Abstract
This study aimed to characterize a technique that assesses the outflow facility (C) efficacy of five kinds of IOP-lowering drugs commonly used clinically in enucleated porcine Eyes. Eyes were perfused at 15 mmHg with GPBS first to establish the baseline outflow facility (C0). Then the anterior chamber contents were exchanged for GPBS with corresponding concentration eye drops (4.9×103 nM Brimonidine, 41.1 nM Latanoprost, 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide, 8.3×103 nM Pilocarpine) in five groups (n = 6 each), while 6 eyes received GPBS alone as control. The mean stable facility obtained after drug administration (C1) was continuously recorded. The changes between C0 and C1 (ΔC = C1-C0) were analyzed. Finally, for drugs among the five experiment groups with statistical significance, the concentration was reduced 3 times, otherwise the drugs’ concentration was increased to 10 times to confirm its effectiveness further using the same methods (n = 6 each). We found that the average baseline outflow facility was 0.24±0.01 μl·min-1·mmHg-1. C increased significantly in Brimonidine and Latanoprost groups, even the concentration of Brimonidine and Latanoprost was decreased 3 times (P < 0.05). However, there was no significantly increase in Levobunolol, Brinzolamide, Pilocarpine and control group (P > 0.05), but when drugs’ concentration was increased to 10 times, the C value of Pilocarpine decreased significantly (P = 0.04). No significant washout effects in porcine eyes were observed. To conclude, outflow facility efficacy of five drugs in enucleated porcine eyes may provide a reference for clinical medicine. A constant-pressure perfusion technique should be useful to evaluate effect of pharmacologic agents or surgical manipulations on aqueous humor dynamics.
Keywords: Aqueous humor outflow facility, ocular perfusion, porcine eyes, ocular hypotensive drugs
Introduction
Glaucoma is characterized by a progressive loss of retinal ganglion cells, a characteristic optic neuropathy and patterns of visual field loss in the more advanced stages. It is a multifactorial disease with several recognized risk factors, of which elevated intraocular pressure (IOP) is a primary contributing factor caused by increased aqueous outflow resistance [1,2]. Lowering IOP by medications or surgery is the only therapeutic modality currently available. Therefore, to precisely describe and understand the regulation of IOP and its mechanism in the eyes is very important for glaucoma research. Notably, the source of increased outflow resistance in primary open angle glaucoma (POAG) has not yet been identified. Studies of the hydrodynamic and morphological correlation of decreasing outflow facility (C), which is defined as the reciprocal of the resistance to aqueous humor outflow as it exits the anterior chamber of the eye, associated with acute and chronic experimental elevation of IOP, have partly improved our understanding of the pathogenesis of this disease [3,4]. For the screening of potential new compounds, five in vitro models have been used: monolayer cell culture, whole eyes, explant culture, and anterior segment culture; both stationary and perfusion systems. In the case of perfusion techniques, IOP and one-way flow of aqueous can be maintained [5]. In the present study, total C is measured, which is equal to the arithmetic sum of trabecular outflow facility and uveoscleral outflow facility. By comparing C before (C0) and after (C1) IOP-lowering drug administration from the same eye, we try to assess the effects of the five pharmacologic agents (Brimonidine, Latanoprost, Levobunolol Brinzolamide and Pilocarpine) on aqueous humor outflow facility, as well as to demonstrate the utility of this approach.
Due to the anatomical differences between humans and animals, human eyes would constitute the most relevant test material in outflow studies; however, their use is limited by their difficult availability. Monkey, bovine, and porcine eyes have been used in whole-eye studies [4-12]. Many previous studies with porcine eyes have shown that the domestic pig is suitable for a variety of ophthalmologic studies [7,8]. The porcine eye was chosen for this study because of its easy availability and low costs. The anterior chamber volume was 300 μl and the globe size was almost equal to the human eye. In addition, the porcine eye contains a shallow scleral sulcus with a wedge-shaped mass of corneoscleral tissue comparable in size to human trabecular meshwork (TM) [8]. Ultrastructural investigations have indicated that subendothelial regions and the cribriform of porcine TM have an architecture similar to that of primate TM [12].
In this study, we use a rapid constant-pressure perfusion technique to measure the outflow facility (C) efficacy of five kinds of eye drops (Brimonidine, Latanoprost, Levobunolol, Brinzolamide, Pilocarpine) commonly used clinically in porcine Eyes. Though the analysis of the results, may it can provide a reference for clinical medicine, and to assess whether this experimental approach can be useful to evaluate effects of pharmacologic agents or surgical manipulations on aqueous humor dynamics in porcine and other animal models.
Methods
Materials
Animal procedures were conducted in compliance with the association for research in vision and ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Sixty-six fresh enucleated porcine eyes were obtained from a local abattoir (Jiading, Shanghai, China), the pigs were 4-5 months old, weighing approximately 70 kg. The eyes delivered were dlivered to our laboratory within 3 hours postmortem. Eyes with discernible damage or accumulated blood in the limbus or anterior chamber (AC) were excluded. The perfusate was Dulbecco’s phosphate-buffered saline (Ph = 7.3; Invitrogen, Grand Island, NY) containing 5.5 mM D-glucose (collectively referred to as GPBS) that was passed through a 0.2 μm cellulose acetate filter prior to use. 0.15% Brimonidine (Allergan, USA); 0.005% Latanoprost (Pfizer Manufacturing Belgium NV); 0.5% Levobunolol (Allergan, USA); 2% Brinzolamide (Alcon Laboratories Ltd, UK); Pilocarpine (Akorn, USA).
Perfusion procedure
Details of the mechanical setup of the perfusion system were described previously [4]. Briefly, the perfusion system consisted of a perfusion chamber and a collection chamber; the perfusion chamber was linked to a pressure transducer (Honeywell model 142 PC; Honeywell Sensing and Control, Freeport, IL), and connected electronically to a computer control system. The computer-controlled syringe pump delivered a variable flow rate (Q) to the anterior chamber to maintain a desired IOP. Outflow facility (C = Q/IOP) was measured at 10 Hz, ensemble averaged over a 10-second window, and electronically recorded every 10 seconds by LabView version 7.1 (National Instrument, USA), the characteristic of the ocular perfusion system is shown in Figure 1.
Figure 1.

Experimental set-up of the perfusion system. The computer- controlled perfusion system consisted of a computer control system (Labview version 7.1), a syringe pump, a pressure transducer, an exchange reservoir, a collection reservoir and a calibration reservoir.
Extraocular tissue was removed from porcine eyes, which were submerged to the limbus in phosphate-buffered saline (PBS) at 34°C. A 23-gauge infusion needle was inserted intracamerally through the peripheral transparent cornea into each eye and connected to the perfusion chamber. This needle was carefully threaded through the pupil and the needle tip positioned within the posterior chamber to prevent deepening of the AC that would otherwise lead to an artificial increase in outflow facility [5]. A second needle was inserted intracamerally into the AC and connected to the collection reservoir. During the perfusion, the collection reservoir tube was clamped except during exchanges. During exchanges, IOP was maintained at 15 mmHg by raising the perfusion reservoir 2 mmHg higher than 15 mmHg above the corneoscleral limbus, and decreasing the collection reservoir 2 mmHg lower than 15 mmHg so that the contents of the AC would flow to the collection reservoir. A volume of 5 ml fluid was exchanged which took about 10 minutes. This amount (5 ml) was chosen for exchange based on our previous experiment, in which the amount of microspheres almost completely disappeared in the perfusate from the AC [4].
Sixty-six enucleated porcine eyes were randomly divided into eleven groups. First, all eyes were perfused at 15 mmHg constant pressure with GPBS at least 30 minutes to establish a stable baseline C0. The perfusion pressure was set by adjusting the height of the reservoir above the surface of the eye. Assuming an episcleral venous pressure of 8 to 10 mmHg, this perfusion pressure would simulate an in vivo IOP of 22 to 25 mmHg [9]. Then the anterior chamber contents were exchanged for GPBS with corresponding concentration eye drops (4.9×103 nM Brimonidine, 41.1 nM Latanoprost, 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide and 8.3×103 nM Pilocarpine) in five groups (N = 6 each), We chose this concentration based on ciliary body and iris tissue concentrations of five drugs observed in topically treated nonhuman primates [13-17], which is the same as Win’s study [18]. While 1 group (N = 6) received GPBS alone as control. Finally, for drugs among the five experiment groups with statistically significant changes, the concentration was reduced 3 times, otherwise the drugs concentration was increased to 10 times to confirm its effectiveness further using the same methods. Because whether porcine eyes exhibit washout is still controversial [5,9,19], the control group was included to account for the washout effect. Finally, the subsequent perfusion of all eyes with corresponding perfusate was at a fixed volume (0.3 ml) and C1 was continuously recorded throughout the experiment.
Statistical analysis
Statistical analysis was performed with statistical software SPSS (Version 17.0; SPSS Inc., USA). The data were expressed as mean ± SEM. The comparison of difference for outflow facility between C0 and C1 was done by a paired two-sample t-test, while the difference for outflow facility within the same drug (changes in ∆C and ∆C%) in different concentration was compared with independent t-test. P < 0.05 were considered to be statistically significant.
Results
Outflow facilities of the eleven groups were shown in Tables 1 and 2. The average baseline facility (C0) was 0.25±0.01 μl·min-1·mmHg-1 (mean ± SEM) in control eyes tested at 15 mmHg. No significant washout effect (i.e., a time-dependent increase in outflow facility) was observed in the GPBS group (P = 0.60).
Table 1.
Summary of facility neasurements, before and after drug treatments (mean ± SEM, μl·min-1·mmHg-1, n = 6)
| Group※ | C0 | C1 | ∆C | ∆C% | P value |
|---|---|---|---|---|---|
|
| |||||
| (C1-C0) | ∆C/C0×100% | ||||
| GPBS | 0.25±0.01 | 0.24±0.03 | -0.01±0.02 | -5.22±6.75 | 0.600 |
| Brimonidine | 0.25±0.02 | 0.33±0.04 | 0.07±0.02 | 26.65±6.32 | 0.010* |
| Latanoprost | 0.24±0.03 | 0.37±0.04 | 0.12±0.02 | 54.2±11.5 | 0.001* |
| Levobunolol | 0.24±0.02 | 0.25±0.02 | 0.01±0.01 | 4.07±4.45 | 0.390 |
| Brinzolamide | 0.22±0.01 | 0.23±0.02 | 0.01±0.02 | 1.23±6.79 | 0.770 |
| Pilocarpine | 0.24±0.02 | 0.25±0.01 | 0.01±0.01 | 2.32±6.06 | 0.380 |
5.5 mM GPBS and GPBS with corresponding concentration eye drops (4.9×103 nM Brimonidine, 41.1 nM Latanoprost, 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide and 8.3×103 nM Pilocarpine1).
For 4.9×103 nM Brimonidine and 41.1 nM Latanoprost showed statistical differences between C0 and C1, their concentration was reduced 3 times, while other drugs concentration was improved 10 times to confirm its effectiveness further. Pre-operation (C0), Post-operation (C1); P < 0.05.
Table 2.
Summary of facility measurements, before and after drug treatments (mean ± SEM, μl·min-1·mmHg-1, n = 6)
| Group※ | C0 | C1 | ∆C | ∆C% | P value |
|---|---|---|---|---|---|
|
| |||||
| (C1-C0) | ∆C/C0×100% | ||||
| GPBS | 0.25±0.01 | 0.24±0.03 | -0.01±0.02 | -5.22±6.75 | 0.600 |
| Brimonidine | 0.26±0.01 | 0.30±0.02 | 0.04±0.01 | 16.15±5.48 | 0.025§ |
| Latanoprost | 0.26±0.02 | 0.32±0.02 | 0.06±0.01 | 22.83±5.57 | 0.005§ |
| Levobunolol | 0.26±0.02 | 0.28±0.02 | 0.02±0.01 | 5.27±3.18 | 0.157 |
| Brinzolamide | 0.24±0.02 | 0.26±0.03 | 0.02±0.01 | 8.62±7.20 | 0.302 |
| Pilocarpine | 0.24±0.02 | 0.22±0.02 | -0.02±0.01 | -10.10±2.22 | 0.004§ |
5.5 mM GPBS and GPBS with corresponding concentration eye drops (4.9×103/3 nM Brimonidine, 41.1/3 nM Latanoprost, 3.4×104 nM Levobunolol, 3.0×104 nM Brinzolamide and 8.3×104 nM Pilocarpine).
Pre-operation (C0), Post-operation (C1);
P < 0.05.
In 4.9×103 nM Brimonidine and 41.1 nM Latanoprost treatment groups, ∆Cbrim-1 = 0.07±0.02 μl·min-1·mmHg-1 (26.65±6.32%), ∆Clata-1 = 0.12±0.02 μl·min-1·mmHg-1 (54.20±11.59%), C increased significantly in Brimonidine (P = 0.010) and Latanoprost (P = 0.001) groups compared with their own baselines (Figure 2), even the concentration of 4.9×103 nM Brimonidine and 41.1 nM Latanoprost was decreased 3 times, it showed the same tendency statistical differences between C0 and C1 (P = 0.025, P = 0.005). However, there was no significantly increase in 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide, 8.3×103 nM Pilocarpine and the control group (P = 0.390, P = 0.770, P = 0.380 and P = 0.600), when drugs’ concentration was increased to 10 times, Levobunolol, and Brinzolamide groups showed no statistical differences between C0 and C1, but high concentration of Pilocarpine decreased C significantly (P = 0.04) with ∆Cpilo = -0.02±0.01 μl·min-1·mmHg-1 (-10.10±2.22%) (Figures 3, 4 and 5).
Figure 2.

Changes in outflow facility between pre-operation, C0 and post-operation, C1. An significantly increase in C was observed in 4.9× 103 nM Brimonidine and 41.1 nM Latanoprost groups compared with their own baselines (n = 6, paired t-test, P < 0.05). In contrast, perfusion with 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide, 8.3×103 nM Pilocarpine, GPBS groups had no significant differences in outflow facility compared to their own baselines (n = 6, paired t-test, P > 0.05). No clear washout effect (i.e., a time-dependent increase in outflow facility) was observed (calculated as a mean of the hourly recorded stable outflow facilities) in the GPBS group (n = 6, paired t-test, P = 0.60). P < 0.05.
Figure 3.

Changes in outflow facility between pre-operation, C0 and post-operation, C1. An significantly increase in C was observed in 4.9×103/3 nM Brimonidine and 41.1/3 nM Latanoprost groups compared with their own baselines (n = 6, paired t-test, P < 0.05). In contrast, perfusion with 3.4×104 nM Levobunolol, 3.0×104 nM Brinzolamide groups had no significant differences in outflow facility compared to their own baselines (n = 6, paired t-test, P > 0.05), while, 8.3×104 nM Pilocarpine showed statistical decrease in C (n = 6, paired t-test, P < 0.05). §P < 0.05.
Figure 4.
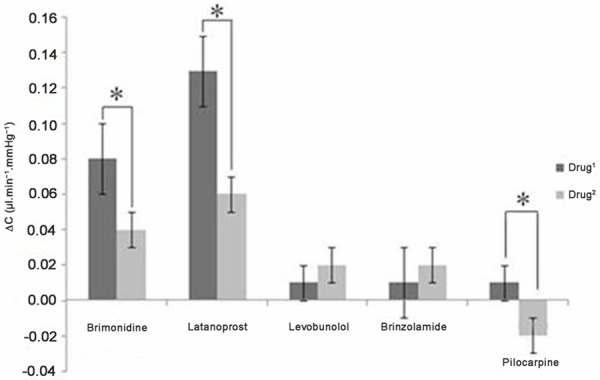
Changes in ΔC (ΔC = C1- C0), Drug1 (4.9×103 nM Brimonidine, 41.1 nM Latanoprost, 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide, 8.3×103 nM Pilocarpine). Drug2 (4.9×103/3 nM Brimonidine, 41.1/3 nM Latanoprost, 3.4×104 nM Levobunolol, 3.0×104 nM Brinzolamide and 8.3×104 nM Pilocarpine) (n = 6, independent t-test, P < 0.05). P < 0.05.
Figure 5.

Changes in ΔC% (ΔC/C0×100%), Drug1 (4.9×103 nM Brimonidine, 41.1 nM Latanoprost, 3.4×103 nM Levobunolol, 3.0×103 nM Brinzolamide, 8.3×103 nM Pilocarpine). Drug2 (4.9×103/3 nM Brimonidine, 41.1/3 nM Latanoprost, 3.4×104 nM Levobunolol, 3.0×104 nM Brinzolamide and 8.3×104 nM Pilocarpine) (n = 6, independent t-test, P < 0.05). P < 0.05.
Discussion
In this study, we used an ex vivo method with the perfused porcine eye suitable for testing clinical antiglaucomatous drugs on aqueous humor outflow facility. In our study, the mean baseline outflow facility was 0.25±0.01 μl·min-1·mmHg-1 (mean ± SEM; n = 6) in control eyes perfused at 15 mmHg, which is very similar to the 0.25±0.01 μl·min-1·mmHg-1 reported by Vaajanen and his coworkers [5]. According to the literature, the basal outflow facility in the enucleated porcine eye has been between 0.25±0.01 and 0.50±0.20 μl·min-1·mmHg-1 [5,9,19-21]. Several reasons may partially explain these differences. Different body weight of pigs (70 kg in the present study versus 80 kg or some papers not described in detail) and regional differences of pig eyes sources (Asia in the present study versus Europe).
Animal models in all perfusion methods are associated with a time-dependent increase in outflow facility, which has not found in human eyes in vitro [22]. This phenomenon is referred to as washout, in accordance with the hypothesis that the progressive decrease in resistance is the result of a perfusion-induced washing away of extracellular material from the outflow pathway tissue [22,23]. The typical washout effect in porcine eyes has been between 6% and 26% [5,19,24]. However, Wagner and coworkers [9] observed no significant washout effect in their porcine whole-eye studies. In our study, No significant washout effect (i.e., a time-dependent increase in outflow facility) was observed either (calculated as a mean of the hourly recorded stable outflow facilities) in the GPBS group (P = 0.60). We suggest that the subsequent perfusion at a fixed volume (0.3 ml) and the total perfusion time in the GPBS group was therefore at most 180 minutes, the volume and time were not enough to cause washout effect, and also strong traction was carefully avoided during enucleation so as not to damage sensitive eye structures.
Five IOP-lowering glaucoma medications were evaluated for their effects on C in the enucleated porcine eyes. We confirmed that the prostaglandin FP agonist latanoprost (41.1 nM), theα2-adrenergic agonist brimonidine (4.9×103 nM) increased C value by 54.20±11.59% and 26.65±6.32%, similarly to previous studies tested by topical ocular delivery of 1 to 2 drops of the drug formulations in mouse eye [25,26], while, there was no significantly different increase in C value when perfusing the β-blocker levobunolol (3.4×103 nM), the carbonic anhydrase inhibitor brinzolamide (3.0×103 nM), and the cholinergic agonist pilocarpine (8.3×103 nM). These results are in agreement with the findings of Millar et al. Latanoprost is a selective FP receptor agonist and it has been reported to increase uveoscleral outflow by remodeling the extracellular matrix in the ciliary muscle and affect intracellular pathways that control cell contractility [27]. It may also increase the conventional outflow facility through changes in the TM [13]. In our study, the C value of latanoprost group significantly increased, followed by brimonidine group, even the concentration of 41.1 nM Latanoprost and 4.9×103 nM Brimonidine was decreased 3 times, it showed the same tendency statistical differences between C0 and C1 (22.83±5.57%, P = 0.025; 16.15±5.48%, P = 0.005). Levobunolol and brinzolamide are compounds known to suppress aqueous humor formation, accordingly, it was ineffective in the porcine eye in the concentration based on ciliary body and iris tissue concentrations observed in topically treated nonhuman primates, there was insignificant as though the concentration of the drugs (Levobunolol and brinzolamide) was improved 10 times in the absence of a functioning circulatory system. Topical application of pilocarpine onto the human glaucomatous eyes lowers IOP, most commonly accepted mechanism of action for muscarinics involves receptor-mediated contraction of the ciliary muscle. Ciliary muscle contraction presumably creates tension on the TM and surrounding tissues. This tension may ultimately effect an enlargement of the intertrabecular spaces, thereby resulting in a decreased resistance to aqueous fluid outflow and a subsequent decrease in IOP [28]. Another muscarinic agent, carbachol, has been shown to induce contraction in isolated TM strips [29]. This direct effect on the TM may very well contribute to a portion of the outflow action of this class of compounds. In our study, 8.3×103 nM pilocarpine perceived no alteration in C value with statistical significance at 15 mmHg, while 8.3×104 nM pilocarpine led to a significant decrease in measured outflow facility. Our findings predict that high concentration of pilocarpine decreased C by inducing a contractile response in trabecular meshwork cells without affecting uveoscleral outflow pathway because of the ciliary muscle poorly developed in nonprimates (such as pig, cat) and there is no clear anatomical connection between the ciliary muscle and the drainage tissues [12].
Techniques for measuring C value have been reported for more than 50 years. In the first report, Baŕany [30] described a two-level, constant-pressure perfusion technique for measuring C in the vervet monkey. In this technique, total C (Ctot) is measured, which is equal to the arithmetic sum of trabecular outflow facility (Ctrab), uveoscleral outflow facility (Cu), and inflow facility (Cps is decreased aqueous humor secretion rate with increasing IOP). However, in practice, Cps is generally disregarded because they are reported to represent only approximately 10% of Ctot [31]. Ctrab and Cu are some 10- to 20-fold more pressure dependent than Cps. Thus, when determined by this Ctot is normally assumed to be equal to Ctrab and Cu. Since publication of paper, this approach for measuring C has been adapted for use in several other species-for example, human beings [1,3,22], the cynomolgus monkey [4,23,30], the pig [5,7-9,24], the bovine [6,10,11]. We applied the whole-globe perfusion system similar to that of Sit’s application in 1977 [32]. The advantages of a constant-flow perfusion method over the more traditional B́aŕany’s constant-pressure perfusion technique are minimal cost and ease of data acquisition. In addition, the small size of the mouse eye with minute changes in fluid volume movement requires greater sensitivity and further magnifies the problem. For the constant-pressure perfusion used in the present study, a strain gauge is not required, because flow is induced with a reliable micro dialysis perfusion pump at the desired pressures (we used 15 mmHg). The resultant pressure generated is determined with a pressure transducer with an output signal in volts (rather than microvolts) with a baseline sufficiently stable for the study period. The apparatus can be set up in a limited bench space, elaborate vibration-dampening and electrical shielding are unnecessary. The procedure is technically straightforward, with a low failure rate (~5%) once the necessary skills have been acquired. This method also allows determination of the pressure-flow rate relationship using several infusion flow rates. We point out that recent studies have successfully exploited this technique to demonstrate the potential role of pharmacologic agent in outflow facility [4,6].
In summary, in this study, a constant-pressure perfusion technique was used to assess outflow facility efficacy of five drugs in enucleated porcine eyes which may provide a reference for clinical medicine. This experimental approach should be useful to evaluate effects of pharmacologic agents or surgical manipulations on aqueous humor dynamics in porcine and other animal models.
Acknowledgements
This work was supported by operating research grants from the Shanghai Pujiang Program 11PJD006.
Disclosure of conflict of interest
None.
References
- 1.Keller KE, Vranka JA, Haddadin RI, Kang MH, Oh DJ, Rhee DJ, Yang YF, Sun YY, Kelley MJ, Acott TS. The effects of tenascin C knockdown on trabecular meshwork outflow resistance. Invest Ophthalmol Vis Sci. 2013;54:5613–5623. doi: 10.1167/iovs.13-11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958;60:523–533. doi: 10.1001/archopht.1958.00940080541001. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Folz SJ, Laryea SN, Overby DR. Multi-scale analysis of segmental outflow patterns in human trabecular meshwork with changing intraocular pressure. J Ocul Pharmacol Ther. 2014;30:213–223. doi: 10.1089/jop.2013.0182. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Toris CB, Liu Y, Ye W, Gong H. Morphological and hydrodynamic correlates in monkey eyes with laser induced glaucoma. Exp Eye Res. 2009;89:748–756. doi: 10.1016/j.exer.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Vaajanen A, Vapaatalo H, Oksala O. A modified in vitro method for aqueous humor outflow studies in enucleated porcine eyes. J Ocul Pharmacol Ther. 2007;23:124–131. doi: 10.1089/jop.2006.0057. [DOI] [PubMed] [Google Scholar]
- 6.Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–281. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann B, Birke M, Kook D, Eichhorn M, Lutjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Ederra J, Garcia M, Hernandez M, Urcola H, Hernandez-Barbachano E, Araiz J, Vecino E. The pig eye as a novel model of glaucoma. Exp Eye Res. 2005;81:561–569. doi: 10.1016/j.exer.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JA, Edwards A, Schuman JS. Characterization of uveoscleral outflow in enucleated porcine eyes perfused under constant pressure. Invest Ophthalmol Vis Sci. 2004;45:3203–3206. doi: 10.1167/iovs.03-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002;43:3455–3464. [PubMed] [Google Scholar]
- 11.Johnson M, Gong H, Freddo TF, Ritter N, Kamm R. Serum proteins and aqueous outflow resistance in bovine eyes. Invest Ophthalmol Vis Sci. 1993;34:3549–3557. [PubMed] [Google Scholar]
- 12.McMenamin PG, Steptoe RJ. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J Anat. 1991;78:65–77. [PMC free article] [PubMed] [Google Scholar]
- 13.Ichhpujani P, Katz LJ, Hollo G, Shields CL, Shields JA, Marr B, Eagle R, Alvim H, Wizov SS, Acheampong A, Chen J, Wheeler LA. Comparison of human ocular distribution of bimatoprost and latanoprost. J Ocul Pharmacol Ther. 2012;28:134–145. doi: 10.1089/jop.2011.0097. [DOI] [PubMed] [Google Scholar]
- 14.Kadam RS, Jadhav G, Ogidigben M, Kompella UB. Ocular pharmacokinetics of dorzolamide and brinzolamide after single and multiple topical dosing: implications for effects on ocular blood flow. Drug Metab Dispos. 2011;39:1529–1537. doi: 10.1124/dmd.111.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Song JZ, Pang GR, Chen ZJ, Zhang JJ, Lu XF. Ocular pharmacokinetics of 0.5% pilocarpine with sodium hyaluronate in rabbits. Zhonghua Yan Ke Za Zhi. 2004;40:87–89. [PubMed] [Google Scholar]
- 16.Acheampong AA, Breau A, Shackleton M, Luo W, Lam S, Tang-Liu DD. Comparison of concentration-time profiles of levobunolol and timolol in anterior and posterior ocular tissues of albino rabbits. J Ocul Pharmacol Ther. 1995;11:489–502. doi: 10.1089/jop.1995.11.489. [DOI] [PubMed] [Google Scholar]
- 17.Acheampong AA, Shackleton M, Tang-Liu DD. Comparative ocular pharmacokinetics of brimonidine after a single dose application to the eyes of albino and pigmented rabbits. Drug Metab Dispos. 1995;23:708–712. [PubMed] [Google Scholar]
- 18.Wan Z, Woodward DF, Cornell CL, Fliri HG, Martos JL, Pettit SN, Wang JW, Kharlamb AB, Wheeler LA, Garst ME, Landsverk KJ, Struble CS, Stamer WD. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48:4107–4115. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80:197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Goldwich A, Ethier CR, Chan DW, Tamm ER. Perfusion with the olfactomedin domain of myocilin does not affect outflow facility. Invest Ophthalmol Vis Sci. 2003;44:1953–1961. doi: 10.1167/iovs.02-0863. [DOI] [PubMed] [Google Scholar]
- 21.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- 22.Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest Ophthalmol Vis Sci. 1990;31:2384–2388. [PubMed] [Google Scholar]
- 23.Kaufman PL, True-Gabelt B, Erickson-Lamy KA. Time-dependence of perfusion outflow facility in the cynomolgus monkey. Curr Eye Res. 1988;7:721–726. doi: 10.3109/02713688809033201. [DOI] [PubMed] [Google Scholar]
- 24.Khurana RN, Deng PF, Epstein DL, Vasantha RP. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp Eye Res. 2003;76:39–47. doi: 10.1016/s0014-4835(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 25.Millar JC, Clark AF, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011;52:685–694. doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- 26.Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther. 2009;25:401–408. doi: 10.1089/jop.2009.0006. [DOI] [PubMed] [Google Scholar]
- 27.Oh DJ, Martin JL, Williams AJ, Peck RE, Pokorny C, Russell P, Birk DE, Rhee DJ. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci. 2006;47:953–963. doi: 10.1167/iovs.05-0516. [DOI] [PubMed] [Google Scholar]
- 28.Shade DL, Clark AF, Pang IH. Effects of muscarinic agents on cultured human trabecular meshwork cells. Exp Eye Res. 1996;62:201–210. doi: 10.1006/exer.1996.0025. [DOI] [PubMed] [Google Scholar]
- 29.Wiederholt M, Lepple WA, Stahl F. In Basic Aspects of Glaucoma Research III. 1993. Contractile properties of trabecular meshwork and ciliary muscle; pp. 287–306. [Google Scholar]
- 30.Barany EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol. 1964;3:135–143. [PubMed] [Google Scholar]
- 31.Kaufman PL. Some thoughts on the pressure dependence of uveoscleral flow. J Glaucoma. 2003;12:89, 93–94. doi: 10.1097/00061198-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Sit AJ, Coloma FM, Ethier CR, Johnson M. Factors affecting the pores of the inner wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1997;38:1517–152. [PubMed] [Google Scholar]


