Abstract
Neuropathic pain is caused by lesion or inflammation of the nervous system and characterized by the symptoms of allodynia, hyperalgesia and spontaneous pain. SIRT1 (Sir2) is a NAD-dependent deacetylase and is reported to regulate a wide variety of cellular processes including inflammation, aging and lifespan extension. Nevertheless, the role of SIRT1 in neuropathic pain is not fully understood. The present study was intended to detect the effect of intrathecal SRT1720, a SIRT1 agonist, using quantitative real-time PCR and western blot analysis over time in rats following chronic constriction injury (CCI) or sham surgery. In addition, the effect of intrathecal injection of SRT1720 on thermal hyperalgesia and mechanical allodynia was evaluated in CCI rats. It was found that daily intrathecal injection of SRT1720 before and 1, 3, 5, 7 days after CCI surgery produced a transient inhibitory effect on thermal hyperalgesia and mechanical allodynia in CCI rats. In addition, an intrathecal injection of STR1-siRNA before SRT1720 administration reversed the anti-nociceptive effect of SRT1720. Furthermore, intrathecal injection of SRT1720 significantly down-regulated the expression of mammalian target of rapamycin (mROT), NF-κB and inflammatory cytokines, such as IL-6, TNF-α and iNOS mRNA. These data indicate that intrathecal SRT1720 may be an alternative strategy for the treatment of neuropathic pain. Our findings suggest that intrathecal SRT1720, a SIRT1 agonist, exerts antihyperalgesic and antiinflammatory effects on CCI-induced neuropathic pain in rats.
Keywords: SRT1720, chronic constriction injury, neuropathic pain, antihyperalgesic, antiinflammatory, SIRT1
Introduction
Neuropathic pain, resulted from lesion or inflammation of the nervous system, is characterized by the symptoms of allodynia, hyperalgesia and spontaneous pain [1,2]. Accumulating evidence demonstrated that a great proportion of mediators involved in the symptoms of neuropathic pain, including cytokines, bradykinin, ATP and adenosine, serotonin, eicosanoids, neurotrophins and so on [1]. However, because of the poorly understanding of the underlying mechanisms in neuropathic pain, it is often severely enervating and largely resistant to treatment.
SIRT1 (Sir2), as a member of the silent information regulator family, is a NAD-dependent deacetylase and is reported to regulate a wide variety of cellular processes including inflammation, aging and lifespan extension [3-5]. Through histones deacetylation and transcription factors, SIRT1 modifies several gene expression, including nuclear factor-κB (NF-κB), p53, FOXOs and hypoxia-inducible factor-1α (HIF-1α) [6,7]. Thus, SIRT1 plays a pivotal role in regulating the inflammatory, immune, and apoptotic responses in mammals. Using a myeloid cell-specific SIRT1 knockout mouse model, some researches showed that ablation of SIRT1 in macrophages rendered NF-κB hyper-acetylated, resulting in increased transcriptional activation of pro-inflammatory target genes [8]. Presently, SIRT1 is gaining increasing importance in the development of innovative treatment strategies for cancer, neurodegenerative disorders and metabolic disease [9,10]. Nevertheless, the role of SIRT1 in neuropathic pain is not fully understood.
Recently, SRT1720 has been reported to be a small molecule activator of SIRT1 and exhibited 1,000-fold potencies than resveratrol. The administration of SRT1720 to monosodium glutamate (MSG) mice significantly ameliorated liver lipid accumulation by directly reducing the expressions of lipogenic genes [11]. Furthermore, recent studies have demonstrated the efficacy of SRT1720 on inflammation, tumor metastasis, and amelioration of fatty liver [12]. However, the mechanisms responsible for the effects of SRT1720 on neuropathic pain have not been fully elucidated.
In this study, we hypothesized that the activation of SIRT1 by SRT1720 may ameliorate neuropathic pain via the antihyperalgesic and antiinflammatory effects. Thus we investigated the effects of SRT1720 treatment on Chronic Constriction Injury (CCI) -induced mechanical allodynia and thermal hyperalgesia in Sprague-Dawley rats. The alteration of cytokines or related factors accounting for SRT1720 injection were also examined in rats. Here, we provide evidence that the treatment with SRT1720 ameliorates the CCI-induced neuropathic pain with antihyperalgesic and antiinflammatory effects.
Materials and methods
Animals
The procedures of animal experiments were performed by Guide for the Care and Use of Laboratory Animals and were approved by Zhejiang Chinese Medical University. All surgical procedures were performed under general anaesthesia, and every effort was made to minimize stress. Specific pathogen free (SPF) male Sprague-Dawley rats weighing 200-220 g were purchased from Animal Experimental Center, Zhejiang Chinese Medical University, China. Animals were housed in standard cage in a temperature-controlled (22±2°C) colony room under a 12 h light/dark cycle regime, with food and water available.
Implantation of intrathecal catheter
For intrathecal drug administration, rats were chronically implanted with catheters as previously described [13]. Briefly, intrathecal polyethylene catheter (PE-10, Becton Dickinson and Company, Sparks, MD, U.S.A.) was inserted caudally from the cistern magna and advanced 7.0-7.5 cm to the level of lumbar enlargement. Proper location was confirmed by a temporary motor block of both hind limbs after injection of 10 μL 2% lidocaine. Only animals with no evidence of neurologic deficit after the operation were studied. All experiments were conducted one week after implantation of intrathecal catheter. For intrathecal administration, drugs were given by using a microinjection syringe over a 60 s interval in a volume of 10 μL, followed by a flush with 10 μL physiological saline. Injections were given daily from day 0 to day 5 after CCI surgery.
Induction of chronic constriction injury
CCI surgery was performed according to methods described previously [14]. Rats were anesthetized with 4% pentobarbital sodium, and a 7 mm segment of the right common sciatic nerve was exposed at the mid-thigh level. Four ligatures (4.0 chromic catgut) threaded at four sites with approximately 1 mm intervals were loosely tied around the nerve proximally to the sciatic trifurcation, while the same procedure in the sham-operated control group was performed by tying the left sciatic nerve without ligation. After suturing the muscle and skin, the major manifestations of neuropathic pain, such as mechanical allodynia and thermal hyperalgesia, would fully develop 14 days after surgery.
Mechanical allodynia
Rats were assessed for behaviors with 1 h before drug administration (baseline) and at 1, 3, 5 and 7 days after drug injection after CCI. Mechanical allodynia was assessed using an automated testing device (dynamic plantar anesthesiometer, UgoBasile, Italy) as described previously [15]. Rats were placed on a metal mesh floor and the mechanical stimulus was delivered to the plantar surface of the right hind paw from below the floor of the test chamber. A steel rod, 0.5 mm in diameter, was pushed against the hind paw with increasing pressure, which increased lineally from 0 to 50 g over a 20 s period. When the animal removed its paw, the pressure threshold of paw withdrawal (PWT) was recorded automatically. The right hind paw was tested at 30 s intervals and three responses were averaged to represent PWT.
Thermal hyperalgesia
A radiant heat paw withdrawal test was used to examine thermal hypersensitivity as described previously [16]. Briefly, each rat was placed on a glass plate (Ugo Basile, Italy) with radiant heat equipment underneath. After a 30 min acclimation period, the radiant heat source under the glass floor was positioned directly under the right hind paw. The controller detected latency to the paw withdrawal (PWL) response when the animal withdrew its hind paw. The intensity of the heat stimulus was set to 30% of the maximum output power. The latency of paw withdrawal after heat stimulation was measured three times at 5 min intervals, and its mean value was used as the latency of the response. The cut-off of heat application was automatically set at 20 s to avoid tissue damage.
Intrathecal delivery of siRNA
SiRNAs were stored in aliquots at -80°C. For intrathecal treatment, the stock siRNAs (2.5 μg of the appropriate siRNA) prepared in double distilled RNase free water were mixed (1:5 v/v) with PEI reagent (Fermentas Inc., Glen Burnie, MD, USA). The siRNA was mixed with PEI (dissolved in 5% glucose) for 10 min before injection to increase cell membrane penetration and reduce degradation. After recovery from the surgery (7 days), the animals received intrathecal injections (2.5 μg siRNA/10 μl/rat) of either SIRT1-selective siRNA mixture (SIRT1 siRNA group) or i-Fect non-targeting dsRNA (control mismatch siRNA group). Intrathecal injections of the siRNAs or the transfection agent alone did not cause any sign of behavioral toxicity.
Western blot analysis
Western blot analysis was performed to confirm the proteins level of NF-κB/Ac-NF-κB and mTOR in the spinal cord. Nuclear extracts were prepared from ipsilateral lumbar spinal cord tissues as previously described [17]. Briefly, proteins were separated on an 8% polyacrylamide SDS-PAGE gel and transferred onto a nitrocellulose membrane. The nitrocellulose membrane was blotted with a primary antibody and GAPDH (mouse monoclonal antibody, Cell Signaling Technology, USA) followed by a secondary antibody conjugated with horseradish peroxidase. Protein signals were detected with an ECL system (Amersham Pharmacia Biotech, Uppsala, Sweden).
Quantitative real-time PCR
Total RNA was extracted from ipsilateral lumbar spinal cord tissues. Extracted RNA was pretreated with DNase I at 37°C for 30 minutes before reverse transcription reaction, which was performed using a high capacity cDNA archived kit (TaKaRa, Japan). A Real-Time PCR Detection System (Roche, Switzerland) was used to continuously monitor the intensity of fluorescence, which was directly proportional to the PCR products.
Statistical analysis
Statistical analysis was performed in SPSS 16.0 (SPSS, Chicago, IL). All data were expressed as mean 6 standard error of mean (SEM). Data from the immunohistochemical analysis, Western blots, and real-time PCR studies were analyzed using a one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s testing. Data from the nociceptive tests were analyzed using ANOVA followed by Bonferroni testing for tactile allodynia testing. P<0.05 was considered significant. The individual investigators responsible for behavioral testing and quantification were blinded to the experimental conditions.
Results
SRT1720 attenuates CCI-induced mechanical allodynia and thermal hyperalgesia
To detect whether SRT1720 could attenuate CCI-induced neuropathic pain, mechanical allodynia and thermal hyperalgesia were examined in Sprague-Dawley rats, which were sacrificed before and at 1, 3, 5, 7 days after CCI or sham surgery. It was found that rats in CCI group developed an evident mechanical allodynia and thermal hyperalgesia in the spinal cord, as compared with sham group (Figure 1). Furthermore, this pain reached the peak at 5 days after CCI surgery. This finding demonstrated the occurrence of neuropathic pain induced by CCI in rats. In comparison to CCI group, mechanical allodynia and thermal hyperalgesia were obviously alleviated in rats intrathecal administrated STR1720 before CCI surgery (P<0.05) (Figure 1). Conversely, sham-operated group had no significant modification of nociceptive threshold. Taken together, these results supposed that intrathecal administration of STR1720 could attenuate CCI-induced mechanical allodynia and thermal hyperalgesia in rats.
Figure 1.

STR1720 attenuates the development of neuropathic pain in rats. An intrathecal administration of 20 mg/kg STR1720 before CCI surgery into rats. The development of mechanical allodynia (A) and thermal hyperalgesia (B) induced by CCI surgery. n=10 in each group for behavior test; Sham operation group, the control group; CCI group, rats experienced CCI surgery; CCI + STR1720 group, rats treated with SRT1720 before CCI surgery. Data are means ± SD in ech group; *P<0.05 vs sham operaton group; #P<0.05 vs CCI group.
SIRT1-siRNA reverses the effects of STR1720 on CCI-induced mechanical allodynia and thermal hyperalgesia
As SRT1720 was known to be a potent and specific activator of SIRT1, we further investigated the analgesic effects of STR1720 through administration SIRT1-siRNA into rats. As illustrated in Figure 2, pretreatment of SIRT1-selective siRNA mixture (2.5 μg/rat, once daily, 4 days) in rats, which administrated STR1720 before CCI surgery, evoked the development of mechanical allodynia and thermal hyperalgesia, in spite it delayed the onset of mechanical allodynia. This finding indicated that SIRT1-siRNA significantly reversed the antihyperalgesic and antiallodynic activity of SRT1720 treatment (P<0.05).
Figure 2.

SIRT1-siRNA reverses the development of neuropathic pain in rats induced by STR1720. An intrathecal administration of si-SIRT1 before CCI surgery into rats. The development of mechanical allodynia (A) and thermal hyperalgesia (B) induced by CCI surgery. n=10 in each group for behavior test; STR1720 + si-control, rats administrated STR1720 and si-control before CCI surgery; STR1720 + si-SIRT1, rats administrated STR1720 and si-SIRT1 before CCI surgery. Data are means ± SD in ech group; *P<0.05 vs STR1720 + si-control.
SRT1720 decreases the expression of CCI-induced mammalian target of rapamycin (mTOR)
Ilona Obara suggested that systemic inhibition of the mTOR pathway reduced neuropathic pain in mice and might be as a potential target for therapeutic intervention, particularly in chronic pain [18]. To investigate the alteration of mTOR effected by SRT1720, western blot was performed and the result was showed in Figure 3. Clearly, up-regulation of mTOR protein level was detected in CCI group compared with sham-operated. Additionally, the protein level of mTOR was lower in CCI + SRT1720 group than in CCI group (Figure 3). These results supported that SRT1720 down-regulated the mTOR protein levels in rats experienced CCI surgery.
Figure 3.
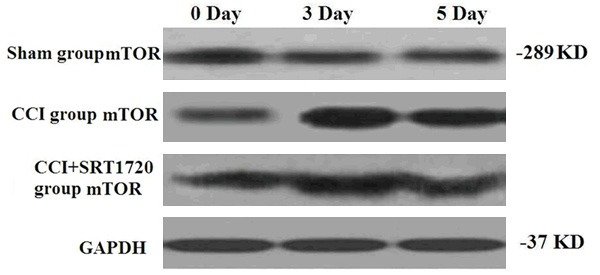
The effects of SRT1720 on CCI-induced mTOR protein levels. Sham operation group, the control group; CCI group, rats experienced CCI surgery; CCI + STR1720 group, CCI group rats treated with SRT1720.
SRT1720 decreases the expression of CCI-induced NF-κB
One of the non-histone cellular substrates of SIRT1 is the transcription factor NF-κB and activation of NF-κB has been detected in dorsal root ganglia in animal models of neuropathic pain [19,20]. As shown in Figure 4, NF-κB and acetylated-NF-κB (Ac-NF-κB) protein levels were increased in rats of CCI group compared with sham-operated group. Moreover, SRT1720 treatment led to decreased NF-κB and NF-κB acetylation in CCI rats.
Figure 4.

The effects of SRT1720 on CCI-induced NF-κB protein levels. Sham operation group, the control group; CCI group, rats experienced CCI surgery; CCI + STR1720 group, CCI group rats treated with SRT1720.
SRT1720 inhibited CCI-induced IL-6, TNF-α, and iNOS activation
Accumulating reports revealed that SRT1720 could decrease the expression of genes involved in inflammatory cytokines. Therefore, RT-PCR was performed to detect the expression of IL-6, TNF-α and iNOS mRNA. As displayed in Figure 5, a significant up-regulation of IL-6, TNF-α and iNOS mRNA expression was found in CCI group compared to the sham group. While, IL-6, TNF-α and iNOS mRNA levels were significantly lower in SRT1720 treated rats than in CCI group rats (P<0.05) (Figure 5).
Figure 5.

The effects of SRT1720 on CCI-induced IL-6, TNF-α, and iNOS mRNA levels. Sham group, the control group; CCI group, rats experienced CCI surgery; CCI + STR1720 group, CCI group rats treated with SRT1720. Data are means ± SD n each group; *P<0.05 vs sham opration group; #P<0.05 vs CCI group.
Discussion
Numerals studies reported that a variety of alterations in pain-related gene expression and modification attributed to symptoms of neuropathic pain. Abnormal histone acetylation, as one of the characteristic alterations in gene modification, is believed to be one of the transcription factor-mediated epigenetic mechanisms underlying neuropathic pain. SIRT1 is a NAD-dependent deacetylase and has been reported to participate in several physiological phenomena, such as longevity, diabetes mellitus, metabolism, cardiac function, neurodegeneration, memory and plasticity, inflammation, circadian rhythms, and cancer. Moreover, SIRT1 is believed to act as an alleviator of systemic, tissue, or cellular dysfunction. Correspondingly, the present results support the ameliorating effects of SIRT1 activators.
In this study, we examined the involvement of SIRT1 in CCI-induced neuropathic pain by using a specific activator, SRT1720. We found that the development of CCI-induced mechanical allodynia and thermal hyperalgesia could be ameliorated through pretreatment of intrathecal SRT1720 in rats. While, this analgesic effect could be reversed by SIRT1-siRNA. Takeshi Yoshizaki et al suggested that SIRT1 served as a negative regulator of inflammatory pathway activation and a positive regulator of insulin signaling in adipocytes [20]. However, Shingo Imanishi’s report indicated that an excess of food containing SIRT1 activators, such as SRT1720, have potentially harmful effects depending on the disease or organ, especially in the case of acute inflammation [21]. Taken together, the findings in our study indicated that as a SIRT1 agonist, SRT1720 could ameliorate CCI-induced neuropathic pain in rats.
mTOR played an established role in controlling local protein synthesis in axons and in the moderation of memory processes [22,23]. Lately, local cutaneous and intrathecal administration of rapamycin, an mTORC1 inhibitor, has been reported to alleviate the increased mechanical hypersensitivity associated with local inflammation or in a model of neuropathic pain [24]. In this regard, we further explored the effect of SRT1720 on mTOR pathway with western blot. The results showed that mTOR could be activated by CCI surgery and SRT1720 down-regulated the mTOR protein levels in rats experienced CCI surgery, which indicated that SRT1720 also alleviated the mechanical hypersensitivity in CCI model of neuropathic pain.
Based on the established role of macrophages and inflammation in causing neuropathic pain, the role of SRT1720 in regulating inflammatory pathways is of importance. In consideration of this respect, we found that SRT1720 also inhibited the activity of CCI-induced NF-κB. For inflammatory cytokines, IL-6, TNF-α and iNOS mRNA levels were also significantly lower in SRT1720 treated rats than in CCI group rats. These data supported that SRT1720 indeed exerted antiinflammatory effect in neuropathic pain.
Sommer et al reported that neuropathic pain was alleviated in nerve-injured mice through administration of neutralizing antibodies to the IL-1 receptor [25]. In addition, intraneural injection of TNF into rat induced thermal mechanical and allodynia hyperalgesia [26]. Nitric oxide was a diffusible free radical that was synthesized by inducible nitric oxide synthases (iNOS), and nitric oxide was also closely related to central mechanisms of hyperalgesia [27]. Therefore, in present study, we could see SRT1720 may exert antiinflammatory effect on neuropathic pain through down-regulating these inflammatory cytokines.
In conclusion, our results have provided some evidence that the development of neuropathic pain can be ameliorated by the administration of SRT1720, a newly synthesized SIRT1 activator, through exerting antihyperalgesic and antiinflammatory effects. This may be a novel mechanism underlying neuropathic pain. It may be possible that the manipulation of SIRT1 activity by SRT1720 in epidural anaesthesia combined with local anesthetics could provide new therapeutic opportunities for the treatment of neuropathic pain induced by nerve injuries associated with some surgical procedures such as amputation or thoracic operation.
Acknowledgements
This research was supported by grant from the Natural Science Foundation of China (81202823) and Zhejiang province health department Foundation (2014KYA156).
Disclosure of conflict of interest
None.
References
- 1.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Warfield CA, Fausett HJ. Manual of Pain Management. Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 3.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641–1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 5.Haigis MC, Guarente LP. Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Charron CE, Adcock IM. Impact of protein acetylation in inflammatory lung diseases. Pharmacol Ther. 2007;116:249–265. doi: 10.1016/j.pharmthera.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB -dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, Kouttab N, Xu A, Wan Y. SIRT1 confers protection against UVB-and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009;13:3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285:13045–13056. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S, Hirata K, Ishiki M, Hayashi R, Urakaze M, Nemoto H, Kobayashi M, Tobe K. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. A J Physiol Endocrinol Met. 2009;297:1179–1186. doi: 10.1152/ajpendo.90997.2008. [DOI] [PubMed] [Google Scholar]
- 12.Matsuya Y, Kobayashi Y, Uchida S, Itoh Y, Sawada H, Suzuki T, Miyata N, Sugimoto K, Toyooka N. Search for a novel SIRT1 activator: Structural modification of SRT1720 and biological evaluation. Bioorga Med Chem Lett. 2013;23:4907–4910. doi: 10.1016/j.bmcl.2013.06.070. [DOI] [PubMed] [Google Scholar]
- 13.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 14.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 15.Muthuraman A, Jaggi AS, Singh N, Singh D. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol. 2008;587:104–111. doi: 10.1016/j.ejphar.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 17.Tegeder I, Niederberger E, Schmidt R, Kunz S, Gühring H, Ritzeler O, Michaelis M, Geisslinger G. Specific inhibition of IκB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci. 2004;24:1637–1645. doi: 10.1523/JNEUROSCI.3118-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obara I, Tochiki KK, Géranton SM, Carr FB, Lumb BM, Liu Q, Hunt SP. Systemic inhibition of the mammalian target of rapamycin (mTOR) pathway reduces neuropathic pain in mice. Pain. 2011;152:2582–2595. doi: 10.1016/j.pain.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Xie W, Liu X, Xuan H, Luo S, Zhao X, Zhou Z, Xu J. Effect of betamethasone on neuropathic pain and cerebral expression of NF-κB and cytokines. Neurosci Lett. 2006;393:255–259. doi: 10.1016/j.neulet.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shingo I. SRT1720, a SIRT1 activator, aggravates bleomycin-induced lung injury in mice. Food and Nutrition Sciences. 2012;3:157–163. [Google Scholar]
- 22.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 23.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Géranton SM, Jiménez-Díaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci. 2009;29:15017–15027. doi: 10.1523/JNEUROSCI.3451-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommer C, Petrausch S, Lindenlaub T, Toyka KV. Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci Lett. 1999;270:25–28. doi: 10.1016/s0304-3940(99)00450-4. [DOI] [PubMed] [Google Scholar]
- 26.Zelenka M, Schäfers M, Sommer C. Intraneural injection of interleukin-1β and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]


