Abstract
The purpose of present study was to investigate the radiosensitivity of lung cancer cells affected by human lung cancer-associated fibroblasts (CAFs) in transwell and direct co-culture in vitro. Human lung CAFs was obtained from fresh human lung adenocarcinoma tissue specimens by primary culture with tissue explants method, and was identified by immunofluorescence staining. The radiosensitivity of A549 and H1299 lung cancer cells in mono-culture, transwell and direct co-culture with human lung CAFs were investigated by clonogenic survival assay, respectively. Human lung CAFs were obtained successfully, and had specific immuno-staining with α-SMA, Vimentin, and FAP, and no cytokeratin-18 expression. The plating efficiency (PE) of A549 cells without receiving irradiation were 0.206±0.031, 0.210±0.007 (>0.05) and 0.352±0.023 (<0.05) in mono-culture, transwell and direct co-culture, respectively. The PE of H1299 cells were 0.200±0.039, 0.259±0.024 (>0.05) and 0.323±0.025 (<0.05) in mono-culture, transwell and direct co-culture, respectively. The clonogenic survival assay showed that the protection enhancement ratio of human lung CAFs in transwell and direct co-culture were 1.11 and 1.29 for A549 cells, and 1.15 and 1.25 for H1299 cells, respectively. The results suggested that human lung CAFs promote the radioresistance of lung cancer cells significantly. Its radioprotective effect may attribute to CAFs stimulating lung cancer cell proliferation.
Keywords: Cancer-associated fibroblasts, lung cancer, co-culture, radiosensitivity
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancers and is the most common cause of cancer-related death worldwide [1,2]. Despite the advance in lung cancer therapeutics in recent years, 5-year survival rate remains largely unaltered [3]. Radiotherapy combined with chemotherapy continues to be the cornerstone in the treatment of local advanced NSCLC. The treatment response depends on the intrinsic radiosensitivity of lung cancer cells for chemo-and radiotherapy. In addition, the tumor stroma surrounding and infiltrating the cancer may have an important impact on the response to chemo- and radiotherapy.
Emerging evidence indicates that tumors are composed of tumor parenchyma and stroma, two discrete but interactive parts that cross-talk to promote tumor growth. Tumor stromal cells play important roles in tumor initiation, progression, and metastasis [5]. Cancer-associated fibroblasts (CAFs) are the most frequent component of tumor stroma [6]. They are considered to stem from resident fibroblasts, bone marrow-derived progenitor cells, or from epigenetic transitions from endothelial or cancer cells through endothelial-mesenchymal transition or epithelial-mesenchymal transition (EMT) [7,8]. There is increasing awareness that CAFs make an important contribution to tumor growth and progression through secretion of soluble factors, growth factors or inflammatory chemokines, as well as remodeling tumor extra-cellular matrix (ECM) and tumor metabolism, regulation of motility and stemness of cancer cells, and preparation of metastatic niche [4,9-12]. So CAFs have recently emerged as a promising therapeutic target to counteract cancer progression.
However, little is known about the role of CAFs in radiation effect for cancer cells, especially in radiosensitivity of lung cancer cells at present. Therefore, in this study CAFs from human NSCLC were isolated and cultured in vitro to establish a model of interaction of human lung CAFs with A549 and H1299 lung cancer cells by transwell and direct co-culture. Their influences on the radiosensitivity of lung cancer cells were further analyzed. The study is likely to aid in understanding the radioresistance mechanisms of lung cancer cells and may provide a reference for clinical treatment.
Materials and methods
Lung cancer cells culture
Tissue culture media and supplements were purchased from GIBCO (Life Technologies, Grand Island, NY). A549 and H1299 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in 100-mm tissue-culture dishes (Coring, USA) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS, Biochrom, Berlin, Germany) and 1% penicillin-streptomycin at 37°C, 5% CO2 incubator.
Primary culture of CAFs from human NSCLC
The fresh specimen from the resected adenocarcinoma tumor tissues was taken from the operating room and sheared into small blocks 1.5-2.0 mm in diameter. The harvested tumor tissues were washed by PBS containing 1% penicillin-streptomycin and then placed uniformly onto the 25-mm tissue culture plates. DMEM (1 ml) containing 10% fetal bovine serum was added, the culture flask was then inverted after 1 h and cultured for 24 h at 37°C, 5% CO2 incubator. Subsequently, the culture flask was overturned and added DMEM (5 ml) containing 10% fetal bovine serum again. The culture liquid was replaced subsequent three days. After cells covered the whole base of the flask, the enzyme digestion method was used to purify the cells. Following the first passage, the medium was replaced with DMEM containing 10% fetal bovine serum to conduct a conventional culture. The cells were used to conduct experiment after 3-7 passages following primary culture.
Identification of human lung CAFs
The third-generation purified CAFs were used to conducted preliminary identification by immunofluorescence staining technique. The slides with CAFs was fixed 4% PFA-PBS for 15 min and permeabilized with 0.3% triton-PBS for 20 min. Slides were then exposed to blocking buffer (2% HAS-PBS) for 1 h. Next primary mono-antibodies including α-smooth muscle actin (α-SMA, Thermo, USA), fibroblast activation protein (FAP, Ebioscience, USA), Vimentin (Abcam, Cambridge, UK) and cytokeratin-18 (Abcam, Cambridge, UK) were diluted in blocking buffer and incubated with CAFs for 10 h. After washing, cells were incubated with secondary antibody for 45 min. A second wash was followed by preparation of slides in DAPI-Fluoromount-G. The staining slides were examined in a fluorescence microscope.
Clonogenic survival assay of lung cancer cells
The 6-well plate (Corning, USA) clonogenic assay was used to measure cell survival ratio. The A549 and H1299 cells cultured with or without human lung CAFs. The co-culture methods included two approaches: transwell and direct co-culture. The ratio of cell numbers between lung cancer cells and CAFs was 1:5. For transwell co-culture assay, lung cancer cells and CAFs were separated by a transwell chamber. CAFs suspended in DMEM plus 10% FBS were seeded in culture inserts (0.4 µm pore size, Corning Inc. Corning, NY) before 24 h and then placed into the wells containing lung cancer cells. For direct co-culture assay, CAFs were allowed to attach plates for 24 h prior to plating lung cancer cells in fresh medium. All lung cancer cells with mono- or co-culture were incubated for 12 h prior to irradiation, and were exposed to graded doses (0, 2, 4, 6, 8 Gy) of 6 Mv X-ray radiation by linear accelerator (Simens, Germany) at a dose rate of 1.95 Gy/min. After 12 days of incubation, the cells were stained with 0.5% crystal violet (Beyotime, China) in absolute ethanol, and colonies with more than 50 cells were counted under dissection microscope. The immunofluorescence double staining with α-SMA and cytokeratin-18 was used to identify lung cancer cells clones from CAFs in direct co-culture condition. Clonogenic survival curves were constructed from three independent experiments by fitting the average survival levels using least squares regression by the linear quadratic (LQ) model [13]. The protection enhancement ratio (PER) of human lung CAFs for A549 and H1299 cells were calculated at the surviving fraction of 0.1 by dividing radiation dose of the co-culture curve with that of the corresponding mono-culture curve.
Statistical analysis
The differences for the all considered parameters between mono- and co-culture were analyzed using by the mean t-test statistical method. A two-tailed value of P<0.05 was considered statistically significant.
Results
Identification of human lung CAFs
Inverted microscope observation showed that purified human lung CAFs were fibroblast-like cells, their sizes were different and their shapes presented as a fusiform or irregular triangle. The nucleus was at the center of cell body, presenting as a circular or elliptical shape (Figure 1). The immunofluorescence staining results revealed that all of the purified cells expressed α-SMA, FAP and Vimentin and had no cytokeratin-18 expression (Figure 2).
Figure 1.

Inverted microscope observation of third-generation human lung CAFs.
Figure 2.
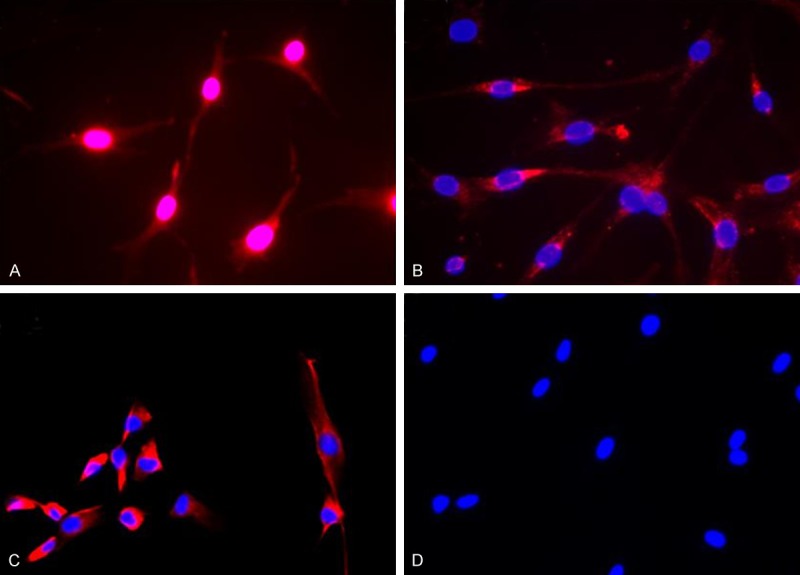
Immunofluorescence staining identification of human lung CAFs. A. α-SMA-positive expression. B. FAP-positive expression. C. Vimentin-positive expression. D. Cytokeratin-18-negative expression.
The radiosensitive differences of lung cancer cells cultured with or without human lung CAFs
For direct co-culture, crystal violet staining showed that lung cancer cells clones formed, and CAFs had no clone formation and grow dispersedly around lung cancer cells clones (Figure 3). Immunofluorescence double staining revealed that A549 and H1299 cells clones expressed cytokeratin-18 and had no α-SMA expression, but CAFs expressed α-SMA and had no cytokeratin-18 expression (Figure 4).
Figure 3.
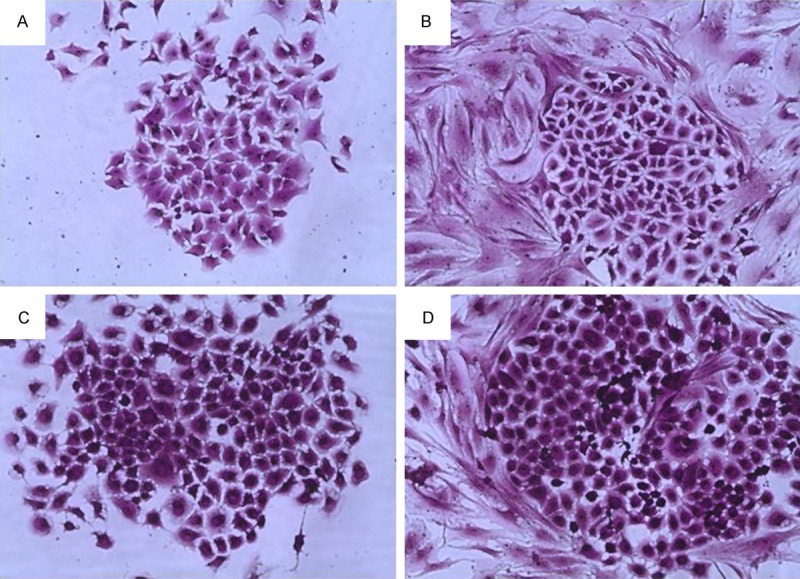
Lung cancer cells clone by crystal violet staining. A. A549 cells clone in mono-culture. B. A549 cells clone in direct co-culture with human lung CAFs. C. H1299 cells clone in mono-culture. D. H1299 cells clone in direct co-culture with human lung CAFs.
Figure 4.
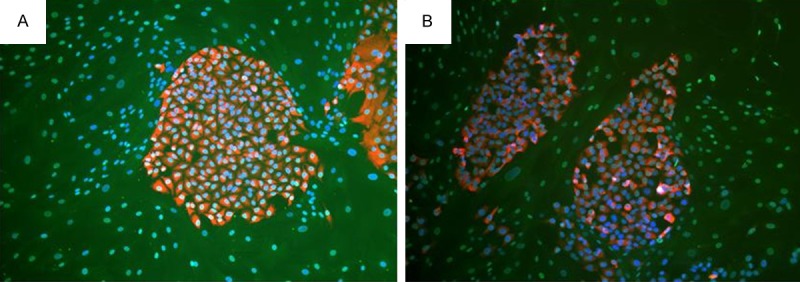
Immunofluorescence double staining revealed that lung cancer cells clone expressed cytokeratin-18 and had no α-SMA expression, and human lung CAFs expressed α-SMA and had no cytokeratin-18 expression in direct co-culture. A. A549 cells. B. H1299 cells.
The plating efficiency (PE) of A549 cells without receiving irradiation were 0.206±0.031, 0.210±0.007 and 0.352±0.023 in mono-culture, transwell and direct co-culture, respectively. The PE of H1299 cells without receiving irradiation were 0.200±0.039, 0.259±0.024 and 0.323±0.025 in mono-culture, transwell and direct co-culture, respectively. There existed significant differences in PE for A549 and H1299 cells in direct co-culture compared to mono-culture. No significant differences existed in PE for A549 and H1299 cells between in mono-culture and in transwell co-culture (Figure 5; Table 1).
Figure 5.
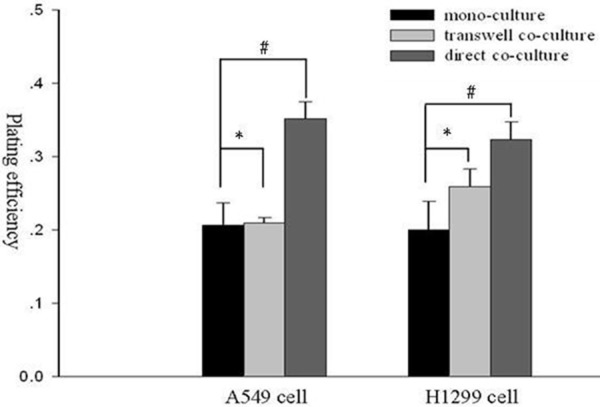
The PE of A549 and H1299 lung cancer cells without receiving irradiation in mono-culture, transwell and direct co-culture. *; p>0.05, #; p<0.05
Table 1.
The PE of A549 and H1299 lung cancer cells without receiving irradiation in mono-culture, transwell and direct co-culture
| Culture condition | A549 cells | H1299 cells | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| PE | T value | P value | PE | T value | P value | |
| Mono-culture | 0.206±0.031 | 0.200±0.039 | ||||
| Transwell co-culture | 0.210±0.007 | 0.219 | >0.05 | 0.259±0.024 | 2.23 | >0.05 |
| Direct co-culture | 0.352±0.023 | 6.577 | <0.05 | 0.323±0.025 | 4.60 | <0.05 |
The clonogenic survival curves of A549 and H1299 cells were shown in Figure 6. The plots showed that lung cancer cells in transwell and direct co-culture with human lung CAFs were more radioresistance than the corresponding cancer cells in mono-culture. The PER of human lung CAFs in transwell and direct co-culture were 1.11 and 1.29 for A549 cells, and 1.15 and 1.25 for H1299 cells, respectively.
Figure 6.
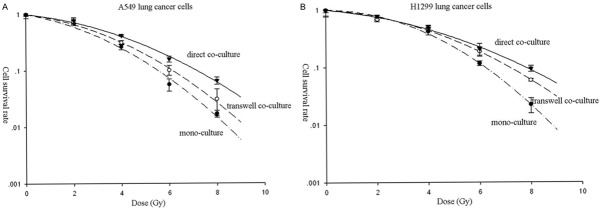
The cell survival curves of lung cancer cells in mono-culture, transwell and direct co-culture. A. A549 cells. B. H1299 cells.
Discussion
CAFs are the extreme abundant cells in the stroma of many tumors and share many similarities with activated fibroblasts found in wound and inflammatory sites [6,9,15]. Currently, the acceptable markers of CAFs consist of high expression of α-SMA, FAP, Vimentin, FSP-1, PDGFR-α, and PDGFR-β or loss of CAV-1, PTEN, p21, or TP53 mutation [5,16]. In the present study, an immunofluorescence technique was used to detect the expression of α-SMA, FAP and Vimentin as human lung CAFs markers. The results revealed that human lung CAFs in vitro expressed α-SMA, FAP and Vimentin (Figure 2A-C). To exclude the possible contamination of epithelial cells, we also performed immunofluorescence staining of these cells by cytokeratin-18. There had not found any cell positively stained for cytokeratin-18 in these cells (Figure 2D). These results indicated that the purified human lung CAFs were obtained successfully.
There is increasing evidence that CAFs play crucial roles in carcinogenesis, proliferation, invasion, and metastasis of lung cancer [17-19]. Saito et al [17] found that NIH3T3 murine fibroblasts stimulated subcutaneous tumor growth of A549 cells in vivo through hedgehog-dependent Forkhead Box F1 signaling. In the present study, we found that clone formation rates of A549 and H1299 cells in direct co-culture with CAFs was significantly higher than that of mono-culture. But clone formation rates had no significant differences between in mono-culture and transwell co-culture (Figure 5; Table 1). The results suggested that human lung CAFs can promote proliferation of lung cancer cells in vitro in direct co-culture, but not in transwell co-culture. So targeting of CAFs could be a novel strategy for cancer treatment.
However, there are few studies focusing on the role of CAFs on the response to radiotherapy until now. In this study, we found that human lung CAFs can promote the radioresistance of A549 and H1299 lung cancer cells. The PER of human lung CAFs in transwell and direct co-culture were 1.11 and 1.29 for A549 cells, and 1.15 and 1.25 for H1299 cells, respectively (Figure 6). Similarly, Mantoni et al [4] found that the survival rate of pancreatic cancer cells (PCC) after radiation was enhanced by direct co-culture with pancreatic stellate cells (PSC), and this PSC-mediated radioprotection of PCC was depended on β1-integrin-FAK signaling.
In conclusion, the results suggested that human lung CAFs promote the radioresistance of lung cancer cells in vitro significantly, and its protective effect may attribute to CAFs stimulating lung cancer cell proliferation.
Acknowledgements
This study was supported by grants from Jiangsu Natural Science Funding (BK20141185) and Jiangsu Province’s Key Medical Person Funding (RC2011144).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Horie M, Saito A, Mikami Y, Ohshima M, Morishita Y, Nakajima J, Kohyama T, Nagase T. Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem Biophys Res Commun. 2012;423:158–163. doi: 10.1016/j.bbrc.2012.05.104. [DOI] [PubMed] [Google Scholar]
- 4.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast. Cancer Metastasis Rev. 2013;32:303–15. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;8:1461–1462. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- 8.Mcanulty RJ. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumor cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 10.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 11.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 12.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Nat Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fertil B, Malaise EP. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981;7:621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- 14.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 15.Pietras K, Ostman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Aboussekhra A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Develop Biol. 2011;55:841–849. doi: 10.1387/ijdb.113362aa. [DOI] [PubMed] [Google Scholar]
- 17.Saito RA, Micke P, Paulsson J, Augsten M, Peña C, Jönsson P, Botling J, Edlund K, Johansson L, Carlsson P, Jirström K, Miyazono K, Ostman A. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res. 2010;70:2644–2654. doi: 10.1158/0008-5472.CAN-09-3644. [DOI] [PubMed] [Google Scholar]
- 18.Wald O, Izhar U, Amir G, Kirshberg S, Shlomai Z, Zamir G, Peled A, Shapira OM. Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non-small cell lung cancer tumor proliferation. J Thorac Cardiovasc Surg. 2011;141:1503–1512. doi: 10.1016/j.jtcvs.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino A, Ishii G, Ito T, Aoyagi K, Ohtaki Y, Nagai K, Sasaki H, Ochiai A. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res. 2011;71:4769–4779. doi: 10.1158/0008-5472.CAN-10-3228. [DOI] [PubMed] [Google Scholar]


