Abstract
Background: Osteopontin (OPN) is overexpressed in many human tumors and involved in promotion of cancer cells by regulating various facets of tumor progression such as cell proliferation, invasion and metastasis. To understand roles of OPN in tumor progression of laryngeal squamous cell carcinoma (LSCC) or develop molecular marker for prognosis and treatment of LSCC, we thus explore biological function of OPN and correlation with p53 in LSCC. Methods: The expression of OPN and p53 in tumor tissues of LSCC was determined immunohistochemically in both LSCC and adjacent normal tissues. Lentivirus vector with RNAi small hairpin gene sequence of OPN (named LV-shOPN) was transfected into Hep-2 cells. OPN expression was detected by Western blotting assay and the viability and invasive ability of Hep-2 cells were examined by MTS and transwell assay. Results: We found that OPN and p53 protein expressions were significantly higher in LSCC tumor tissues than adjacent normal tissues (76.2% vs. 23.8% for OPN and 63.8% vs. 15.2% for p53, all P < 0.001). OPN expression was also significantly correlated with p53 expression, tumor stage, grade and the presence of lymph node. The constructed LV-shOPN effectively inhibited the OPN expression, viability and invasive ability of Hep-2 cells (all P < 0.050). Conclusion: Taken together, OPN is overexpressed in LSCC. OPN expression is correlated with p53 expression, tumor progression and lymph node metastasis. Additionally, RNAi silencing of OPN expression can significantly inhibit tumor viability and invasion ability of Hep-2 cells. Thus, OPN may be considered as a marker and potential gene targeting therapy in LSCC.
Keywords: Osteopontin, p53, RNA interference, laryngeal squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma is the sixth most common cancer worldwide. Laryngeal squamous cell carcinoma (LSCC) is one of the most common types of HNSCC [1-3]. Unfortunately, patients with the same diagnostic and prognostic profile can have markedly different clinical outcomes. It is likely that the classifications of current taxonomy of LSCC patients with different molecular profiles and distinct clinical phenotypes are based mainly on morphology [4]. Although various biological prognostic markers have been identified for LSCC, little is known about the molecular mechanisms that govern the neoplastic phenotypes of LSCC. Thus, better understanding the molecular mechanisms of LSCC progression may help identify more effective targets for improving the outcomes for patients with LSCC.
Osteopontin (OPN) is a highly acidic calcium-binding glycosylated phosphoprotein [5-7] which functions as a cell attachment protein and cytokine that signals through two cell adhesion molecules, αvβ3-integrin and CD44 [8]. Binding of OPN to these cell surface receptors stimulates cell adhesion, chemotaxis, proliferation, migration, and specific signaling functions [9,10]. Therefore, it involved in promotion of cancer cells by regulating various facets of tumor progression such as cell proliferation, invasion, angiogenesis and metastasis. OPN may play multiple roles in promoting tumor progression, including inhibiting macrophage function and enhancing growth or survival of metastases [11]. Overexpression of OPN has been found in a variety of cancers, including breast cancer, lung cancer, colorectal cancer, stomach cancer, ovarian cancer, esophageal cancer and melanoma [12-14]. Moreover, OPN is present in elevated levels in some patients with metastatic cancers [15-17]. Elevated OPN level was significantly associated with poor survival [18]. Therefore, suppression of the action of OPN may confer significant therapeutic activity, for which several strategies have been identified [19,20].
Although OPN is overexpressed in many human malignancies, the molecular function of OPN in LSCC has not been fully determined. Thus, further studies of the role of OPN in carcinogenesis and its clinicopathologic characteristics in LSCC are needed. p53, a tumor suppressor gene, has been considered as an early important incident in malignant transformation of head and neck cancers [21], but its correlation with OPN has not been examined. Thus, in this study, we investigated OPN expression in LSCC specimens and determined its correlation with p53 and clinicopathologic characteristics. Furthermore, we used small hairpin RNA (shRNA), a powerful therapeutic potential tool by silencing oncogenes, to detect the potential function by RNA interference (RNAi) silencing OPN expression in Hep-2 cells of LSCC in vitro.
Material and methods
Patients and specimens
All 105 tumor samples were obtained from patients who had undergone surgery and were diagnosed with primary LSCC in General Hospital of Jinan Military Region from January 2003 to May 2007. The adjacent nontumor tissues were obtained about 1 cm from primary carcinoma. The Ethics Committee of General Hospital of Jinan Military Region approved the protocol of this study. All written informed consent for participation in this study was obtained from all study patients. Histopathological diagnoses were made according to the pathological classification system of the World Health Organization [22], and the tumor was staged following the tumor-node-metastasis classification of the International Union Against Cancer (UICC 2002). The clinicopathological information of patients was available, including gender, age, tumor site, tobacco, alcohol, tumor stage, histological grade, and metastasis. All patients didn’t receive radiotherapy, chemotherapy and immunotherapy before the surgery.
Immunohistochemical staining
Immunohistochemical staining was performed on thin sections (~4 μm) of paraffin-embedded archival poly-L-lysine-coated glass slides. The samples were dewaxed in 100% xylene and rehydrated in descending ethanol series and water according to standard protocols. Heat-induced antigen retrieval was performed in 0.001 mol/L EDTA buffer for two minute at 100°C. The slides were fixed and the endogenous peroxidase activity was quenched by incubation in methanol with 3% hydrogen peroxide for 10 minutes. The slides were then washed with phosphate-buffered saline. Nonspecific binding was blocked by incubation with 2% bovine serum in tris-buffered saline for 30 minutes at room temperature. The slides were first incubated with the primary monoclonal mouse-anti-human antibody against OPN (Zhongshan Technology Co., Beijing, China) and primary monoclonal mouse-anti-human antibody against p53 (Zhongshan, Godbridge, China) respectively overnight at 4°C, and the slides were then washed 3 times in phosphate-buffered saline containing 3%, 2%, and 1% of normal human serum. Biotinylated anti-mouse-IgG (Zhongshan Technology Co., Beijing, China) avidin-biotin complex was applied as a secondary antibody for 30 min at room temperature, followed by the application of peroxidase-conjugated strepavidin for 30 min. The antibody binding was visualized with 3, 3-diaminobenzidine tetrahydrochloride before brief counterstaining with Mayer’s hematoxylin. For monoclonal antibodies of mouse origin, negative controls were obtained using isotypic mouse immunoglobulin in the same dilution as the primary antibody of concern. All control experiments gave negative results.
Evaluation of immunostaining
Evaluation of OPN and p53 protein staining was independently performed by 2 experienced pathologists in a blinded fashion. Five representative microscopic areas at × 400 magnifications were randomly selected for examination. Expression of OPN and p53 was determined by both the intensity of staining and the proportion of tumor cells that had an unequivocal positive reaction using a semi-quantitative and subjective grading system [23]. A proportion score was assigned, which represented the estimated proportion of positively stained tumor cells (0, none; 1, < 10%; 2, ≥ 10% to < 50%; 3, ≥ 50% to < 80%; 4, ≥ 80% to < 100%). An intensity score was assigned that represented the average intensity of the positive tumor cells (0, none; 1, weak; 2, intermediate; 3, strong). Multiplication of the intensity and the proportion scores gave rise to the ultimate immunohistochemical scores: a total score greater than or equal to 3 was taken to indicate a high expression and a sum score below 3 indicated a low expression.
Cell culture and lentiviral infection
The Hep-2 cells of human LSCC were provided by the Center Laboratory, Second Military Medical University. Cells were cultured in DMEM medium containing 10% fetal bovine serum (Gibco) and incubated in a humidified (37°C, 5% CO2) incubator. Small hairpin RNA (shRNA) of human OPN lentivirus gene transfer vector (named LV-shOPN) encoding green fluorescent protein (GFP) sequence was constructed and provided by Dr. Zhu Minghui (Changhai Hospital of the Second Military Medical University, Shanghai, China). Lentivirus vector carried GFP but without OPN was named LV-shNon as a control group. Hep-2 cells were plated in 24-well plates (2 × 104 cells/well) overnight. The lentiviruses were diluted in 0.2 mL (108 TU/mL) complete medium containing polybrene (8 mg/mL) and added to the cells for incubation for 1 h at 37°C, followed by incubation in 0.3 mL of freshly prepared polybrene-DMEM for another 24 h, then the medium was replaced with fresh DMEM and the cells were cultured for another 48 h.
Western blot analysis
Cells were washed in phosphate buffered saline, and lysed directly using RIPA buffer. Proteins at the same amount were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. After probing with antibodies, the signals were visualized by super signal enhanced chemiluminescence reagent (Pierce, Rockford, I L). The antibodies used were anti-osteopontin (R&D Systems, Minneapolis, MN) and anti-glyceraldehyde-3-phosphate dehydrogenase (KangChen Bio-tech, Shanghai, P. R. China). Gel bands were then developed with ECL Western blotting detection reagents (Amersham, USA)
Cell viability assays
Cell viability was assessed by using the 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS) assay (Promega, Madison, WI) in 96-well plates (5000 cells/well) following the instructions of the manufacturer. Each experiment was done in triplicate and repeated three times.
Matrigel invasion assays
Cell invasion assays were quantified in vitro using Transwell chambers with polycarbonate membrane filters (8 µm pore size; Corning, NY) coated with a Matrigel (Sigma) according to the manufacturer’s instructions, as previously described [24]. The number of cells invading through Matrigel was counted by randomly selecting five visual fields, and the extent of invasion was expressed as the average number of cells per microscopic field at a magnification of × 200. All experiments were performed for three times. Two independent investigators were blinded when reading the assay for Matrigel invasion.
Statistical analysis
The difference in OPN expression between LSCC and adjacent nontumor tissues was performed using the Student’s t-test and one-way analysis of variance for multiple comparisons. A Pearson’s correlation coefficient was used to determine the correlation between OPN and p53 protein expression. Chi-square test was used to analyze the associations between OPN expression and p53 and several clinicopathologic parameters. In vitro assays, the student’s t-test was used to determine the differences between the groups with different assays. The SPSS software (for Windows, version 19.0) was used for statistical calculations. A P value of less than 0.05 was considered statistically significant.
Results
Expression of OPN and p53 in LSCC
The age of 105 LSCC patients, including 92 men and 13 women, ranged from 33 to 76 years old, with a mean of 61.4 years. Of 105 patients, OPN positive staining was detected in 80 (76.2%) and 28 (26.7%) in LSCC and adjacent nontumor tissues, respectively (Table 1). The staining of OPN was observed in cytoplasm and cell membrane, but no nuclear staining was found in all tissues studied. The brown or yellow-brown grana or bolus was shown in cytoplasm of positive staining cells. OPN expression was higher in LSCC than in adjacent normal tissue (P < 0.001) (Figure 1A, 1B). The p53 protein expression showed a yellow-brown nuclear positive staining pattern in 67 (63.8%) tumor tissues of 105 cases and 16 (15.2%) in adjacent nontumor tissues. However, no evidence of p53 expression was found in cytoplasm (Figure 2A, 2B).
Table 1.
TIP30 and P53 expression in LSCC and adjacent normal tissues
| Groups | OPN | Total | P * value | P53 | Total | P * value | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| High | Low | High | Low | |||||
| LSCC | 80 | 25 | 105 | < 0.001 | 67 | 38 | 105 | < 0.001 |
| Adjacent normal tissues | 28 | 77 | 105 | 16 | 89 | 105 | ||
| Total | 108 | 102 | 210 | 83 | 127 | 210 | ||
Two-sided c2 test.
Figure 1.
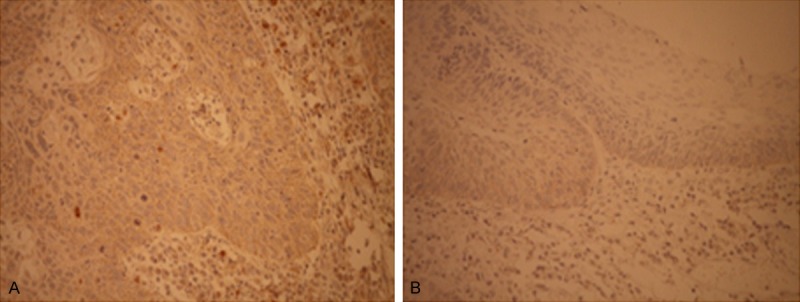
OPN expression in LSCC tissues (A) and adjacent nontumor tissues (B), immunohistochemical staining (× 400).
Figure 2.
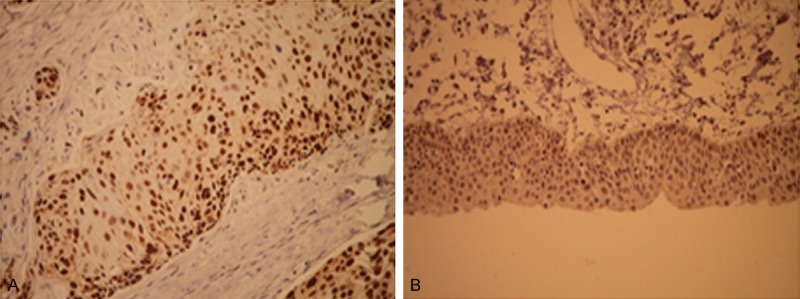
P53 expression in LSCC tissues (A) and adjacent nontumor tissues (B), immunohistochemical staining (× 400).
Correlation between expression of OPN and p53
Of 67 tumors with high p53 expression, 57 (85.1%) were OPN positive. Of 38 tumors with low p53 expression, 23 (60.5%) were OPN positive (Table 2). The expression of OPN was positively associated with p53 expression (r = 0.277, P = 0.004).
Table 2.
Association between OPN and p53 expressions
| P53 | OPN | Total | P * | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| High | 57 | 10 | 67 | 0.005 |
| Low | 23 | 15 | 38 | |
| Total | 80 | 25 | 105 | |
Two-sided c2 test.
Correlation of OPN expression with selected clinicopathologic characteristics of LSCC
The clinicopathologic characteristics of 105 patients with LSCC are listed in Table 3. A significant correlation was found between the OPN expression and overall stage, tumor differentiation, and lymph node metastasis (P = 0.042; P = 0.004 and P = 0.019, respectively). However, such significant correlation was not observed for other variables including age, gender, smoking, alcohol use, tumor sites, and treatment (all P > 0.05).
Table 3.
Associations between OPN expression and clinicopathological characteristics of LSCC
| Variables | N | OPN expression | P values | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Age (yr) | 0.554 | |||
| < 55 | 17 | 12 | 5 | |
| ≥ 55 | 88 | 68 | 20 | |
| Gender | 0.947 | |||
| Male | 92 | 70 | 22 | |
| Female | 13 | 10 | 3 | |
| Tobacco | 0.890 | |||
| Ever | 85 | 65 | 20 | |
| Never | 20 | 15 | 5 | |
| Alcohol | 0.825 | |||
| Ever | 61 | 46 | 15 | |
| Never | 44 | 34 | 10 | |
| Tumor sites | 0.306 | |||
| Supraglottic | 36 | 26 | 10 | |
| Glottic | 55 | 45 | 10 | |
| Infraglottic | 14 | 9 | 5 | |
| Overall stage | 0.042* | |||
| I-II | 64 | 46 | 20 | |
| III-IV | 41 | 34 | 5 | |
| Tumor differentiation | 0.004* | |||
| Well | 49 | 31 | 18 | |
| Moderate/poor | 56 | 49 | 7 | |
| Lymph node metastasis | 0.019* | |||
| Positive | 63 | 53 | 10 | |
| Negative | 42 | 27 | 15 | |
| Treatment | 0.511 | |||
| Surgery only | 48 | 38 | 10 | |
| Combined | 57 | 42 | 15 | |
Two-sided c2 test.
LV-shOPN reduced OPN expression in Hep-2 cells
The OPN expression was further analyzed by western blotting in Hep-2 cells (Figure 3A-C). Hep-2 cells infected with LV-shOPN significantly decreased OPN expression, in comparison to cells with LV-shNon transfection as control (P < 0.05). These results clearly indicated that the constructed LV-shOPN might effectively infect Hep-2 cells and significantly reduced OPN expression.
Figure 3.
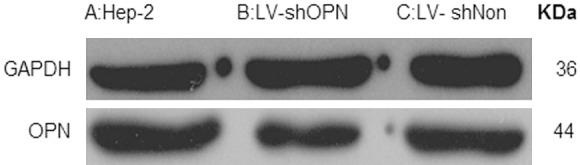
Inhibition of LV-shOPN decreased OPN expression by Western blot.
Decreased viability of Hep-2 cells with LV-shOPN transfection
As shown in Figure 4, after LV-shOPN transfection, the viability of Hep-2 cells was decreased in comparison with that of cells with LV-shNon transfection (P < 0.05). However, the viability was not significantly different between the cells with LV-shNon transfection and the cells with blank control (Hep-2 cells only) (P > 0.05). The time-effect curves indicated that LV-shOPN transfection could decrease the viability of Hep-2 cells in vitro.
Figure 4.
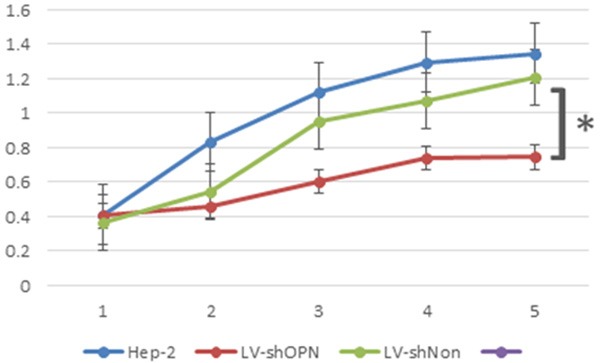
Inhibition of LV-shOPN decreased viability of Hep-2 cells.
LV-shOPN suppressed the invasive ability of Hep-2 cells
Hep-2 cells were incubated with Matrigel for 72 hours to detect the invasion ability (Figure 5A-C). The invasive abilities through Matrigel were significantly decreased in LV-shOPN-infected Hep-2 cells, relative to that in LV-shNon-infected cells and in Hep-2 cells (Figure 5D).
Figure 5.
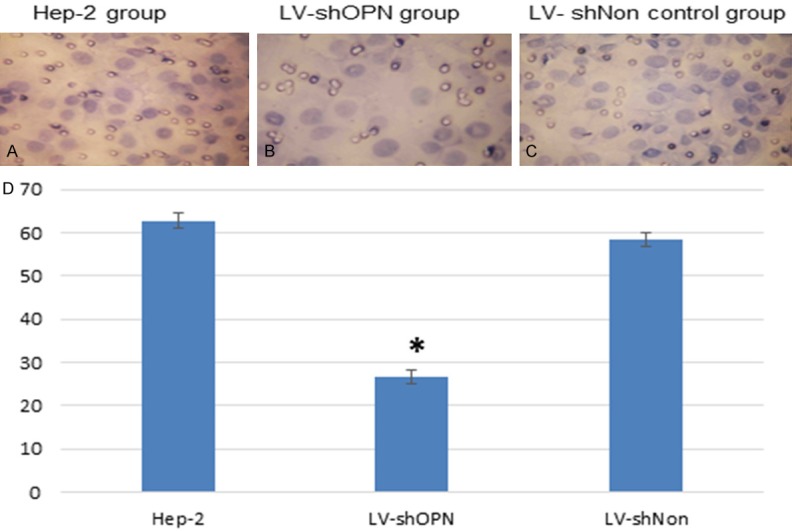
LV-shOPN Inhibited invasion viability of Hep-2 cells (A-C) and Invading cell numbers in tranwell Matrigel assay: Hep-2, LV-shOPN and LV-shNon group (62.78 ± 1.70, 26.72 ± 1.30, and 58.43 ± 1.50, respectively) (D).
Discussion
Despite modern detection and treatment strategies, primary prevention, screening, surgical treatment, and radiotherapy, the long-term survival of LSCC patients has remained substantially unchanged in the last two decades [25,26]. A tumor marker for early detection before the clinical manifestation of this cancer would be essential to better treatment and survival [3,27]. Recently, it was reported that OPN may be a candidate of such markers for early detection of head and neck cancers [28]. In LSCC, the OPN expression is positively correlated with degree of dysplasia but negatively with survival [29]. In tumor tissue samples, the elevated OPN expression was also significantly associated with metastasis and survival of LSCC [18] and was highly in all the invasive carcinomas than in patient-matched normal mucosa [30]. These findings may support that OPN may serve as one of useful indicators of tumorigenesis and prognosis for LSCC.
In the present study, we have also found that the OPN expression in LSCC tissues was significantly higher than that in adjacent normal tissues. The expression of OPN was significantly associated with tumor stage, lymph node metastasis and poor differentiation, but such similar correlations were not observed for age, gender, smoking, alcohol use, tumor sites and treatment. It is suggested that OPN may be a potential marker for tumor progression and may play important roles in invasion and metastasis of LSCC. It is likely that OPN may be a potentially therapeutic target for LSCC therapy.
The p53 tumor suppressor gene regulates cellular homeostasis through induction of cell cycle arrest, apoptosis, immune surveillance, cell senescence and is frequently mutated in most human malignant tumors [31]. It is found that induction of OPN expression by p53 is conserved across multiple species, and the endogenous OPN gene was induced in mouse and rat embryo fibroblasts in a p53-dependent manner. The OPN gene has a functional p53-responsive element in its promoter region, and was confirmed that there is an interaction between the OPN promoter and p53 protein in vivo. These results suggest that OPN is a direct transcriptional target of p53 [32]. Our data on immunohistochemical staining demonstrated that expression of p53 was significantly correlated with OPN, which is an indirect proof of p53-regulated OPN expression.
OPN in patients with tumor metastasis is significantly increased in comparison to normal sera [18,33,34], thus its prospects as a marker for evaluating tumor genesis and metastasis are expected. Methods for applying RNAi to the treatment of cancers as an antiviral therapy agent are currently under development [35]. Lentiviral vectors are optimal tools for the delivery of shRNAs into dividing and nondividing cells, making their application promising for the treatment of malignancy tumors [36]. In this study of LSCC, we used the lentivirus vector system to deliver a specially designed shRNA for human OPN gene into LSCC cell line, Hep-2, to silence the expression of OPN. We investigated the effect of decreased OPN on viability and invasive ability of LSCC cells and found that in vitro the Hep-2 cells with transfected LV-shOPN were obviously inhibited the viability and invasive ability. Thus, it was likely that OPN knocked down by shRNA in LSCC might inhibit tumor progression and development.
In summary, the present study provides evidence that OPN is overexpressed in LSCC and is correlated with tumor progression and lymph node metastasis which could be regulated by p53. The delivery of shOPN using lentivirus may have potential promise in gene targeted therapy for human LSCC. Additionally, future studies are needed to elucidate the mechanisms behind the interactions between OPN and p53 in the development and progression of LSCC.
Acknowledgements
We thank Yongjian Cao for assistance with patient samples recruitment. The authors gratefully thank MS. Da Liu and Dr. Tian He for article laboratory support and the funding from the Nature Science Foundation of Shandong Province, China, No. ZR2011.
Disclosure of conflict of interest
None.
Abbreviations
- RNAi
RNA interference
- OPN
osteopontin
- LSCC
laryngeal squamous cell carcinoma
- LV-shOPN
lentivirus vector with RNAi small hairpin gene sequence of OPN
- MTS
3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium
References
- 1.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 3.Licitra L, Bernier J, Grandi C, Locati L, Merlano M, Gatta G, Lefebvre JL. Cancer of the larynx. Crit Rev Oncol Hematol. 2003;47:65–80. doi: 10.1016/s1040-8428(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 4.Sotiriou C, Lothaire P, Dequanter D, Cardoso F, Awada A. Molecular profiling of head and neck tumors. Curr Opin Oncol. 2004;16:211–214. doi: 10.1097/00001622-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552:61–85. doi: 10.1016/s0304-419x(01)00037-3. [DOI] [PubMed] [Google Scholar]
- 6.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 9.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 11.Crawford HC, Matrisian LM, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res. 1998;58:5206–5215. [PubMed] [Google Scholar]
- 12.Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie MT, Thomas RJ, Pu ZY, Zhou H, Martin TJ, Findlay DM. Calcitonin receptors, bone sialoprotein and osteopontin are expressed in primary breast cancers. Int J Cancer. 1997;73:812–815. doi: 10.1002/(sici)1097-0215(19971210)73:6<812::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Skates SJ, Uede T, Wong KK, Schorge JO, Feltmate CM, Berkowitz RS, Cramer DW, Mok SC. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA. 2002;287:1671–1679. doi: 10.1001/jama.287.13.1671. [DOI] [PubMed] [Google Scholar]
- 15.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–4066. [PubMed] [Google Scholar]
- 16.Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer. 2002;95:506–512. doi: 10.1002/cncr.10709. [DOI] [PubMed] [Google Scholar]
- 17.Shimada Y, Watanabe G, Kawamura J, Soma T, Okabe M, Ito T, Inoue H, Kondo M, Mori Y, Tanaka E, Imamura M. Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology. 2005;68:285–292. doi: 10.1159/000086961. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Li L, Wang JT, Kan X, Lu JG. Elevated content of osteopontin in plasma and tumor tissues of patients with laryngeal and hypopharyngeal carcinoma associated with metastasis and prognosis. Med Oncol. 2012;29:1429–1434. doi: 10.1007/s12032-011-0012-z. [DOI] [PubMed] [Google Scholar]
- 19.El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Meerovitch K, Bergeron F, Leblond L, Grouix B, Poirier C, Bubenik M, Chan L, Gourdeau H, Bowlin T, Attardo G. A novel RGD antagonist that targets both alphavbeta3 and alpha5beta1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol. 2003;40:77–89. doi: 10.1016/s1537-1891(02)00339-7. [DOI] [PubMed] [Google Scholar]
- 21.van Houten VM, Tabor MP, van den Brekel MW, Kummer JA, Denkers F, Dijkstra J, Leemans R, van der Waal I, Snow GB, Brakenhoff RH. Mutated p53 as a molecular marker for the diagnosis of head and neck cancer. J Pathol. 2002;198:476–486. doi: 10.1002/path.1242. [DOI] [PubMed] [Google Scholar]
- 22.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. [PubMed] [Google Scholar]
- 23.Bache M, Reddemann R, Said HM, Holzhausen HJ, Taubert H, Becker A, Kuhnt T, Hansgen G, Dunst J, Vordermark D. Immunohistochemical detection of osteopontin in advanced head-and-neck cancer: prognostic role and correlation with oxygen electrode measurements, hypoxia-inducible-factor-1alpha-related markers, and hemoglobin levels. Int J Radiat Oncol Biol Phys. 2006;66:1481–1487. doi: 10.1016/j.ijrobp.2006.07.1376. [DOI] [PubMed] [Google Scholar]
- 24.Tong X, Li K, Luo Z, Lu B, Liu X, Wang T, Pang M, Liang B, Tan M, Wu M. Decreased TIP30 expression promotes tumor metastasis in lung cancer. Am J Pathol. 2009;174:1931–1939. doi: 10.2353/ajpath.2009.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 26.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Van Der Wal JE, Snow GB, Leemans CR, Braakhuis BJ. Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am J Pathol. 2002;161:1051–1060. doi: 10.1016/S0002-9440(10)64266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 28.Eto M, Kodama S, Nomi N, Uemura N, Suzuki M. Clinical significance of elevated osteopontin levels in head and neck cancer patients. Auris Nasus Larynx. 2007;34:343–346. doi: 10.1016/j.anl.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Staibano S, Merolla F, Testa D, Iovine R, Mascolo M, Guarino V, Castellone MD, Di Benedetto M, Galli V, Motta S. OPN/CD44v6 overexpression in laryngeal dysplasia and correlation with clinical outcome. Br J Cancer. 2007;97:1545–1551. doi: 10.1038/sj.bjc.6604070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celetti A, Testa D, Staibano S, Merolla F, Guarino V, Castellone MD, Iovine R, Mansueto G, Somma P, De Rosa G. Overexpression of the cytokine osteopontin identifies aggressive laryngeal squamous cell carcinomas and enhances carcinoma cell proliferation and invasiveness. Clin Cancer Res. 2005;11:8019–8027. doi: 10.1158/1078-0432.CCR-05-0641. [DOI] [PubMed] [Google Scholar]
- 31.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto I, Sasaki Y, Ishida S, Imai K, Tokino T. Identification of the osteopontin gene as a direct target of TP53. Genes Chromosomes Cancer. 2002;33:270–278. doi: 10.1002/gcc.10020. [DOI] [PubMed] [Google Scholar]
- 33.Cristaudo A, Foddis R, Bonotti A, Simonini S, Vivaldi A, Guglielmi G, Ambrosino N, Canessa PA, Chella A, Lucchi M. Comparison between plasma and serum osteopontin levels: usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int J Biol Markers. 2010;25:164–170. doi: 10.1177/172460081002500307. [DOI] [PubMed] [Google Scholar]
- 34.Sreekanthreddy P, Srinivasan H, Kumar DM, Nijaguna MB, Sridevi S, Vrinda M, Arivazhagan A, Balasubramaniam A, Hegde AS, Chandramouli BA. Identification of potential serum biomarkers of glioblastoma: serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol Biomarkers Prev. 2010;19:1409–1422. doi: 10.1158/1055-9965.EPI-09-1077. [DOI] [PubMed] [Google Scholar]
- 35.Van den Haute C, Eggermont K, Nuttin B, Debyser Z, Baekelandt V. Lentiviral vector-mediated delivery of short hairpin RNA results in persistent knockdown of gene expression in mouse brain. Hum Gene Ther. 2003;14:1799–1807. doi: 10.1089/104303403322611809. [DOI] [PubMed] [Google Scholar]
- 36.Lebedev TD, Spirin PV, Prassolov VS. Transfer and Expression of Small Interfering RNAs in Mammalian Cells Using Lentiviral Vectors. Acta Naturae. 2013;5:7–18. [PMC free article] [PubMed] [Google Scholar]


