrupt blastocyst hatching. Excessive NO leads to apoptosis of blastocyst cells.
Keywords: Mouse, nitric oxide, NO/cGMP pathway, blastocyst hatching, apoptosis
Introduction
Nitric oxide (NO) is a gaseous free radical that plays a vital role in female reproductive system as the second messenger and a regulatory factor of cell functions [1-3]. NO participates in physiological activities such as follicular development [1], secretion of ovarian hormones [2], implantation and delivery [3]. NO is generated from L-arginine and oxygen by the catalysis of nitric oxide synthase (NOS). There are three types of NOS, including epithelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). NOS activity may indirectly reflect NO production and its biological effects.
Blastocyst hatching is a very important and precisely regulated physiological phenomenon. Successful blastocyst hatching determines subsequent embryo survival and development. Any adverse factors that affect blastocyst hatching can lead to failure in embryo implantation, which ultimately leads to female infertility [4-6]. In the whole process of hatching, blastocysts are regulated by a variety of cytokines and biological molecules [7-9], but their types and mechanisms of action are still unclear.
A study found that eNOS is highly expressed at the stage of blastocyst development, indicating its important regulatory effect in the process of blastocyst development [10]. eNOS can promote the production of NO. Proper amount of NO is needed in the development of blastocyst to regulate the proliferation of blastocyst cells [11]. Only when blastocyst cells proliferate to enough numbers and blastocyst size becomes big enough, blastocysts begin to hatch [6]. Therefore, NO is likely to exert certain regulatory effects in the process of blastocyst hatching.
The main biological effect of NO is to increase the concentration of intracellular 3’5’-cyclic guanosine monophosphate (cGMP) by activating guanylate cyclase. cGMP stimulates cGMP-dependent protein kinase to regulate phosphodiesterase activity and ion channel openings and hence, regulating the physiological activities of cells. Tranguch et al. found that NO/cGMP pathway played important roles in the regulation of development arrest in mouse embryos. Inhibition of NO/cGMP pathway will result in delayed development of mouse embryos [12]. However, it still needs further investigation whether NO, as a signaling molecule, can regulate blastocyst hatching process via NO/cGMP pathway. N-nitro-L arginine methyl ester (L-NAME) is the competitive inhibitor of nitric oxide synthase, which could reduce the production of nitric oxide through the inhibition of nitric oxide synthase [12]. Sodium nitroprusside (SNP), as an NO donor, could increase the concentration of NO in cells [11]. In this study, we investigate the regulatory effect of NO in blastocyst hatching through adding L-NAME, SNP and cGMP analogue 8-Br-3’-5’-cyclic guanosine monophosphate (8-Br-cGMP) to embryo culture medium.
Materials and methods
Animals
A total of 130 Kunming mice were used for the present study. The weight for female mice ranged 30-35 g, and that for male mice ranged 40-45 g. The mice were raised at 20 ± 3°C, and 50 ± 10% humidity, with controlling light and dark indoor environment (14 h:10 h). The animals had free feeding and drinking all the time. Male mice were raised in individual cages.
After one week of adaptive breeding, female mice were injected with 10 IU pregnant mare serum gonadotrophin intraperitoneally. After 48 h, 10 IU human chorionic gonadotrophin was injected intraperitoneally into these female mice. Afterwards, one male mouse and one female mouse were put into the same cage. On the next morning, female mice with vaginal plugs were defined as pregnant for 0.5 day. All animal experiments were conducted according to the ethical guidelines of Jilin Medical University.
In vitro culture and hatching of embryos
On day 2.5 of pregnancy, female mice were sacrificed by cervical dislocation. After removing blood with filter paper, the uteruses were placed onto petri dishes. After washing uterus inner wall with KSOMAA medium [13], developing morulae were obtained. After being washed by KSOMAA medium for three times, the morulae were placed into pre-balanced KSOMAA medium droplets for incubation at 37°C and 5% CO2 for 48 h. Subsequently, blastocyst development was observed and hatching rate of blastocysts was calculated.
Of note, morulae were cultured in media containing different concentrations of N-nitro-L arginine methyl ester (L-NAME), sodium nitroprusside (SNP), 8-Br-3’-5’-cyclic guanosine monophosphate (8-Br-cGMP) or the combination of L-NAME and SNP or 8-Br-cGMP for 48 h. All morulae were divided into 6 groups, including i) control group: morulae in KSOMAA medium without L-NAME, SNP or 8-Br-cGMP; ii) L-NAME group: morulae in KSOMAA medium containing 125 μM L-NAME, 250 μM L-NAME, 500 μM L-NAME, or 5 mM L-NAME; iii) SNP group: morulae in KSOMAA medium containing 10 nM SNP, 100 nM SNP, 500 nM SNP, or 2 μM SNP; iv) L-NAME + SNP group: morulae in KSOMAA medium containing 5 mM L-NAME + 10 nM SNP, 5 mM L-NAME + 500 nM SNP, or 5 mM L-NAME + 2 μM SNP; v) 8-Br-cGMP group: morulae in KSOMAA medium containing 10 nM 8-Br-cGMP, 100 nM 8-Br-cGMP, 500 nM 8-Br-cGMP, or 2 μM 8-Br-cGMP; vi) L-NAME + 8-Br-cGMP group: morulae in KSOMAA medium containing 5 mM L-NAME + 10 nM 8-Br-cGMP, 5 mM L-NAME + 500 nM 8-Br-cGMP, or 5 mM L-NAME + 2 μM 8-Br-cGMP.
Immunofluorescence staining
Hatched blastocysts were examined on day 5. Blastocysts were fixed in 4% paraformaldehyde for 40 min, and then permeated with 0.1% Triton X-100/phosphate-buffered saline for 15 min. Fixed embryos were incubated with anti-eNOS antibody (1:400 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-activated cysteinyl aspartate specific proteinase (caspase) 3 antibody (1:400 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) for 2 h. After thorough washing with phosphate-buffered saline, the samples were incubated with isothiocyanate-fluorescein-tagged second antibody (1:400 dilution; Life Technologies, Grand Island, NY, USA) for another 1 h. Then, nuclear DNAs of blastocyst blastomeres were stained with 1 μmol/L Hoechst33342 (Sigma-Aldrich, Munich, Germany). The mounted embryos were visualized using a laser scanning confocal microscope (FV-500; Olympus, Tokyo, Japan). All pictures were taken using the same laser intensity. Image J image analysis software (http://rsb.info.nih.gov/ij/) was used to analyze the average fluorescence intensity of eNOS or active caspase 3 in each picture. For each group, a total of 10 embryos were analyzed.
Statistical analysis
Analysis of results was performed with SPSS 13.0 (IBM, Armonk, NY, USA). The data were expressed as means ± standard deviation. Single factor analysis of variance (one-way ANOVA) and LSD method were used for comparison between groups. P < 0.05 indicates significant difference.
Results
Higher concentrations of L-NAME inhibit eNOS expression and blastocyst hatching
To investigate the effect of L-NAME on eNOS expression and blastocyst hatching, morulae were treated with different concentrations of L-NAME. The data showed that eNOS was expressed in the cytoplasm of blastocyst cells, which presented stronger fluorescence in hatching blastocyst cells (Figure 1A). Compared with control, morulae treated with 125 μM, 250 μM, 500 μM and 5 mM L-NAME had significantly reduced fluorescence intensity of eNOS in blastocyst cells, exhibiting a dose-dependent manner. As the increase of L-NAME concentration, the expression of eNOS was decreased remarkably (Figure 1B). In addition, 125 μM L-NAME had no significant effect on blastocyst hatching rate compared with control. By contrast, 250 μM, 500 μM and 5 mM L-NAME reduced blastocyst hatching rate in a dose-dependent manner. With the increase of L-NAME doses, blastocyst hatching rate was significantly reduced. Of note, L-NAME (5 mM) completely suppressed blastocyst hatching (Figures 2 and 3). These results suggest that higher concentrations of L-NAME inhibit eNOS expression and blastocyst hatching.
Figure 1.
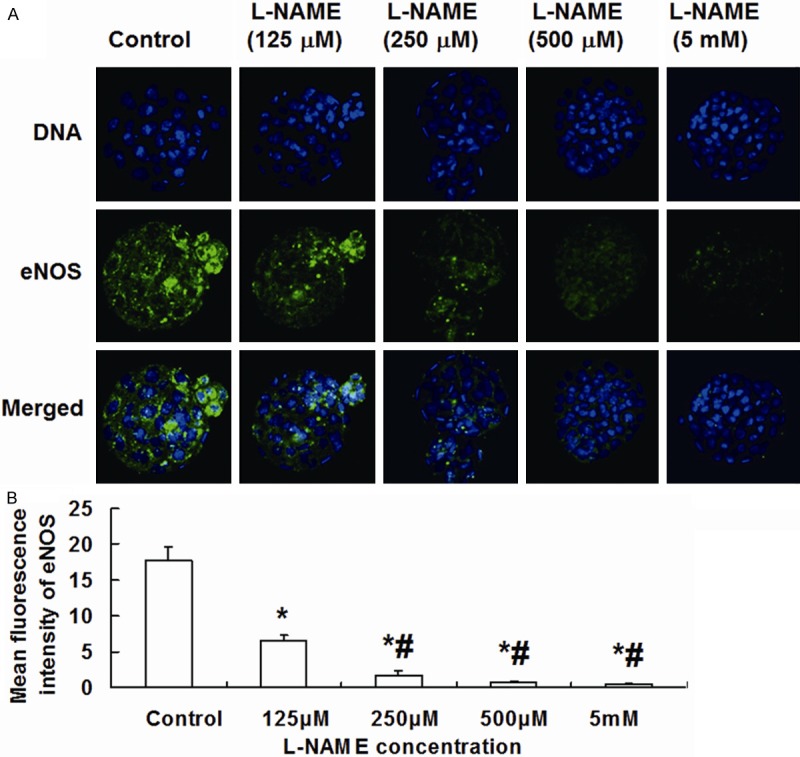
Effect of L-NAME on the expression of eNOS in blastocysts. A. Imaging of blastocysts treated with different concentrations of L-NAME (800×). DNA was dyed with Hoechst 33342 (blue). eNOS was dyed with FITC (green). Images of stained DNA and eNOS were merged in the third row. eNOS, endothelial nitric oxide synthase; FITC, fluorescein isothiocyanate. B. Mean fluorescence intensity of eNOS in blastocyst cells. *, P < 0.05 compared with control group; #, P < 0.05 compared with 125 μM L-NAME group.
Figure 2.

Effect of L-NAME on blastocyst hatching on Day 5. On day 2.5 of pregnancy, female mice were sacrificed by cervical dislocation to obtain developing morulae. After being washed by KSOMAA medium for three times, the morulae were placed into pre-balanced KSOMAA medium droplets for incubation at 37°C and 5% CO2 for 48 h. Subsequently, blastocyst development was observed and hatching rate of blastocysts was calculated. *, P < 0.05 compared with control group; #, P < 0.05 compared with 250 μM L-NAME group; Δ, P < 0.05 compared with 500 μM L-NAME group.
Figure 3.
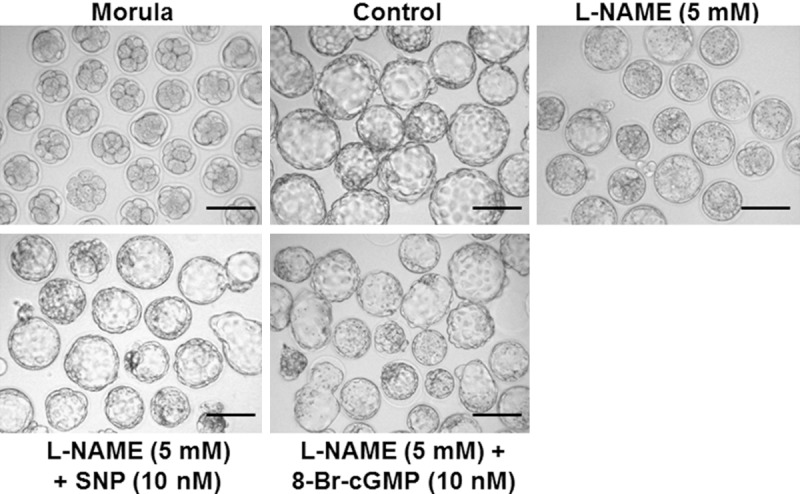
Imaging of hatched blastocysts on Day 5. Embryos were visualized using a laser scanning confocal microscope (scale bar = 100 μm). All pictures were taken using the same laser intensity and analyzed using Image J software (http://rsb.info.nih.gov/ij/).
NO regulates blastocyst hatching by cGMP
In order to investigate whether NO regulates blastocyst hatching by cGMP, we incubated embryos in vitro with SNP alone or SNP in combination with L-NAME (5 mM), and 8-Br-cGMP alone or 8-Br-cGMP in combination with L-NAME (5 mM), respectively. The data showed that 10 nM SNP had no significant impact on blastocyst hatching rate, compared with control. However, 100 nM, 500 nM and 2 μM SNP significantly reduced blastocyst hatching rate. With the increase of SNP doses, blastocyst hatching rate was significantly reduced in a dose-dependent manner, with 2 μM SNP completely inhibiting blastocyst hatching (Figure 4A). In addition, 10 nM SNP reversed the inhibition of blastocyst hatching induced by 5 mM L-NAME (Figures 3 and 4B). Of note, with the increase of SNP concentrations with 5 mM L-NAME, blastocyst hatching rate was decreased significantly (Figure 4B). Compared with control, treatment with 10 nM, 100 nM, 500 nM and 2 μM 8-Br-cGMP significantly reduced blastocyst hatching rate. With the increase of 8-Br-cGMP doses, blastocyst hatching rate was reduced markedly in a dose-dependent manner, with 2 μM 8-Br-cGMP completely suppressing blastocyst hatching (Figure 5A). Furthermore, treatment with 5 mM L-NAME in combination with 10 nM or 500 nM Br-cGMP significantly promoted blastocyst hatching by partially reversing the inhibitory effect of L-NAME. However, after treatment with 5 mM L-NAME in combination with 2 μM 8-Br-cGMP, no blastocyst hatching was observed (Figures 3 and 5B). These results indicate that NO regulates blastocyst hatching by cGMP.
Figure 4.
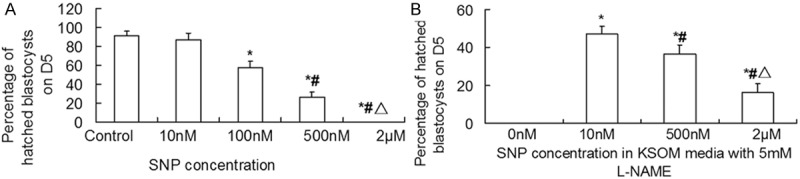
Effect of (A) SNP and (B) the combination of SNP with 5 mM L-NAME on blastocyst hatching on Day 5. Morulae were cultured in KSOMAA media containing 10 nM, 100 nM, 500 nM, or 2 μM SNP alone or the combination of 5 mM L-NAME and 10 nM, 500 nM, or 2 μM SNP for 48 h. In (A) *, P < 0.05 compared with control group; #, P < 0.05 compared with 100 nM SNP group; Δ, P < 0.05 compared with 500 nM SNP group. In (B) *, P < 0.05 compared with 5 mM L-NAME group; #, P < 0.05 compared with 5 mM L-NAME + 10 nM SNP group; Δ, P < 0.05 compared with 5 mM L-NAME + 500 nM SNP group.
Figure 5.
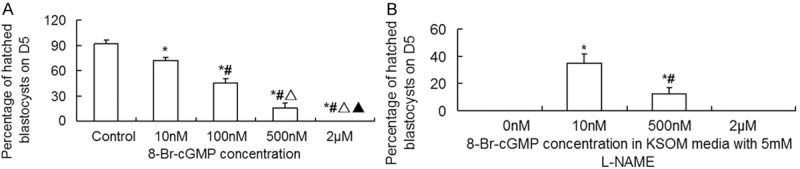
Effect of (A) 8-Br-cGMP and (B) the combination of 8-Br-cGMP with 5 mM L-NAME on blastocyst hatching on Day 5. Morulae were cultured in KSOMAA media containing 10 nM, 100 nM, 500 nM, or 2 μM 8-Br-cGMP alone or the combination of 5 mM L-NAME and 10 nM, 500 nM, or 2 μM 8-Br-cGMP for 48 h. In (A) *, P < 0.05 compared with control group; #, P < 0.05 compared with 10 nM 8-Br-cGMP group; Δ, P < 0.05 compared with 100 nM 8-Br-cGMP group; ▲, P < 0.05 compared with 500 nM 8-Br-cGMP group. In (B) *, P < 0.05 compared with 5 mM L-NAME group; #, P < 0.05 compared with 5 mM L-NAME + 10 nM 8-Br-cGMP group.
Higher concentrations of SNP promote apoptosis of blastocyst cells
To observe the apoptosis of blastocyst cells, active caspase 3 was labeled in blastocyst cells using immunofluorescence staining. Our results showed that 5 mM L-NAME, 2 μM 8-Br-cGMP and 10 nM SNP had no significant effect on fluorescence intensity of active caspase 3 in blastocyst cells, while 500 nM SNP and 2 μM SNP significantly enhanced the average fluorescence intensity of active caspase 3 in a dose-dependent manner (Figure 6A and 6B). These results suggest that higher concentrations of SNP promote apoptosis of blastocyst cells.
Figure 6.
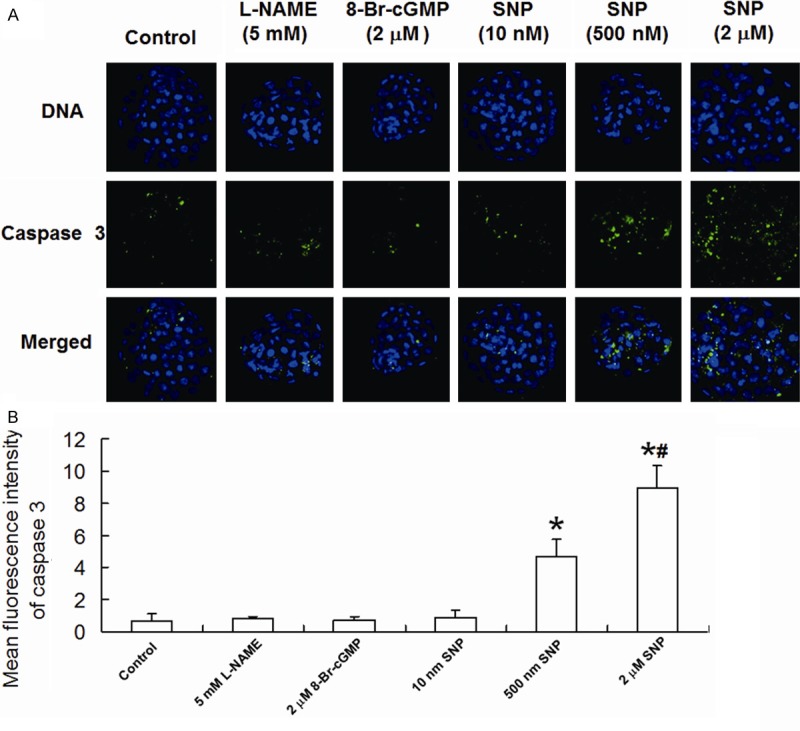
Expression of active caspase 3 in blastocyst cells. A. Imaging of blastocysts treated with different conditions (800×). DNA was dyed with Hoechst 33342 (blue). Active caspase 3 was dyed with FITC (green). Images of stained DNA and active caspase 3 were merged in the third row of images. FITC, fluorescein isothiocyanate. B. Mean fluorescence intensity of active caspase 3 in blastocyst cells. *, P < 0.05 compared with control group; #, P < 0.05 compared with 500 nM SNP group.
Discussion
Our results show that NO plays an important role in regulating mouse blastocyst hatching process via NO/cGMP pathway, in which appropriate NO concentration is necessary. With the increase of concentrations of L-NAME in culture medium, the expression levels of eNOS in mouse blastocyst cells were reduced. It has been demonstrated that eNOS is an important enzyme in NO production at early stage of embryonic development. Chen et al. [14] and Tranguch et al. [12] showed that eNOS could be detected in blastocyst cells, and eNOS knockout led to reproductive dysfunction, resulting in decreased ovulation rate in female mice [15,16]. Therefore, our results reveal that eNOS is involved in the regulation of mouse blastocyst hatching as an important enzyme for NO synthesis.
In this study, embryos were treated with different concentrations of L-NAME in vitro. Blastocyst hatching rate was significantly reduced with the increase of L-NAME concentrations, and 5 mM L-NAME completely inhibited this process. In addition, increasing concentrations of SNP significantly reduced blastocyst hatching rate in a dose-dependent manner, but significantly increased the expression of active caspase 3 in blastocyst cells at concentrations above 500 nM, indicating enhanced degrees of cell apoptosis. By contrast, 5 mM L-NAME alone did not induce significant apoptosis of blastocyst cells. This may be due to the generation of excessive NO by high concentrations of SNP. Excessive NO can react with oxygen to generate peroxynitrite, which is then converted to peroxynitrous acid, a strong oxidant. Peroxynitrous acid can cause lipid peroxidation and severe cytotoxicity, eventually leading to cell apoptosis [17,18].
In the present study, blastocyst hatching rate was significantly increased in the presence of low concentrations of SNP ( ≤ 500 nM) and 5 mM L-NAME. However, blastocyst hatching rate was not increased by 2 μM SNP due to its toxicity [19]. These data indicate that reduced blastocyst hatching rate caused by inadequate secretion of NO can be improved by addition of appropriate concentrations of NO donor.
NO exerts its effect through cGMP pathway in many tissues [20-22]. It remains unclear whether NO also acts in this way during blastocyst hatching. L-NAME inhibits NO generation. If NO exerts its effect by cGMP pathway in blastocyst hatching, addition of 8-Br-cGMP into culture medium containing L-NAME will possibly reverse the inhibition of blastocyst hatching induced by L-NAME. Our results showed that 8-Br-cGMP (< 500 nM) partially reversed the inhibition of blastocyst hatching induced by 5 mM L-NAME and hence, significantly improving blastocyst hatching. These results demonstrate that NO/cGMP pathway plays an important role in the regulation of blastocyst hatching process.
The present study showed that high concentrations of L-NAME (5 mM) or 8-Br-cGMP (2 μM) did not significantly elevate the expression of active caspase 3 in blastocyst cells. However, SNP (> 500 nM) significantly increased the expression of active caspase 3 in blastocyst cells and induced its apoptosis. These results indicate that blastocyst cell apoptosis induced by excessive NO is not achieved through cGMP pathway, but via other apoptotic pathways that involve NO [23-25]. Lee et al. found that after addition of SNP, proteins in mitochondria and endoplasmic reticulum in mouse embryonic cells were nitrosylated, ATP generation in blastocyst cells was reduced and embryonic cell apoptosis occurred; by contrast, addition of cGMP analogues could not induce significant protein nitrosylation, and no obvious embryonic cell apoptosis was observed [17]. These results are consistent with the present study. Therefore, further studies are needed to clarify the mechanisms by which NO/cGMP pathway exerts its effect in blastocyst hatching, including its regulatory effect on molecules related to zona pellucida rupture and blastocyst hatching.
Acknowledgements
This study was supported by the “Twelfth Five-Year” Scientific and Technological Research Projects of Education Department of Jilin Province [No. (2013) 349], Natural Science Foundation Projects of Shandong Province (No. ZR2011HL005), Scientific and Technological Research Projects of Jilin Province (No. 201201077), and Open Foundation Projects from State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (No. KF2014-12).
Disclosure of conflict of interest
None.
References
- 1.Dubey PK, Tripathi V, Singh RP, Saikumar G, Nath A, Pratheesh , Gade N, Sharma GT. Expression of nitric oxide synthase isoforms in different stages of buffalo (Bubalus bubalis) ovarian follicles: effect of nitric oxide on in vitro development of preantral follicle. Theriogenology. 2012;77:280–291. doi: 10.1016/j.theriogenology.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Sagar P, Prasad JK, Prasad S, Gupta HP, Das A. Effect of L-arginine methyl ester (L-NAME) on hormonal profile and estrous cycle length in buffaloes (Bubalus bubalis) Trop Anim Health Prod. 2012;44:1697–1702. doi: 10.1007/s11250-012-0126-0. [DOI] [PubMed] [Google Scholar]
- 3.Maul H, Longo M, Saade GR, Garfield RE. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des. 2003;9:359–380. doi: 10.2174/1381612033391784. [DOI] [PubMed] [Google Scholar]
- 4.Nakasuji T, Saito H, Araki R, Nakaza A, Kuwahara A, Ishihara O, Irahara M, Kubota T, Yoshimura Y, Sakumoto T. Validity for assisted hatching on pregnancy rate in assisted reproductive technology: analysis based on results of Japan Assisted Reproductive Technology Registry System 2010. J Obstet Gynaecol Res. 2014;40:1653–1660. doi: 10.1111/jog.12403. [DOI] [PubMed] [Google Scholar]
- 5.Wu D, Huang SY, Wu HM, Chen CK, Soong YK, Huang HY. Monozygotic twinning after in vitro fertilization/intracytoplasmic sperm injection treatment is not related to advanced maternal age, intracytoplasmic sperm injection, assisted hatching, or blastocyst transfer. Taiwan J Obstet Gynecol. 2014;53:324–329. doi: 10.1016/j.tjog.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Park SB, Kim HJ, Choi YB, Ahn KH, Lee KH, Yang JB, Yu CS, Seo BB. The effect of various assisted hatching techniques on the mouse early embryo development. Clin Exp Reprod Med. 2014;41:68–74. doi: 10.5653/cerm.2014.41.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thongkittidilok C, Tharasanit T, Sananmuang T, Buarpung S, Techakumphu M. Insulin-like growth factor-1 (IGF-1) enhances developmental competence of cat embryos cultured singly by modulating the expression of its receptor (IGF-1R) and reducing developmental block. Growth Horm IGF Res. 2014;24:76–82. doi: 10.1016/j.ghir.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura K, Chen Y, Shu Y, Cheng Y, Qiao J, Behr B, Pera RA, Hsueh AJ. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS One. 2012;7:e49328. doi: 10.1371/journal.pone.0049328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Wang YS, Wang LJ, Zhang H, Li RZ, Cui CC, Li WZ, Zhang Y, Jin YP. Vitamin C supplementation enhances compact morulae formation but reduces the hatching blastocyst rate of bovine somatic cell nuclear transfer embryos. Cell Reprogram. 2014;16:290–297. doi: 10.1089/cell.2013.0088. [DOI] [PubMed] [Google Scholar]
- 10.Rameshbabu K, Sharma R, Singh KP, George A, Chauhan MS, Singla SK, Manik RS, Palta P. Presence of nitric oxide synthase immunoreactivity and mRNA in buffalo (Bubalus bubalis) oocytes and embryos. Reprod Domest Anim. 2012;47:e22–25. doi: 10.1111/j.1439-0531.2011.01884.x. [DOI] [PubMed] [Google Scholar]
- 11.Santana PD, Silva TV, da Costa NN, da Silva BB, Carter TF, Cordeiro Mda S, da Silva BJ, Santos Sdo S, Herculano AM, Adona PR, Ohashi OM, Miranda Mdos S. Supplementation of bovine embryo culture medium with L-arginine improves embryo quality via nitric oxide production. Mol Reprod Dev. 2014;81:918–927. doi: 10.1002/mrd.22387. [DOI] [PubMed] [Google Scholar]
- 12.Tranguch S, Steuerwald N, Huet-Hudson YM. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol Reprod. 2003;68:1538–1544. doi: 10.1095/biolreprod.102.009282. [DOI] [PubMed] [Google Scholar]
- 13.Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol. 1993;225:153–164. doi: 10.1016/0076-6879(93)25012-q. [DOI] [PubMed] [Google Scholar]
- 14.Chen HW, Jiang WS, Tzeng CR. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil Steril. 2001;75:1163–1171. doi: 10.1016/s0015-0282(01)01780-0. [DOI] [PubMed] [Google Scholar]
- 15.Sengoku K, Takuma N, Horikawa M, Tsuchiya K, Komori H, Sharifa D, Tamate K, Ishikawa M. Requirement of nitric oxide for murine oocyte maturation, embryo development, and trophoblast outgrowth in vitro. Mol Reprod Dev. 2001;58:262–268. doi: 10.1002/1098-2795(200103)58:3<262::AID-MRD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Kusinski LC, Stanley JL, Dilworth MR, Hirt CJ, Andersson IJ, Renshall LJ, Baker BC, Baker PN, Sibley CP, Wareing M, Glazier JD. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol. 2012;303:R86–93. doi: 10.1152/ajpregu.00600.2011. [DOI] [PubMed] [Google Scholar]
- 17.Lee TH, Lee MS, Huang CC, Tsao HM, Lin PM, Ho HN, Shew JY, Yang YS. Nitric oxide modulates mitochondrial activity and apoptosis through protein S-nitrosylation for preimplantation embryo development. J Assist Reprod Genet. 2013;30:1063–1072. doi: 10.1007/s10815-013-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinerman JL, Lowenstein CJ, Snyder SH. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993;73:217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- 19.Rahman MS, Kwon WS, Lee JS, Kim J, Yoon SJ, Park YJ, You YA, Hwang S, Pang MG. Sodium nitroprusside suppresses male fertility in vitro. Andrology. 2014;2:899–909. doi: 10.1111/j.2047-2927.2014.00273.x. [DOI] [PubMed] [Google Scholar]
- 20.Byun EB, Sung NY, Yang MS, Song DS, Byun EH, Kim JK, Park JH, Song BS, Lee JW, Park SH, Byun MW, Kim JH. Procyanidin C1 causes vasorelaxation through activation of the endothelial NO/cGMP pathway in thoracic aortic rings. J Med Food. 2014;17:742–748. doi: 10.1089/jmf.2013.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabri DR, Hott SC, Reis DG, Biojone C, Correa FM, Resstel LB. The expression of contextual fear conditioning involves activation of a NMDA receptor-nitric oxide-cGMP pathway in the dorsal hippocampus of rats. Eur Neuropsychopharmacol. 2014;24:1676–1686. doi: 10.1016/j.euroneuro.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Silva RO, Lucetti LT, Wong DV, Aragao KS, Junior EM, Soares PM, Barbosa AL, Ribeiro RA, Souza MH, Medeiros JV. Alendronate induces gastric damage by reducing nitric oxide synthase expression and NO/cGMP/K(ATP) signaling pathway. Nitric Oxide. 2014;40:22–30. doi: 10.1016/j.niox.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Yang S, Wang X, Gan R. Matrine improved the function of heart failure in rats via inhibiting apoptosis and blocking beta3adrenoreceptor/endothelial nitric oxide synthase pathway. Mol Med Rep. 2014;10:3199–3204. doi: 10.3892/mmr.2014.2642. [DOI] [PubMed] [Google Scholar]
- 24.Rathnasamy G, Sivakumar V, Rangarajan P, Foulds WS, Ling EA, Kaur C. NF-kappaB-mediated nitric oxide production and activation of caspase-3 cause retinal ganglion cell death in the hypoxic neonatal retina. Invest Ophthalmol Vis Sci. 2014;55:5878–5889. doi: 10.1167/iovs.13-13718. [DOI] [PubMed] [Google Scholar]
- 25.Scheit K, Bauer G. Synergistic effects between catalase inhibitors and modulators of nitric oxide metabolism on tumor cell apoptosis. Anticancer Res. 2014;34:5337–5350. [PubMed] [Google Scholar]


