Abstract
Objectives: This study sought to determine the current effectiveness and safety of spironolactone for resistant hypertension. Methods: Pubmed, EMBASE and Cochrane databases were searched to identify studies that used spironolactone as add-on treatment in resistant hypertension patients. The major outcome is reduction of blood pressure. Results: Three randomized controlled trials, 5 observational study without control group have been identified. At follow-up review, there was a reduction in mean systolic and diastolic blood pressure (BP) respectively, of -20.56 mmHg (95% confidence interval [CI]: -36.47 to -4.65 mmHg) and -6.04 mm Hg (95% CI: -10.24 to -1.85 mm Hg) in controlled study. In uncontrolled studies, at 6-month follow-up, systolic and diastolic BP were reduced -20.73 mm Hg (95% CI: -25.62 to -15.83 mm Hg) and -8.94 mm Hg (95% CI: -10.82 to -7.05 mm Hg, respectively. Conclusion: Spironolactone resulted in the reduction of mean BP in patients with resistant hypertension. The sustained efficacy and safety of spironolactone need to further confirm by large randomized controlled trials with long-term follow-up.
Keywords: Spironolactone, resistant hypertension, meta analysis
Introduction
Resistant hypertension (RHTN) is a common clinical problem faced by both primary care clinicians and specialist worldwide. It is defined as blood pressure (BP) that remains above the goal, despite the concurrent use of 3 antihypertensive agents of different classes prescribed at optimal dosage; one of the 3 agents used should be a diuretic [1,2]. It is unknown the exact prevalence of resistant hypertension, but large clinical trials is estimated that resistant hypertension affects at least 10% to 15% of all hypertensive patients [3,4]. If no secondary cause of hypertension is found, the use of multidrug treatment regimens including 3, 4, or more antihypertensive drugs is usually necessary to lower BP and thus prevent future cardiovascular events [5].
The classic regimen for patients with treatment-resistant hypertension is the combination therapy including a diuretic, a long-acting calcium channel blocker, an angiotensin-converting enzyme inhibitor, a beta blocker, and a mineralocorticoid receptor antagonist. Evidence suggests that hyperaldosteronism is an important underlying cause of RHTN. The contention is strongly supported by studies evaluating aldosterone antagonists in RHTN patients [6]. Spironolactone, mineralocorticoid receptor antagonists, is a potassium-sparing diuretic (water pill) that prevent body from absorbing too much salt and potassium levels from getting too low. Several studies have shown that the addition of spironolactone as a treatment option for RHTN patients provided benefit in African American and Caucasian patients with RHTN [7-9]. Despite the efficacy of spironolactone in patients with RHTN, available evidences of comprehensive review have been supported and used to guide the clinical treatment. We have systematically reviewed the current available evidence for spironolactone and qualified it BP-lowing effect in patients with RHTN using a random effects meta-analysis model.
Methods
Data source and search strategy
PubMed, EMBASE, and the Cochrane Collaboration database have been searched till January 2014 for available study. These search restricted to English Language articles publisher. The following search terms were used: resistant hypertension, spironolactone. If same study had been published in different journals or in various years, our analysis only selected the most recent publication. If different experiments of multiple studies were done and published by same group of researchers, then each study was included in the analysis. There was no overlap in patients included in our analysis in this study.
Study selection
We included randomized, prospective, placebo-controlled studies comparing BP response in patient treated with spironolactone. Inclusion criteria were: 1) spironolactone was used as add-on therapy drug in RHTN; 2) patient populations with RHTN (not meeting BP goal despite therapy with 3 or more antihypertension agents from at least 3 different classes). Measurement of blood pressure could use manual, automated or invasive BP recording, as long as the same method was used before and after treatment. Reviews, editorial and abstracts presented at conference were excluded.
Data extraction
The following information was collected for our analysis: (1) First author and publication year; (2) study design and sample size; (3) treatment regimens (dosage of spironolactone); (4) the primary outcome was mean systolic blood pressure (BP) and diastolic blood pressure (BP) reduction.
Methodological quality
To determine the quality of the included studies, Guidelines in the Cochrane Handbook for systematic Reviews of Interventions 5.0.25 was used to direct the qualitative classification of studies.
Statistical analysis
The analysis was performed by using Cochrane Collaboration Review Manager (version 5.1.7, Cochrane Collaboration, Copenhagen, Denmark) software package. Mean difference (MD) was used to represent the continuous outcomes, with a 95% confidence interval (CI). The presence of heterogeneity was determined by Cochran’s Q test with the I 2 statistic [10]. When P<0.05 and I 2>50%, heterogeneity was considered significant across selected studies, and meta analysis was performed in a random-effects model, otherwise, fixed-effects model was applied. Publication bias was detected by the Begge’s test and funnel plots [11].
Results
Study selection and characteristics
154 potentially relevant studies as shown in the flow diagram (Figure 1) were identified. Of these studies, nine studies met the inclusion criteria. We excluded one additional study. The Krieger study [12], which is a controlled study to compare efficacy of spironolactone and Clonidine, was not included in controlled study and was not included in our analysis. Oxlund et al’s study [4] was placebo-controlled study on effect of spironolactone add-on treatment on RHTN with type 2 diabetes mellitus patients study, these two study was still included in our analysis since except this study, Vaclavik et al study [2] was the only one placebo-control study reported. Thus, we systematically reviewed 8 studies, encompassing 2051 patients treated between 2002 and 2013. The follow-up duration varied between 1 month and 6 months.
Figure 1.
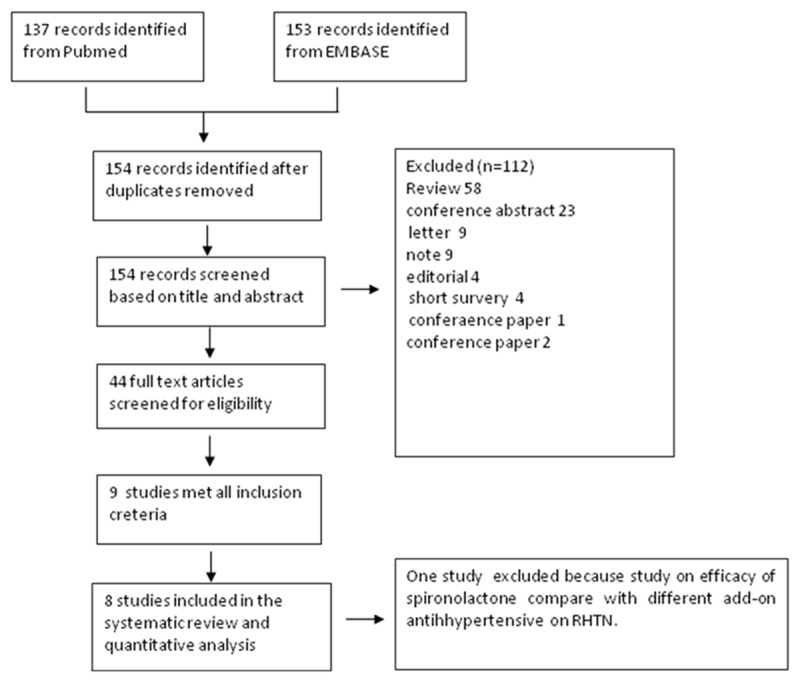
Flow diagram for study selection.
Table 1 summarized the baseline characteristics of all included studies. Among 8 studies included in this study, four were specified as prospective studies [7-9,13]; one was reported as Surveillance study [14]; Three were reported as randomized placebo-control trial [7,15,16].
Table 1.
Characteristics and inclusion criteria of all included studies
| study/first author | year | type of study | method of BP measurement for analysis | include/exclude criteria | RHTN patients n | control patients n | length of follow-up | Dose (mg/day) |
|---|---|---|---|---|---|---|---|---|
| Ouzen et al | 2002 | Prospective study | clinical BP in triplicate and 24 h AMBP | standart creteria, exclusion: prior use of spironolactone, reanal insufficiency (creatinine >15 mg/L, or potassium level >5 meq/L | 25 | 0 | 1 month | 1 mg/kg/D (medium: 25) |
| Nishizaka et al | 2003 | Prospective study | clinical BP in triplicate | standard creteria | 76 | 0 | 3 month 6 month | 12.5-50 |
| Chapman et al | 2007 | Prospective study | clinical BP in triplicate | standard creteria | 1411 | 0 | 6 month | 25 (median), 35 (mean initial), 45 (mean final) |
| Lane et al | 2007 | Surveillance study | clinical BP in triplicate and 24 h AMBP | standard creteria, exclusion: patients with primary or secondary aldosterone excess, underlying renal or nenovascular causes of hypertension, a history or clinical evidence of heart failure, and chronic renal impairment with a serum creatinine level greater than 175 umol/L | 119 | 0 | 3 month, 6 month | 25-50 (median: 25) |
| Souza. et al | 2010 | Prospective study | clinical BP in triplicate and 24 h AMBP | standard creteria | 175 | 0 | 6 month | 25-100 (medium 50) |
| Vaclavik et al | 2011 | Randomized control-plecebo trial | clinical BP in triplicate and 24 h AMBP | standard creteria | 55 | 56 | 4-week, 8-week | 25 |
| Oxlund et al | 2013 | Randomized control-placebo trial | Clinical BP in triplicate and 24 h AMBP | Standard creteria, all patients with type-2 diabetes millitus | 61 | 58 | After 16-week | 25 |
| Abolghasmi et al | 2011 | Randomized control-placebo trial | Clinical BP in triplicate | Standard creteria, all patients with Chronic kidney disease | 19 | 22 | 6-week, 12-week | 25-50 |
Standard creteria: 1) clinical BP measurement or mean 24 h ambulatory BP (AMBP) monitoring >140/90 mm Hg despite treatment with at least two antihypertensive drugs; 2) no prior therapy with spironolactone.
The total number of patients per study ranged from 25 to 1411, with a total of 2051 patients accrued. There were 3 randomized placebo-controlled group (n 135) and 6 observational uncontrolled studies. The dosing of spironolactone ranged from 25 mg/day to 1 mg/kg/day, medium dosage was 25 mg/day. There were a big similarity between the inclusion criteria and exclusion criteria. The clinical BP measurements, as primary outcome measurement, were employed for the meta-analysis in all studies since ambulatory BP monitors were relatively lower than clinical measurements [7-9,15,16].
Table 2 summarized the patient characteristics. Around 40% patients were male with average age of 60 years. The average SBP of patients before add-on spironolactone was 161.9 mmHg and the average of DBP were 90.34 mmHg.
Table 2.
Baseline characteristics of the included study subjects
| Study/first author | Treatment group | age. Yrs | Male, % | BMI, kg/m2 | SBP, mm Hg | DBP, mm Hg | serum creatinine, umol/L | serum K, mEq/L |
|---|---|---|---|---|---|---|---|---|
| Observational uncontrolled study | ||||||||
| Ouzen et al | Spironoclatone | 65±11 | 40 | NR | 152±2 | 86±2 | NR | NR |
| Nishizaka et al | Spironoclatone | 55±12 | 40.7 | 33.6±8.7 | 163 ±18 | 91±14 | NR | 4.0±0.5 |
| Chapman et al | Spironoclatone | 63.5±8.4 | 77 | 29.4±4.6 | 156.9±18 | 85.3±11.5 | 99 | 4.18±0.5 |
| Lane et al | Spironoclatone | 62.5±12.1 | 31.9 | NR | 182.3±23.2 | 94.9±18.5 | 88.7 | 4.3±0.5 |
| Souza et al | Spironoclatone | 62±10 | 27.6 | 30.2±5.1 | 169±27 | 92±18 | 70 | 4.2±0.5 |
| Randomized controlled study | ||||||||
| Vaclavik et al | Spironoclatone | 61.4±9.6 | 67.3 | 32.3±5.1 | 154.9±10.4 | 92.6±10.7 | 81 | 4.2±0.5 |
| Placebo | 60.1±9.4 | 57.1 | 32.3±5.3 | 153.5±12 | 90.6±10.9 | 83 | 4.2±0.5 | |
| Oxlund et al | Spironoclatone | 62.9±7.1 | 75 | 32 (25 to 43) | 144±15 | 79±11 | 81.2 | 3.9±0.4 |
| Placebo | 63.9±6.9 | 78 | 31.5 (26 to 43) | 139±15 | 76±8 | 79 | 3.8±0.3 | |
| Abolghasmi et al | Spironoclatine | 49±13,2 | 53 | 31.2±4.3 | 171±10 | 95±4 | 2.2±0.8 mg/dL | 4.3±0.8 |
| Placebo | 50±10.1 | 54.5 | 30.0±3.2 | 170±10 | 93±5 | 2.3±0.6 mg/dL | 4.5±0.6 | |
Efficacy of Spironolactone
In controlled studies, we observed the reduction in mean systolic and diastolic BP at follow-up endpoint of -20.56 mmHg (95% confidence interval [CI]: -36.47 to -4.65 mmHg) and -6.04 mm Hg (95% CI: -10.24 to -1.85 mm Hg), respectively, compared spironolactone add-on treatment with placebo (for both, P<0.00001) (Figure 2A, 2B). There was a high amount of heterogeneity (I2 98% and 90%) among the included studies.
Figure 2.
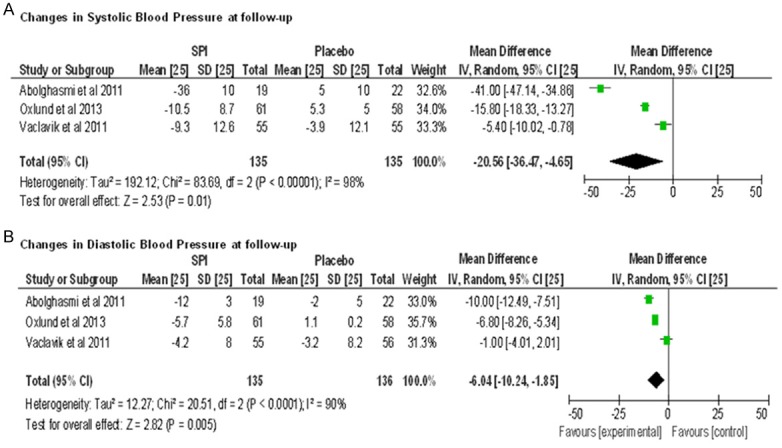
Forest plots comparing the systolic blood pressure (A) and diastolic blood pressure (B) between spironolactone and control group of controlled studies.
In uncontrolled studies, we observed the reduction in mean systolic and diastolic BP at 3 month follow-up with -19.65 mm Hg (95% CI: -26.60 to -12.70 mmHg) and -9.88 mm Hg (95% CI: -10.94 to -8.83 mm Hg), respectively, compared before spironolactone add-on treatment with after add-on treatment, (for both, P<0.00001) (Figure 3A-D). There showed a significant amount of heterogeneity for SBP at the 3 month follow-up (I2 85%), The heterogeneity for DBP was not appear (I2 = 0), whereas the DBP change was still maintained (-9.88 mm Hg), At 6-months follow-up, there were reductions of -20.73 mm Hg (95% CI: -25.62 to -15.83 mm Hg) and -8.94 mm Hg (95% CI: -10.82 to -7.05 mm Hg (for both, P<0.00001). Statistical heterogeneity was observed for both the SBP and DBP response. (I2 81% for SBP and 73% for DBP).
Figure 3.
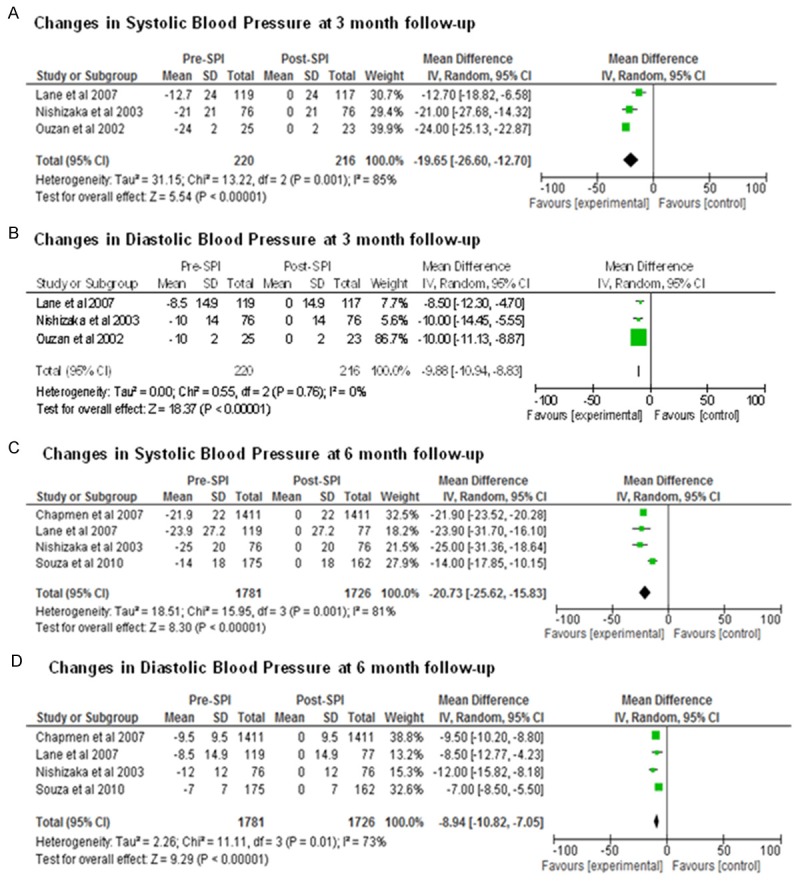
Forest plots comparing the systolic blood pressure (A) and diastolic blood pressure (B) between spironolactone and control group at 3-month follow-up, the systolic blood pressure and diastolic blood pressure between spironolactone and control group at 6-month follow-up are shown in (C) and (D), respectively.
Sensitivity analyses and publication bias assessment
The result stability was assessed by sensitivity analysis, in which one study was removed at a time. The pooled mean differences were not significantly changed. Begg’s funnel plot was performed to access qualitative publication bias of the studies. The result showed that there was no evidence of obvious asymmetry (Figure 4). Therefore, no bias of publications was found.
Figure 4.
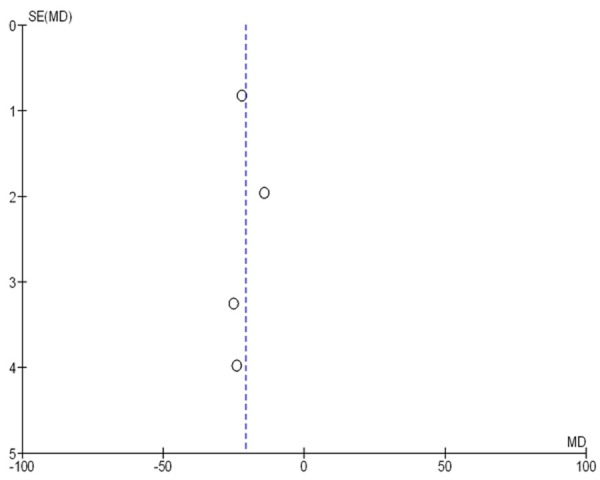
Funnel plot of the studies selected. The plot is for qualitative estimation of publication bias of the studies. No bias was found.
Discussion
The goal of this study was to evaluate the effect of spironolactone on BP reduction in a resistant hypertension population. We found that reduction in BP after add-on spironolactone was appeared as early as 3 months. There was a substantial reduction in BP at 6-months.
We pooled the results of the studies according to the study design. One randomized placebo-controlled study on RHTN patients with type 2 diabetes milieus and one randomized placebo-controlled study on RHTN patients with chronic kidney disease were pooled with only one placebo-controlled study on RHTN patients. Resistant hypertension is particularly associated with a number of conditions such as obesity, older age, diabetes mellitus and chronic kidney disease and can make a large number of patients be at high risk for cardiovascular morbidity and mortality [17]. The pathogenesis of resistant hypertension is still not completely known, but there is a large amount of evidence for aldosterone and enlarged mineralocorticoid receptor activity to be important factors [18,19]. In our study, the reduction of placebo-corrected clinical BP of 15.8 mm Hg in Oxlund study [4], and 41 mm Hg in Abolghasmi study [20] were higher than the 6.5 mm Hg observed in ASPIRANT trial [2], but patients administrated with higher doses of spironolactone, on average 35 vs. 25 mg per day. With a placebo control group, the cause-and-effect relationship as well as safety could be established.
We pooled the uncontrolled observational studies together. Confounding by indication in observational studies causes to overestimate treatment effects. However, in our analysis, all the studies have shown the consistent BP reduction regardless of study design.
In this analysis, the study population is composed of 270 patients for controlled study and 1781 patients for uncontrolled study. In controlled study, the number of study population is small for a meta-analysis. Placebo-controlled study on effect of spironolactone on RHTN patients started on 2011. So far only three studies was published, Oxlund et al’s study [4], ASPIRANT trial [2] and Abolghasmi et al’s study [20]. In a meta-analysis, the number of included studies is more important than the number of patients, especially when the outcome is continuous. As spironolactone has been employed for add-on treatment on resistant hypertension, the understanding of the current extent of effectiveness of spironolactone, and the source of the evidence, is of utmost important. Therefore, current clinical data for analysis must inflect the quality of the evidence and effectiveness and safety of spironolactone.
The short-term and intermediate term data suggests that spironolactone is safe and well-tolerated, with some adverse effect, gynecomatia, elevation of serum creatinine and increase of serum patossium. In Ouzan’s study [5], no patients required discontinuation of spironolactone due to adverse renal effects. Chapman et al’s study [17] recorded that 6% participants discontinued the drug due to gynecomatia and biochemical abnormal. Lane et al’s study [7] recorded that two participants discontinued due to an increase of serum patossium above to 6.0 mmol/L, whereas the mean increase in serum potassium was 0.3 mmol/L. In Souza’s study, 7.4% participants had adverse effects attributed to spironolactone, gynecomastia. Oxlund et al’s study [4] and ASPIRANT trial [2] showed comparable frequency of adverse events in two group. However, there were no sufficient data to make meaningful conclusion of adverse effects on RPTN patients treated with add-on spironolactone.
Study limit
First, Most of the included studies were observational in nature and thus confounding by indication or selection bias may affect the result of analysis. Secondly, the BP was reduced in both placebo-controlled studies and uncontrolled studies. The participants in some studies were patients with primary aldosteromism, type-2 diabetes. In addition, included studies in this analysis were restricted to published studies, and may therefore be affected by publication bias.
Conclusions
The current available data indicate that spironolactone can result in a substantial BP reduction in patients with RPN at 3-month follow-up. Even though few adverse have been reported, available data also showed that spironolactone has a favorable safety profile. Nonetheless, to further confirm the sustained efficacy and safety of spironolactone in patients with resistant hypertension, long-term follow-up and large randomized controlled trials are needed.
Disclosure of conflict of interest
None.
References
- 1.Colussi G, Catena C, Sechi LA. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens. 2013;31:3–15. doi: 10.1097/HJH.0b013e3283599b6a. [DOI] [PubMed] [Google Scholar]
- 2.Vaclavik J, Sedlak R, Plachy M, Navratil K, Plasek J, Husar R, Kocianova E, Taborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT)-study protocol. Hypertension. 2011;57:1069–75. doi: 10.5507/bp.155.2011.004. [DOI] [PubMed] [Google Scholar]
- 3.Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2010;25:514–23. doi: 10.1038/ajh.2011.245. [DOI] [PubMed] [Google Scholar]
- 4.Oxlund CS, Henriksen JE, Tarnow L, Schousboe K, Gram J, Jacobsen IA. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31:2094–2102. doi: 10.1097/HJH.0b013e3283638b1a. [DOI] [PubMed] [Google Scholar]
- 5.Ouzan J, Pérault C, Lincoff AM, Carré E, Mertes M. The role of spironolactone in the treatment of patients with refractory hypertension. Am J Hypertens. 2002;15:333–9. doi: 10.1016/s0895-7061(01)02342-1. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Alvarez B, Abad-Cardiel M, Fernandez-Cruz A, Martell-Claros N. Management of resistant arterial hypertension: role of spironolactone versus double blockade of the renin-angiotensin-aldosterone system. J Hypertens. 2010;28:2329–35. doi: 10.1097/HJH.0b013e32833d4c99. [DOI] [PubMed] [Google Scholar]
- 7.Lane DA, Shah S, Beevers DG. Low-dose spironolactone in the management of resistant hypertension: a surveillance study. J Hypertens. 2007;25:891–4. doi: 10.1097/HJH.0b013e328014954d. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Hypertens. 2007;25:1105–87. [Google Scholar]
- 9.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–30. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Statistics in medicine. 1996;15:1237–48. doi: 10.1002/(SICI)1097-0258(19960630)15:12<1237::AID-SIM301>3.0.CO;2-N. discussion 49-52. [DOI] [PubMed] [Google Scholar]
- 11.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 12.Krieger EM, Drager LF, Giorgi DM, Krieger JE, Pereira AC, Barreto-Filho JA, da Rocha Nogueira A, Mill JG. Resistant hypertension optimal treatment trial: a randomized controlled trial. Clin Cardiol. 2014;37:1–6. doi: 10.1002/clc.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–52. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 14.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 15.ReHOT Investigators. Krieger EM, Drager LF, Giorgi DM, Krieger JE, Pereira AC, Barreto-Filho JA, da Rocha Nogueira A, Mill JG. Resistant hypertension optimal treatment trial: A randomized controlled trial. Clin Cardiol. 2014;37:1–6. doi: 10.1002/clc.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–58. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman N, Dobson J, Wilson S, Dahlöf B, Sever PS, Wedel H, Poulter NR Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–45. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 18.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH Jr, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A, Anders RJ CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–82. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 20.Abolghasmi R, Taziki O. Efficacy of low dose spironolactone in chronic kidney disease with resistant hypertension. Saudi J Kidney Dis Transpl. 2011;22:75–8. [PubMed] [Google Scholar]


