Abstract
Bone mesenchymal stem cells (BMSCs) are able to differentiate into multi types of lineages, so they have been widely applied in the stem cell transplantation. The BMSCs are usually needed to be expanded before transplantation due to their limited content in bone marrow. It has recently been reported that Icariin (ICA), a major constituent of flavonoids from the Chinese medical herb Epimedium brevicornum Maxim, promotes the proliferation of various types of differentiated cells. However, whether ICA can enhance BMSCs proliferation and the possible underlying mechanisms are still unknown. After being isolated and purified from rat bone marrow, cultured BMSCs are stimulated with different concentrations of ICA. The cytotoxicity of ICA is evaluated by the Cell Counting Kit-8 (CCK-8) assay method and the ICA optimal concentration for BMSCs proliferation is determined at 320 μg/L. Our work reveals that ICA induces an obvious phosphorylation of ERK and p38 kinases in BMSCs, no matter serum exists or not. Inhibition of ERK or p38 MAPK signaling by their specific inhibitors PD98059 or SP600125, respectively, not only prevents the activation of these kinases, but also attenuates cell proliferation induced by ICA. Furthermore, the downstream transcription factors of MAPK pathway, Elk1, Stat3, c-Myc and Fos, are also monitored by RT-PCR, and our results show that among them, Elk1 and c-Myc are significantly upregulated after ICA treatment. Taken together, our results demonstrate that ICA promotes the proliferation of rat BMSCs through activating ERK and p38 MAPK signaling which further leads to upregulation of their downstream transcription factors Elk1 and c-Myc. Our work provides a novel effective way to expand the content of BMSCs in vitro, which casts light on clinical applications of stem cell transplantation in the future.
Keywords: Icariin, bone mesenchymal stem cells, ERK, p38 MAPK
Introduction
In the bone stromal environment, there exist two types of cells, hematopoietic stem cells (HSCs) and bone marrow stromal cells (BMSCs, also known as bone marrow derived mesenchymal stem cells) [1,2]. BMSCs are multipotent progenitor cells and can differentiate into multi-type of cells like the adipocytic, chondrocytic and osteocytic lineages [3]. It is promising to exploit the broad differentiation potential of BMSCs for the therapeutic treatment of human diseases such as cardiac, genetic, hematological, metabolic, and neurologic diseases [4-8]. However, low cell engraftment success and expansion in vitro, and low cell survival after transplantation, impair BMSCs therapeutic efficiency [9]. Therefore, it is necessary to develop a novel way to multiply BMSCs effectively.
Icariin (ICA), a major constituent of flavonoids from the Chinese medical herb Epimedium brevicornum Maxim, has originally been proven to be protective for male reproductive ability, but the underlying mechanism is largely unknown [10]. Recently, ICA has been found to stimulate the proliferation of different type of differentiated cells including Sertoli cells [10], human periodontal ligament cells [11], osteoblasts [12], MC3T3-E1 cells [13] and osteoblast-like UMR 106 cells [14]. Whether ICA can promote proliferation of stem cells or progenitor cells is, however, not investigated yet.
Mitogen-activated protein kinase (MAPK) signal transduction pathway is one of the most frequently involved pathways for eukaryotic cell regulation. In mammalian cells, the MAPK family is consisted of three major members: the extracellular regulated kinase (ERK or p42/p44 MAPK), the p38 kinase and the jun amino-terminal kinases/stress-activated protein kinase (JNK) [12,15]. Mammalian MAPK can be activated by a wide variety of stimuli including hormones (e.g., insulin), growth factors (e.g., PDGF, EGF and FGF), inflammatory cytokines of tumor necrosis factor (TNF) family and environmental stresses (e.g., radiation, osmotic shock and ischemic injury). Pile of evidence supports the notion that MAPK pathway is frequently involved in the proliferation of human cells like uterine leiomyoma cell [16], human breast cancer cells [17] and human umbilical vein endothelial cell [18].
In this study, we demonstrate the crucial role of ICA in stimulating proliferation of BMSCs-a promising type of progenitor cells for clinical transplantation. Given that MAPK pathway is the most frequently involvement contributing to the effects of ICA [19,20], we further demonstrate that ICA promotes proliferation of BMSCs through ERK and p38 MAPK signaling.
Materials and methods
Animals/ethics statement
Healthy Sprague-Dawley (SD) male rats at 80~100 g are provided by the department of medical animal science in Peking University. (Certification number: SCXK (Jing) 2006-0008). All animals are treated in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health.
Isolation and culture of BMSCs
To establish the BMSCs culture, the animals are anesthetized with 10% chloral hydrate through single intraperitoneal injection and killed by cervical dislocation method. Under sterile conditions, both femur and tibiae from each rat are excised. Muscle and the entire connective tissue are detached [21-23]. The ends of the bones are cut away and a 21-gauge needle inserted into shaft of the bone marrow is extruded by flushing with Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone, USA) and100 IU/ml penicillin-100 mg/ml streptomycin (Solarbio, Beijing, China). Marrow plug suspension is dispersed by pipetting, successively filtered through 70-μm mesh nylon filter. Then cell suspension is transferred to flasks and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 72 h. The mesenchymal stem cells are isolated on the basis of their ability to adhere to the culture plates. On the third day, red blood cells and other non-adherent cells are removed and fresh medium is added to allow further growth. The adherent cells growing to 70% confluence are defined as passage zero (P0) cells. For each passage, the cells are plated similarly and allowed to grow to 60-80% confluence. Finally, passage 3 (P3) cells are chosen for subsequent experiments [20,24].
Flow cytometry analysis
P3 BMSCs are subjected to flow cytometry analysis in a FACS Calibur machine from BD Biosciences (USA). Briefly, P3 cells are harvested and suspended in culture medium at a concentration of 1×106 cells/ml. After a brief centrifugation, cells are resuspended in PBS and 500 μl of cell suspension is incubated with FITC-conjugated antibodies for 15 min at 37°C. Immunophenotyping of rat BMSCs is performed with antibodies against antigens CD90, CD44, CD45 and CD31 [21,23,24].
Cell proliferation assay
The influence of ICA on cell proliferation is evaluated by CCK-8 assay. P3 BMSCs are plated in 96-well plate with 5×103 cells per well. 24 h later, the culture medium is changed to complete medium with serial concentrations of ICA (20 μg/L, 40 μg/L, 80 μg/L, 160 μg/L or 320 μg/L). After 72 h treatment, the medium is replaced with CCK-8 solution (Dojindo Molecular Technologies, Japan; 10 μl in 100 μl growth medium). After 4 h treatment of CCK-8 solution, the cells are washed twice with PBS (PH7.2), and then incubated at 37°C for 1 to 4 hours. The OD value (absorbance at 450 nm) is detected by an enzyme linked immunosorbent assay (ELISA) reader (BIO-RAD, USA) [20].
Blockade of ERK, p38 and JNK MAPK signaling
In order to determine the role of ERK, p38 or JNK signaling in mediating the effects of ICA on BMSCs proliferation, the CCK-8 assay is performed in the presence of specific inhibitors of above mentioned kinases. P3 BMSCs are plated at 96-well plate. 24 h later, pathway inhibitors (final concentration: the ERK inhibitor PD98059, 30 μmol/L; the p38 inhibitor SB203580, 20 μmol/L; the JNK inhibitor SP600125, 10 μmol/L) are added to the medium. After one hour treatment, the cells are washed twice with PBS and then incubated in 100 μl growth medium with ICA (320 μg/L) for 48 hours. The absorbance is determined at 450 nm by the CCK-8 assay.
Western blot
After washed twice by cold PBS, cells are harvested and lysed in WIP cell lysis buffer (Beyotime Institute of Biotechnology, China) at 4°C for 1 h. Then cell lysates are centrifuged at 12000×g at 4°C for 15 min to obtain supernatant. Protein concentrations are determined using the Bradford protein assay kit (Solarbio, Beijng City, China) with BSA as a standard. Proteins from the different experimental groups are separated by 12% sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis and transferred to PVDF membranes (MILLIPORE, USA) by electroblotting (300 mA for 1 h). The membranes are blocked in TBS-T (137 mM NaCl, 20 mM Tris (pH 7.6), and 0.1% (v/v) Tween 20) containing 5% (w/v) BSA (Solarbio, Beijng City, China) at room temperature for 1 h. After two times washes with TBS-T, the membranes are then incubated with primary antibodies diluted at 1:1000 in TBS-T at 4°C overnight. After washed three times with TBS-T, the membranes are incubated with appropriate second antibodies HRP-IgG at room temperature for 1 h, then washed three times with TBS-T. At last, immunoreactive bands are visualized with DAB reagent.
Total RNA isolation and RT-PCR
Total RNA is extracted by Trizol Reagent (Invitrogen, USA). cDNA is synthesized using Takara RNA PCR kit (Takara biotechnology) following the manufacturer’s instruction. Subsequently, the DNA amplification is carried out in a DNA thermal cycler (TP3000, Takara), with initial denaturation at 94°C for 1min and each cycle consisting of denaturation at 94°C for 30 s, annealing at 55-65°C for 1 min and extension at 72°C for 1 min. The endogenous housekeeping gene Gapdh is used to evaluate the efficiency of reverse transcription. Elk1, Stat3, c-Myc and Fos, a few of the MAPK downstream genes regulating cell proliferation, are amplified and the sequences of the gene-specific primers used for RT-PCR are listed as follows:
Elk1: F: gtttgtgtcctacccagaggtt, R: gcagggactgtattgtgaaggt; Stat3: F: cacaaaagtcaggttgctggt, R: caatcaaggaggcatcacaat; c-Myc: F: gatgtggtgtctgtggaaaaga, R: gttgttgctgatctgtttcagg; Fos: F: gtccgtctctagtgccaacttt, R: cttattcctttcccttcggatt; Gapdh: F: acagcaacagggtggtggac, R: tttgagggtgcagcgaactt.
Statistical analysis
Statistical analysis is run with SPSS17.0 software. All values are expressed as mean ± SEM. Comparisons among the groups are made using Kruskal-Wallis oneway ANOVA. Comparisons between two groups are made using LSD and S-N-K test.
Results
Isolation and culture of rat BMSCs
BMSCs are isolated from rat marrow aspirates as described previously. BMSCs attaching to the culture flasks sparsely display a fibroblast-like, spindle-shaped morphology during the initial days of incubation. After a week of culture, proliferation starts and the cells gradually grow into small colonies. As growth continues, adjacent colonies interconnect with each other and a monolayer confluence is obtained. In the third passage, BMSCs exhibit large, flattened or fibroblast-like morphology (Figure 1A). BMSCs are characterized by their ability to adhere to plastic and to proliferate. P3 BMSCs are chosen for subsequent experiments, since this passage is nearly homogeneous as reported [25]. To confirm the phenotypic characterization of the isolated BMSCs, the flow cytometry is carried out to detect the membrane antigens CD90, CD44, CD45 and CD31. As shown in Figure 1B and 1C, about 90% of the cell population expresses CD90 and CD44, two common surface markers of rat BMSCs. Meanwhile, less than 1% of the cell population expresses CD45 and CD31, indicating that these cells are not of hematopoietic origin.
Figure 1.
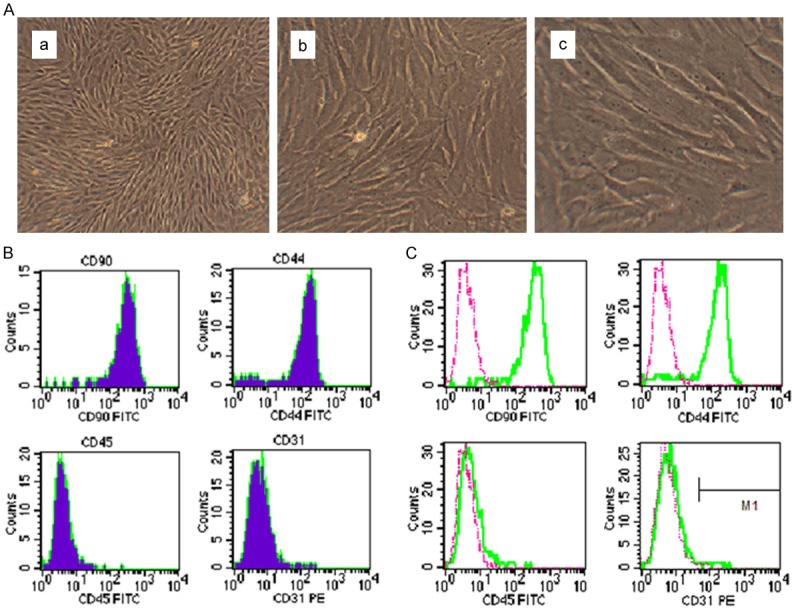
Morphological observation and the immunophenotype of rat P3 BMSCs. (A) The morphology of cultured rat P3 BMSCs. (a) 40×; (b) 200×; (c) 400×. As shown in (B and C), CD90 and CD44 (the stem cell markers) are expressed, but CD45 (the hematopoietic cell marker) and CD31 (the endothelial cell marker) are not expressed in rat P3 BMSCs.
ICA stimulates the proliferation of BMSCs through ERK and p38 MAPK signaling
The CCK-8 assay is performed to evaluate the effects of ICA on BMSCs proliferation. As shown in Figure 2A, the OD value of each group with ICA treatment is significantly higher than that of the group without ICA treatment (the control) (P<0.01). Further observation demonstrates that the OD value is gradually going up with the increase of ICA concentration. Among five concentration groups (20 µg/L, 40 µg/L, 80 µg/L group 160 µg/L and 320 µg/L), 320 µg/L of ICA has the best effect on the proliferation of rat BMSCs (Figure 2A). As more than 320 µg/L of ICA has side effect on cell growth (data not shown), so this concentration is chosen for subsequent experiments.
Figure 2.
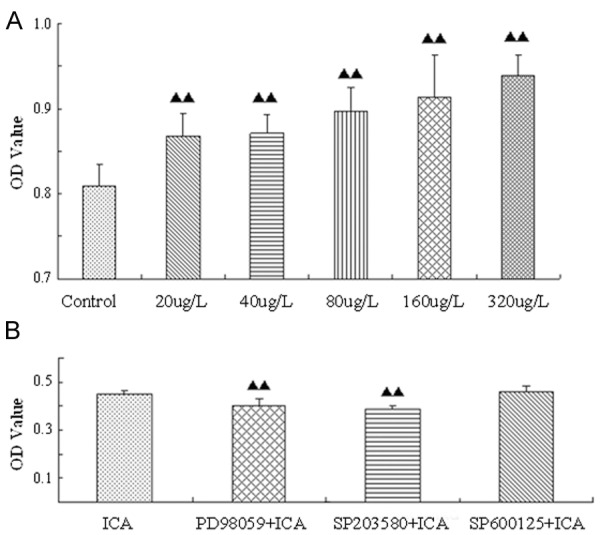
ICA promotes BMSCs proliferation through ERK and p38 MAPK signaling. A: The relative numbers of survival BMSCs treated with different concentrations of ICA for 48 h, as measured by the CCK-8 assay. The OD values represent units of absorbance at 450 nm per well. B: BMSCs are pretreated with ERK-specific inhibitor PD98059, p38-specific inhibitor SB203580, or JNK-specific inhibitor SP600125. One hour later, 320 μg/L of ICA is administrated. Then cells are subjected to the CCK-8 assay. ▲▲P<0.01 when compared to the control group.
MAPKs are frequently involved in the regulation of mammalian cells. To evaluate their potential roles in influencing on the proliferation of BMSCs induced by ICA, the specific inhibitors of MAPKs (PD98059 for ERK1/2, SB203580 for p38 and SP600125 for JNK, respectively) [26,27] are administrated followed by ICA treatment. The results of CCK-8 assays show that the OD value of the PD98059 + ICA group or the SB203580 + ICA group is obviously lower than that of the ICA only group (P<0.01); the OD value of the SP600125 + ICA group is slightly higher than the ICA only group ,but there is no statistical significance (P>0.05) (Figure 2B). These findings indicate that ICA-induced proliferation of BMSCs is mediated by ERK and p38 rather than by JNK signaling.
ICA activates ERK and p38 kinases in rat BMSCs
To evaluate whether ICA induces activation of ERK and p38 MAPK pathways, the levels and ratios of phosphorylated and total MAPKs are determined by SDS-PAGE/Western blotting. When BMSCs are cultured without serum (Figure 3D-F), the influence of the original serum on MAPK pathway is excluded. Activation of ERK and p38 kinases determined by the ratios of their phosphorylated/total proteins, respectively, reaches at the highest level as early as 5 min and lasts up to 30 min after ICA treatment. When BMSCs are cultured with serum (Figure 3A-C), there exists influence of the original serum on MAPK activation. Under this condition, the time of ICA-induced phosphorylation of ERK and p38 significantly prolonged. During our observation, the phosphorylated/total protein ratios of both ERK and p38 go up from 15 min from the beginning and reach maximum 24 h after ICA treatment (Figure 3A-C). Meanwhile our experiments also demonstrate that ICA has no influence on activation of JNKs (data not shown).
Figure 3.
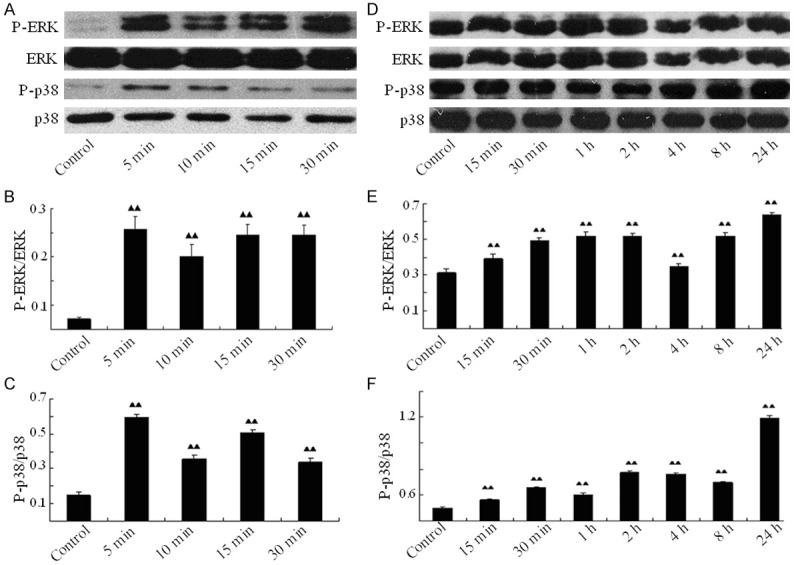
ICA stimulates phosphorylation of ERK and p38 kinases in rat BMSCs. When cultured without serum, BMSCs are treated with ICA up to 30 min, then harvested at every indicated time point. Total and phosphorylated ERK and p38 proteins are analyzed by Western blotting (A) and the ratio of phosphorylated ERK to total ERK (B) or of phosphorylated p38 to total p38 (C) is calculated; when cultured with serum, BMSCs are treated with ICA up to 24 h, then harvested at every indicated time point. Total and phosphorylated ERK and p38 proteins are analyzed by Western blotting (D) and the ratio of phosphorylated ERK to total ERK (E) or of phosphorylated p38 to total p38 (F) is also calculated). ▲▲P<0.01 when compared to the control.
The specific inhibitors of ERK or p38 are administrated to further observe whether they can block or attenuate the activation of these sub-pathways induced by ICA. When BMSCs are cultured without serum, the P-ERK/ERK ratio of the PD98059 + ICA group is obviously lower than that of the ICA group (P<0.01) and the P-p38/p-38 ratio of the SB203580 + ICA group is also lower than that of the ICA group (P<0.01) (Figure 4). These results show that the inhibition of ERK or p38 proteins significantly reduces their phosphorylation induced by ICA, further demonstrating that ICA activates ERK and p38 MAPK signaling in BMSCs.
Figure 4.
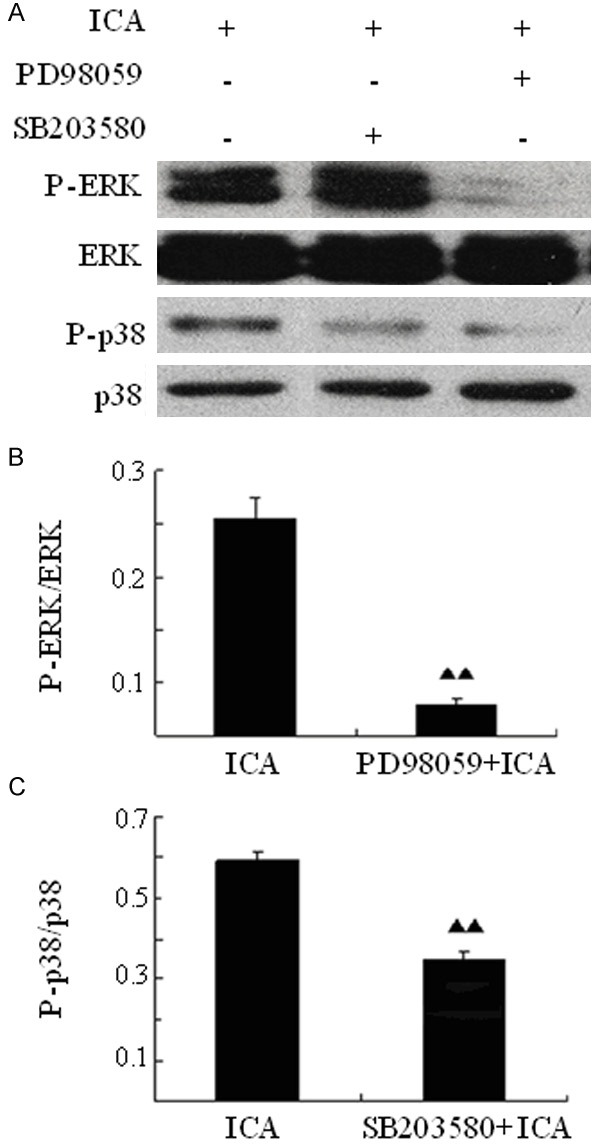
ICA-induced activation of ERK and p38 is attenuated by inhibitors of the MAPK pathway. BMSCs are pre-treated with PD98059 (ERK specific inhibitor) or SP203580 (p38 specific inhibitor) for 1 hour and then cultured in the presence of ICA for 48 h. Cells are harvested for Western blotting analysis (A), then the ratio of the phosphorylated ERK to total ERK (B) or of the phosphorylated p38 to total p38 (C) is calculated. ▲▲P<0.01 compared to the control (ICA treatment only).
ICA upregulates the expression of MAPK downstream transcription factors Elk1 and c-Myc
To further dissect the mechanisms of ICA-induced proliferation of rat BMSCs, the expression levels of a few of MAPK downstream transcription factors (Elk1, Stat3, c-Myc and Fos) frequently involved in cell proliferation are detected by RT-PCR. After ICA treatment, our results show that the mRNA levels of both Elk1 and c-Myc are significantly higher than those of the control group (P<0.05), while there are no dramatically changes for the expression of Stat3 (P=0.273) and Fos (P=0.693) compared to the control (Figure 5). Taken together, these findings indicate that upregulation of MAPK targets Elk1 and c-Myc induced by ICA might promote the proliferation of rat BMSCs.
Figure 5.
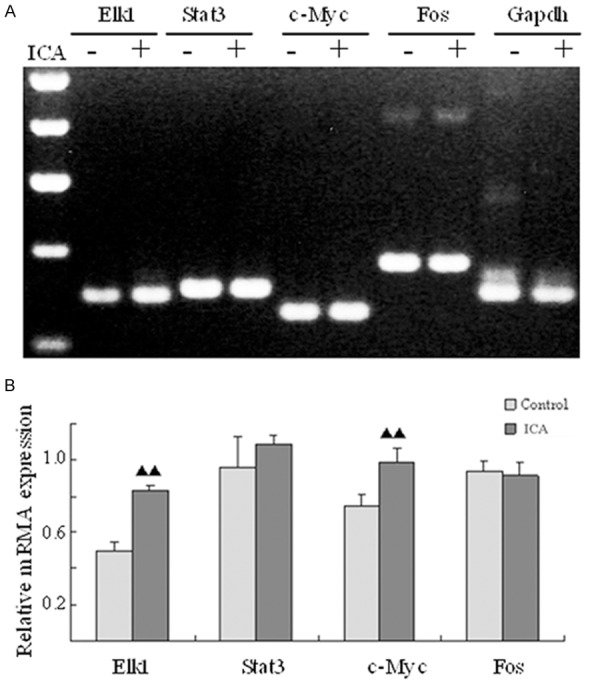
Up-regulation of the MAPK downstream transcription factors Elk1 and c-Myc in ICA-treated BMSCs. A: RT-PCR: the mRNA expression of Elk1, Stat3, c-Myc and Fos. B: The relative mRNA expression of Elk-1, Stat3, c-Myc and Fos.
Discussion
In this study, we have demonstrated that ICA stimulates the proliferation of rat BMSCs in vitro. Meanwhile, our results clearly show that the activity of ICA is positively associated with the phosphorylation levels of ERK and p38 kinases. The specific inhibitors of ERK or p38, not only block the activation of these proteins induced by ICA, but also significantly attenuates the proliferation of rat BMSCs stimulated by ICA. Taken together, these results suggest that ICA possesses potent proliferative effect through the activation of ERK and p38 signaling.
BMSCs within the bone marrow microenvironment are a population of pluripotent cells with the ability to differentiate into cells of the osteogenic, chondrogenic, tendonogenic, adipogenic, and myogenic lineages [2]. Myocardial regeneration of tissue engineering has become a major cutting-edge issue in current medical field. The works of Polychronis [28] and Makino [29] show that 5-azacytidine (5-aza) induce BMSCs differentiate into cardiomyocyte-like cells, which throws light on the clinical applications of BMSCs in myocardial regeneration. However, the percentage of BMSCs is estimated at about only 0.001%-0.01% in the bone marrow [3]. Thus, it is very necessary and extremely urgent to explore more effective and lower side effect compounds for stimulating the proliferation of BMSCs.
It has been reported that ICA promotes the synthesis of DNA of bone marrow and promotes MSCs differentiate into cardiomyocytes with 5-aza through increasing the expression of zinc finger transcription factor (GATA-4) [28]. However, mechanisms of proliferation and differentiation of BMSCs has not been fully elucidated. MAPKs, serine/threonine protein kinases, are consisted of three sub-pathways including ERK, p38 and JNKs. They play important roles in the regulation of cell growth, proliferation, differentiation, death and other activities [30]. So far, there have been a few publications reporting MAPKs are related to the effects of ICA [31,32], indicating that they might be involved in the proliferation of BMSCs induced by ICA. Our work discloses that ERK’s specific inhibitor PD98059 and p38’s specific inhibitor SP600125 significantly inhibit MAPK activation and BMSCs proliferation stimulated by ICA, clearly suggesting that the activation of ERK or p38 MAPK signaling is crucial for the proliferation of BMSCs. Further supporting evidence comes from the results showing that MAPK downstream transcription factors Elk1 and c-Myc frequently involved cell proliferation are significantly up-regulated after ICA treatment. Meanwhile, the JNK sub-pathway is found probably not to mediate the cells’ response to stimuli of ICA, based on the facts that ICA cannot activate JNKs and that the specific inhibitor of JNKs cannot block the effect of ICA.
In summary, our study investigates the effects of ICA in promoting the proliferation of rat BMSCs, and we find it is associated with phosphorylation of ERK and p38, and with the upregulation of MAPK targets Elk-1 and c-Myc. As our knowledge, it is the first time for us to demonstrate that ICA promotes the proliferation of BMSCs via ERK and p38 MAPK signaling. Although these findings need to be further confirmed in vivo, our data here suggest that ICA should be considered as a potential therapeutic drug in regeneration medicine.
Acknowledgements
This work is supported by a grant from the Beijing Key Disciplines Project of Traditional Chinese Medicine (No. JZZ-312) to Dr Hongjin Wu.
Disclosure of conflict of interest
None.
References
- 1.Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003;34:565–571. doi: 10.1016/j.arcmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Majumdar M, Thiede M, Mosca J, Moorman M, Gerson S. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger M, Mackay A, Beck S, Jaiswal R, Douglas R, Mosca J, Moorman M, Simonetti D, Craig S, Marshak D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz E, Prockop D, Fitzpatrick L, Koo W, Gordon P, Neel M, Sussman M, Orchard P, Marx J, Pyeritz R, Brenner M. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 8.Strauer BE. Repair of Infarcted Myocardium by Autologous Intracoronary Mononuclear Bone Marrow Cell Transplantation in Humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 9.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Nan Y, Zhang X, Yang G, Xie J, Lu Z, Wang W, Ni X, Cao X, Ma J, Wang Z. Icariin stimulates the proliferation of rat Sertoli cells in an ERK1/2-dependent manner in vitro. Andrologia. 2012 doi: 10.1111/and.12035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Zhang F. Effect of icariin on cell proliferation and the expression of bone resorption/formation-related markers in human periodontal ligament cells. Mol Med Rep. 2013;8:1499–504. doi: 10.3892/mmr.2013.1696. [DOI] [PubMed] [Google Scholar]
- 12.Song L, Zhao J, Zhang X, Li H, Zhou Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur J Pharmacol. 2013;714:15–22. doi: 10.1016/j.ejphar.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Cao H, Ke Y, Zhang Y, Zhang CJ, Qian W, Zhang GL. Icariin stimulates MC3T3-E1 cell proliferation and differentiation through up-regulation of bone morphogenetic protein-2. Int J Mol Med. 2012;29:435–439. doi: 10.3892/ijmm.2011.845. [DOI] [PubMed] [Google Scholar]
- 14.Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine. 2005;12:189–193. doi: 10.1016/j.phymed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65:3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Yu L, Moore AB, Kissling GE, Waalkes MP, Dixon D. Cadmium and Proliferation in Human Uterine Leiomyoma Cells: Evidence of a Role for EGFR/MAPK Pathways but Not Classical Estrogen Receptor Pathways. Environ Health Perspect. 2014;123:331–6. doi: 10.1289/ehp.1408234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Xie N, Yang J, Guan H, Chen W, Wu H, Yuan Z, Wang K, Li G, Sun J, Yu L. SiRNA-mediated suppression of synuclein gamma inhibits MDA-MB-231 cell migration and proliferation by downregulating the phosphorylation of AKT and ERK. J Breast Cancer. 2014;17:200–206. doi: 10.4048/jbc.2014.17.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song H, Wu F, Zhang Y, Zhang Y, Wang F, Jiang M, Wang Z, Zhang M, Li S, Yang L, Wang XL, Cui T, Tang D. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS One. 2014;9:e110273. doi: 10.1371/journal.pone.0110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Wang H, Liang L, Zhao WC, Chen Y, Deng HZ. Total alkaloids of Sophora alopecuroides increases the expression of CD4+ CD25+ Tregs and IL-10 in rats with experimental colitis. Am J Chin Med. 2010;38:265–277. doi: 10.1142/S0192415X1000783X. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Wang NL, Cai GP. Maohuoside A promotes osteogenesis of rat mesenchymal stem cells via BMP and MAPK signaling pathways. Mol Cell Biochem. 2011;358:37–44. doi: 10.1007/s11010-011-0918-y. [DOI] [PubMed] [Google Scholar]
- 21.Karaoz E, Aksoy A, Ayhan S, Sariboyaci AE, Kaymaz F, Kasap M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol. 2009;132:533–546. doi: 10.1007/s00418-009-0629-6. [DOI] [PubMed] [Google Scholar]
- 22.Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid Signaling Defines a Novel Commitment State during Adipogenesis In Vitro. Mol Biol Cell. 2008;19:4032–4041. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayed M, Drummond CA, Evans KL, Haller ST, Liu J, Xie Z, Tian J. Effects of Na/K-ATPase and its ligands on bone marrow stromal cell differentiation. Stem Cell Res. 2014;13:12–23. doi: 10.1016/j.scr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin MS, Shi S, Zhang Y, Yan Y, Sun XD, Liu W, Liu HW. Icariin-mediated differentiation of mouse adipose-derived stem cells into cardiomyocytes. Mol Cell Biochem. 2010;344:1–9. doi: 10.1007/s11010-010-0523-5. [DOI] [PubMed] [Google Scholar]
- 25.Harting M, Jimenez F, Pati S, Baumgartner J, Cox C Jr. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10:243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- 26.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 27.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 28.Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007;6:593–597. doi: 10.1510/icvts.2007.157875. [DOI] [PubMed] [Google Scholar]
- 29.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsden M, Henderson Z, Pearson H. Modulation of Ca channel currents in primary cultures of rat cortical neurones by amyloid b protein (1-40) is dependent on solubility status. Brain Res. 2002;956:254–261. doi: 10.1016/s0006-8993(02)03547-3. [DOI] [PubMed] [Google Scholar]
- 31.Ding L, Liang X, Zhu D, Lou Y. Icariin promotes expression of PGC-1α, PPARα, and NRF-1 during cardiomyocyte differentiation of murine embryonic stem cells in vitro. Acta Pharmacol Sin. 2007;28:1541–1549. doi: 10.1111/j.1745-7254.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Zhang L, Chen ZB, Wu JY, Zhang X, Xu Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur J Pharmacol. 2009;609:40–44. doi: 10.1016/j.ejphar.2009.03.033. [DOI] [PubMed] [Google Scholar]


