Abstract
The major surface lipoglycan of Mycobacterium tuberculosis (M. tb), mannose-capped lipoarabinomannan (ManLAM), is an immunosuppressive epitope of M. tb. Interleukin (IL)-37, is a newly identified anti-inflammatory cytokine, which reduces systemic and local inflammation. However, the correlation between ManLAM and IL-37 remains unknown. Therefore, in this study, we investigate the possible role and relative molecular mechanism of ManLAM in IL-37 production of human type II alveolar epithelial cells by using A549 cell line. Here, we report that M. tb induced IL-37 mRNA and protein expression in a time-dependent manner. We next fractionated components of M. tb using chloroform: methanol (C:M) and water. In sharp contrast to the C:M phase, water phase was mainly responsible for the production of IL-37. Since ManLAM is the major component of water phase, we found that ManLAM induced IL-37 mRNA and protein expression in a time and dose-dependent manner, while this activity was almost totally abolished by the ERK1/2 (U0126) and p38 (SB203580) inhibitor. ManLAM stimulation significantly induced ERK1/2 and p38 phosphorylation in A549 cells, as well as cell surface TLR2 expression. After interfering TLR2 expression, ERK1/2 and p38 phosphorylation levels were markedly decreased, and also IL-37 production. Though ManLAM also promoted TLR4 expression on A549 cells, TLR4 interference showed no influence on ManLAM-induced IL-37 production. Our results indicate that ManLAM induces IL-37 production in human type II alveolar epithelial cells via up-regulating TLR2/p38 or ERK1/2 pathway, and this provide an important evidence to explain the pathological role of ManLAM that contribute to the persistence of M. tb.
Keywords: ManLAM, tuberculosis, IL-37, TLR2, ERK1/2, p38
Introduction
Tuberculosis (TB), usually attacks the lungs, resulting in the pulmonary TB (PTB) [1]. Mycobacterium tuberculosis (M. tb), the etiologic agent of TB, is a ubiquitous and extraordinarily aggressive human pathogen which infects an estimated one-third of the world’s population and causes millions of TB-associated deaths yearly [2]. Mannose-capped lipoarabinomannan (ManLAM), as a kind of lipoglycan, is a major cell wall component and virulence factor of M. tb [3]. It has long been known to have both inhibitory and stimulatory effects on host immunity [4]. Its function has been described to inhibit phagosome-lysosome fusion in macrophages, dendritic cells maturation, CD4+ T-cell activation and recruitment [5-8]. As for the influence of cytokines production, ManLAM increases interleukin (IL)-10 production by dendritic cells (DCs), and depresses its IL-12 cytokine production [7].
IL-37, as a new member of IL-1 family, recently, it has been proved to be a natural suppressor of innate immunity and inflammatory responses in autoimmune diseases and tumors [9-11], and also plays a pivotal role in regulating adaptive immunity by inducing regulatory T cells and impairing activation of effector T-cell responses [12]. Importantly, in bacterial diseases, IL-37 functions as a broad spectrum inhibitor of the innate response to infection-mediated inflammation, and could be considered to be therapeutic in reducing the pulmonary damage [13].
It has been demonstrated that IL-37 mainly expresses on epithelial cells and can be induced by several toll-like receptor (TLR) ligands, like TLR2 and TLR4 [14-16]. Furthermore, intracellular signaling cascades analysis reveals that the expression of IL-37 can be dampened by the extracellular signal-regulated kinase 1 and 2 (ERK1/2) and p38 inhibitors [17]. As another important inhibitory cytokine, enhanced IL-10 production also can be induced by TLRs stimulation and the activation of MAPK family components, ERK1/2 and p38 in DCs [18,19]. Furthermore, ManLAM is a ligand of TLRs and regulates multiple host immune responses through ERK1/2 and p38 activation, like promoting IL-8 and iNOS production with presence of IFN-γ [4,20-24]. Thus, we speculated that ManLAM from M. tb could induce IL-37 production via binding to TLR2 and promoting the activation of p38, ERK1/2 in human type II alveolar epithelial cells.
In the present study, we assessed whether ManLAM purified from M. tb were able to induce IL-37 production by human type II alveolar epithelial cells, namely A549 cells, and examined the relative molecular pathways involved in IL-37 production. Our results showed that ManLAM induced IL-37 production in a time and dose-dependent manner, more importantly, this effect depended on the unregulated expression of TLR2, as well as enhanced p38 and ERK1/2 phosphorylation.
Materials and methods
Cell line and bacteria
The human type II alveolar epithelial cell line A549 (Institute of Biochemistry and Cell Biology, China) was maintained in RPMI-1640 (Hyclone, USA) supplemented with 10% fetal calf serum (FBS, Gibco) at 37°C. M. tb H37Rv (strain American Type Culture Collection (ATCC) 93009) was purchased from the Beijing Biological Product Institute (Beijing, China) and maintained on Lowenstein-Jensen medium and harvested while in the log phase of growth. Bacilli were washed in PBS containing 0.05% Tween-80 and triturated uniformly before use [25].
ManLAM preparation
ManLAM was extracted and purified from delipidated cells as previously described [26]. Purified ManLAM were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, silver staining.
Real-time PCR
Total RNA was extracted from A549 cells using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA was reversely transcribed into cDNA using First Strand cDNA Synthesis Kit (Toyobo). cDNA was quantified using specific primers using a real-time RT-PCR system (Step-one Real-Time PCR system, Applied Biosystems) with the SYBR Green real-time PCR Master Mix kit (Toyobo). Amplification of the IL-37 gene was performed with the following primers: forward, 5’-CAGCCTCTGCGGAGAAAGGAAGT-3’, and revers, 5’-GTTTCTCCTTCTTCAGCTGAAGGGATGGAT-3’ [27]. β-actin [28], forward (5’-TCGTCGACAACGGCTCCGGCATGT-3’) and reverse (5’-CATTGTAGAAGGTGTGGTG-3’), was used for housekeeping gene control.
Western blot
IL-37 was detected using monoclonal antibody [11] and a horseradish peroxidase-labeled goat anti-mouse secondary antibody (Santa Cruz Biotechnology). Phosphorylated and total p38 and ERK1/2 MAPKs were examined by Western blot analysis using MAPK antibody kits (Cell Signaling Technology). β-actin was set as endogenous control. Bands were visualized with Fluor S-MultiImager MAX system (Bio-Rad Laboratories) and quantified by image analysis software (Quantity One, Bio-Rad Laboratories).
ELISA
The level of IL-37 was measured by ELISA following the manufacturer’s instructions respectively. Human IL-37 ELISA reagent Kits were purchased from Cusabio (Wuhan, China), all of the samples were measured in triplicate.
siRNA transfection
siRNA to TLR2 or TLR4 or control siRNA were obtained from life technologies [29,30]. A549 were transfected with 60 pmol siRNA per 5×105 cells and 3 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in serum-free Dulbecco’s modified Eagle’s medium for 4 h, rinsed, and then placed in fresh RPMI-1640 supplemented with 10% FBS.
Flow cytometry
All staining reactions were performed at 4°C on cells first exposed to Fc receptor mAb (Human TruStain FcX™, Biolegend) in order to reduce nonspecific binding. PE labelled anti-TLR2 (TL2.1) and APC labelled anti-TLR4 (HTA125) were purchased from Biolegend. Cells were analyzed with a BD Accuri C6 flow cytometer (BD).
Statistical analysis
Data were expressed as mean ± SD or median (range) and analyzed by Graphpad Prism V.5.00 software (GraphPad Software, San Diego CA, USA). Differences among different groups were tested by one-way ANOVA followed by Neuman-Keuls post hoc test. A two-side p values under 0.05 were considered statistically significant.
Results
Mycobacterium tuberculosis stimulation increases IL-37 expression in A549 cells
Human type II alveolar epithelial cell line A549 cells were stimulated with inactivated M. tb strain H37Rv at a ratio of 10:1 (bacteria to cells), IL-37 mRNA and protein expression were detected at different time-period. As showed in Figure 1A, RT-PCR revealed that the IL-37 mRNA expression level in A549 cells was significantly increased after 12 h of H37Rv stimulation (an approximate 7.6-fold enhancement). Furthermore, protein production analysis also identified that H37Rv promoted IL-37 production in a time-dependent manner (Figures 1B and 3A). These findings suggest that the IL-37 as an inhibitory cytokine might play a role in the response of type II alveolar epithelial cells to M. tb infection.
Figure 1.
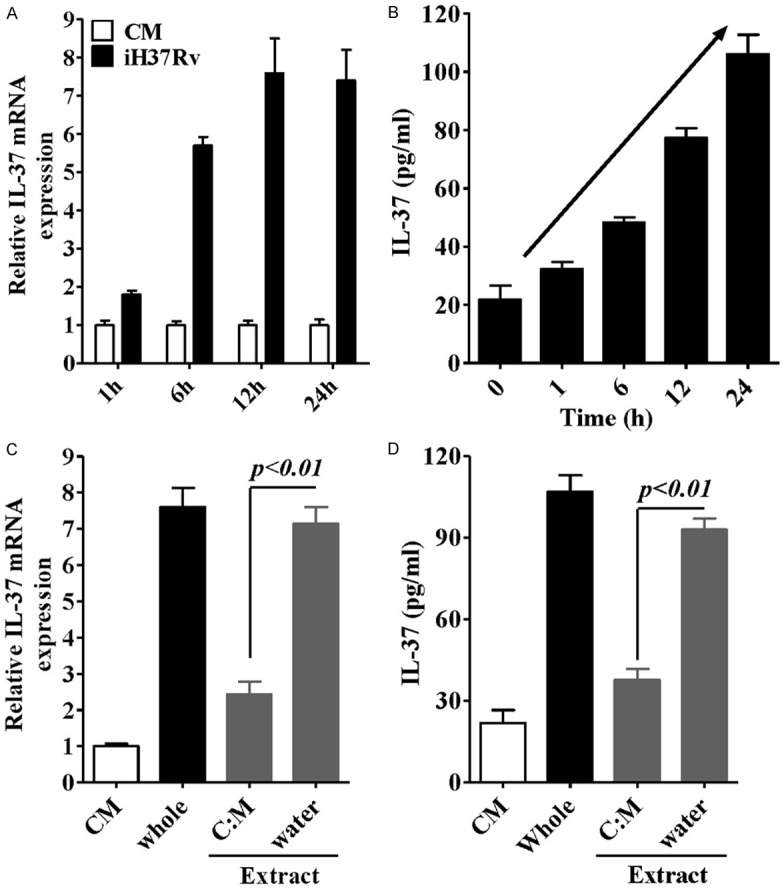
Analysis of the effects of inactivated H37Rv (M.tb strain) on the expression of IL-37 in A549 cells. Real-time PCR (A) and ELISA (B) analysis of IL-37 mRNA or protein expression induced by iH37Rv at different incubation periods. Real-time PCR (C) and ELISA (D) analysis of IL-37 mRNA or protein expression induced by plate-coated water extract or (C) M extract from iH37Rv for 24 h. Data are expressed as mean ± SD.
Figure 3.
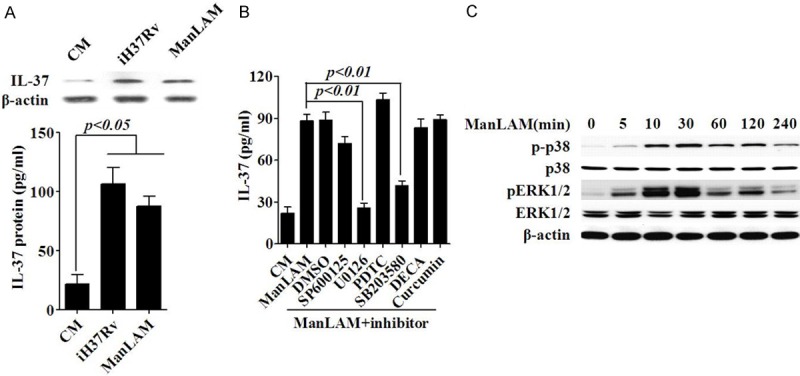
p38 MAPK and ERK1/2 are involved in ManLAM-induced IL-37 production. A. IL-37 production induced by medium (CM), iH37Rv or ManLAM inA549 cells for 24 h were examined by western blotting. B. ELISA analysis of the effect of various signal proteins’ inhibitors on the production of IL-37 in A549 cells induced by ManLAM for 24 h. C. Western blot analysis for detection of ERK1/2, p38, and β-actin activation in A549 cells stimulated by ManLAM. Data are expressed as mean ± SD.
H37Rv cell wall component ManLAM mainly respond for the H37Rv-induced IL-37 expression
We next fractionated components of H37Rv using lipophilic and hydrophilic solvents, such as chloroform: methanol (C:M) and water, The IL-37 expression activity for each of the extracts was assessed in a plate-coated form [4]. We found that only the water phase demonstrated a stimulatory activity for IL-37 expression (Figure 1C and 1D), which showed no significantly difference with whole bacteria stimulation, in sharp contrast to the C:M phase that only showed minor increase for IL-37 expression. These results suggest that the hydrophilic components of mycobacteria are candidates for the IL-37 expression.
Among mycobacterial hydrophilic components, ManLAM constitutes the most abundant hydrophilic lipoglycan [31], we thus further evaluated the ability of ManLAM in inducing IL-37 expression by A549 cells. As shown in Figure 2A, ManLAM upregulated IL-37 mRNA level in a dose-dependent manner, and the maximum up regulation of IL-37 (an approximate 7.1-fold enhancement) was observed in the presence of 10 μg/ml of ManLAM at the 12 h incubation period. More importantly, IL-37 protein production induced by ManLAM showed time and dose-dependent manner (Figure 2B and 2C), and showed no apparent difference with H37Rv treatment (Figure 3A). These findings indicate that ManLAM is the main component of H37Rv with the ability to induce IL-37 production in human type II alveolar epithelial cells.
Figure 2.
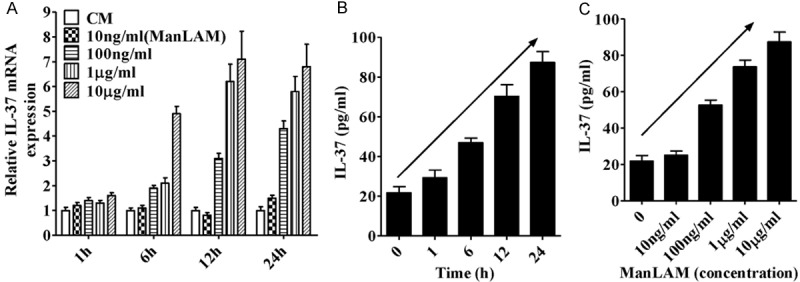
Analysis of the effects of ManLAM extracted from H37Rv on the expression of IL-37 in A549 cells. (A) Real-time PCR analysis of IL-37 mRNA expression induced by ManLAM at different concentrations for various periods. ELISA analysis of IL-37 production induced by ManLAM at different concentrations (C) for various periods (B). Data are expressed as mean ± SD.
p38 and ERK1/2 activation involved in ManLAM induced IL-37 production
To further understand the mechanism by which ManLAM induced the expression of IL-37, inhibitors target to multiple important signal proteins were investigated, like JNK or MAPK pathways. A549 cells were pre-treated with various inhibitors (Cell Signaling Technology) for 30 min, and then, the treated cells were stimulated with ManLAM for 24 h. The results showed that p38 and ERK1/2 inhibitors (SB203580 and U0126) markedly abolished the production of IL-37 induced by ManLAM (Figure 3B), moreover, ManLAM significantly promoted the phosphorylation of p38 and ERK1/2 MAPKs in A549 cells. These results suggest that the p38 MAPK and ERK1/2 pathways may be involved in the upregulation of ManLAM-induced IL-37.
TLR2 involves in ManLAM-induced IL-37 production
The TLR ligands lipopolysaccharide (LPS) and Pam3CSK4 were highly effective in inducing IL-37 [16,32,33], it is worth mentioning that ManLAM is also a ligand of TLRs [4,34]. In A549 cells, ManLAM stimulation significantly induced TLR2 and TL4 expression, more importantly, after knockdown TLR2 expression, ManLAM-induced IL-37 production was almost completely abrogated, as well as the phosphorylation of p38 and ERK1/2. While TLR4 knockdown only showed negligible influence in ManLAM-induced IL-37 production (Figure 4A and 4B). Thus, TLR2 deficiency resulted in decreased IL-37 production presumably due to the downregulation of its downstream signal proteins p38 and ERK1/2 activation (Figure 4C). Collectively, these results suggest that ManLAM enhances IL-37 production in human type II alveolar epithelial cells mainly by regulating TLR2 expression as well as p38 and ERK1/2 phosphorylation.
Figure 4.
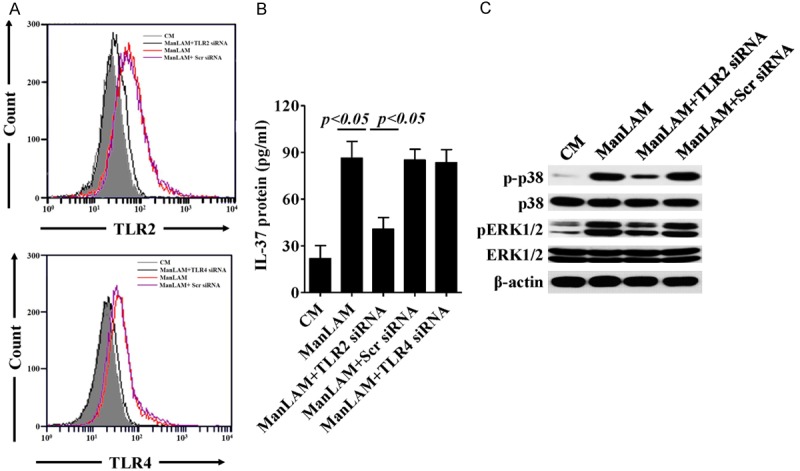
TLR2 involves in ManLAM-induced IL-37 production in A549 cells. (A) Flow cytometry analysis of TLR2 or TLR4 expression in A549 cells stimulated by ManLAM for 24 h, TLR2 or TLR4 expression in A549 cells were intervened by its specific siRNA before ManLAM stimulation, respectively. (B) ELISA analysis of IL-37 production in cell supernatant of A549 cells same treated as (A). (C) Western blot analysis for detection of ERK1/2, p38, and β-actin activation in A549 cells stimulated by ManLAM, with or without the presence of TLR2 siRNA. Data are expressed as mean ± SD. Scr, scramble; CM, control medium.
Discussion
In this study, we firstly demonstrate that a M. tb virulence factor, ManLAM, is capable of inducing IL-37 production in human type II alveolar epithelial cells. This activity was accomplished by the promotion of TLR2 expression, and also the upregulation of p38 and ERK1/2 phosphorylation. Since the first description of IL-37 [35], IL-37 serve as a fundamental inhibitor of innate immunity [16], its mRNA and protein have been detected in inflammatory and autoimmune diseases, as well as tumors [11], such as rheumatoid arthritis [16], atopic dermatitis [36], inflammatory bowel disease [15] and systemic lupus erythematosus [37]. Nowadays, the inhibitory activity of IL-37 has been expanded in adaptive immunity [12] and fungi infection [13], especially, IL-37 reduces the pulmonary damage in invasive aspergillosis infection.
In mycobacterium tuberculosis (M. tb) infection, M. tb is typically restricted in lung and causes pathological injury. Recently, alveolar epithelial cells were identified as important mediators of the immune response to respiratory pathogens. Several in vitro studies have demonstrated that type II alveolar pneumocytes are capable of internalizing M. tb, and once inside the type II alveolar cells, the bacteria have been shown to replicate extensively [28,38], we thus speculated that M. tb may facilitate its replication by promoting IL-37 production of alveolar cells.
Human type II alveolar cell line A549 cells were stimulated with inactivated M. tb strain H37Rv for different time period. From the results, we found that H37Rv surely promoted the mRNA expression and protein production of IL-37. As for the first to report M. tb has the ability to induce IL-37 production, we further want to identify which component was attributed to H37Rv-induced IL-37.
H37Rv cells were fractionated as two components by using lipophilic and hydrophilic solvents, such as chloroform: methanol (C:M) and water, we found that only the water phase demonstrated an apparent stimulatory activity for IL-37 production. LPS has been reported to induce IL-37 in A549 cells [16], although mycobacteria do not possess LPS, the cell wall of M. tb contains a complex lipid glycoprotein called ManLAM which shares many physicochemical properties with LPS [4,21,39]. Among mycobacterial hydrophilic components, ManLAM constitutes the most abundant hydrophilic lipoglycan, which has been shown to suppress host immune system [40], one of the key events is the production of immunosuppressive cytokine IL-10.
In A549 cells, ManLAM induced IL-37 mRNA and protein expression in time and concentration-dependent manner. Furthermore, though IL-37 production is slightly lower in ManLAM-treated A549 cells, there is no significant difference in IL-37 production between H37Rv and ManLAM after 24 h incubation, this slight decrease may own to other inhibitory components of H37Rv, like mycolylarabinogalactan peptidoglycan (mAGP) complex [41] and LprG [42], which need to be further identified. IL-37 inhibits pro-inflammatory cytokines production, like IL-1β, IL-6 and TNF, in autoimmune diseases and fungi infection [13,16,43], and also the effects of ManLAM or H37Rv induced IL-37 on various pro-inflammatory cytokines could be determined in future studies.
The MAPK signaling pathway is one of the major signaling pathways in inflammation. As for IL-37 production, it can be inhibited by p38 and ERK1/2 inhibitors in THP1 cells [17]. ManLAM not only activated p38 MAPK and MEK/ERK phosphorylation to arrest mycobacterial phagosome maturation [44,45] but also inhibited pro-inflammatory cytokines production, like IL-8 [23], and stimulated IL-10 production [46]. Our experimental results showed that ManLAM promoted p38 and ERK1/2 phosphorylation in A549 cells, and also inhibitors specific to these two proteins inhibited ManLAM-induced IL-37 production (Figure 4A and 4B). However, JNK inhibitor SP600125 also inhibited IL-37 production and further detailed investigation is required to address the mechanism of IL-37 upregulation induced by ManLAM.
ManLAM can be recognized by TLR2 and TLR4 [21,47], which have been found to mediate IL-37 production [16]. Furthermore, TLR ligands also promote IL-10 production by dendritic cells (DC) through p38 and ERK1/2 MAPK pathways [19,48]. After stimulated with ManLAM for 24 h, TLR2 and TLR4 expression in cell surface of A549 cells all significantly enhanced, while only TLR2 knockdown decreased ManLAM-induced IL-37 production. More importantly, TLR2 interfere also markedly reduced p38 and ERK1/2 phosphorylation level with the stimulation of ManLAM.
In conclusion, this study was the first to demonstrate that ManLAM is the main cell component of M. tb with the ability to induce IL-37 in human type II alveolar cells, which is mediated by upregulating TLR2 expression, ERK1/2 and p38 MAPK phosphorylation. These findings have revealed previously unrecognized immunoregulatory effects of ManLAM from M. tb.
Disclosure of conflict of interest
None.
References
- 1.Rasolofo Razanamparany V, Menard D, Auregan G, Gicquel B, Chanteau S. Extrapulmonary and pulmonary tuberculosis in antananarivo (madagascar): high clustering rate in female patients. J Clin Microbiol. 2002;40:3964–3969. doi: 10.1128/JCM.40.11.3964-3969.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zumla A, George A, Sharma V, Herbert RH, Oxley A, Oliver M. The WHO 2014 Global tuberculosis report-further to go. Lancet Glob Health. 2015;3:e10–12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 3.Jozefowski S, Sobota A, Pawlowski A, Kwiatkowska K. Mycobacterium tuberculosis lipoarabinomannan enhances LPS-induced TNF-alpha production and inhibits NO secretion by engaging scavenger receptors. Microb Pathog. 2011;50:350–359. doi: 10.1016/j.micpath.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Yonekawa A, Saijo S, Hoshino Y, Miyake Y, Ishikawa E, Suzukawa M, Inoue H, Tanaka M, Yoneyama M, Oh-Hora M, Akashi K, Yamasaki S. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity. 2014;41:402–413. doi: 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci U S A. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahon RN, Sande OJ, Rojas RE, Levine AD, Harding CV, Boom WH. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol. 2012;275:98–105. doi: 10.1016/j.cellimm.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweet L, Singh PP, Azad AK, Rajaram MV, Schlesinger LS, Schorey JS. Mannose receptor-dependent delay in phagosome maturation by Mycobacterium avium glycopeptidolipids. Infect Immun. 2010;78:518–526. doi: 10.1128/IAI.00257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DE, Renshaw BR, Ketchem RR, Kubin M, Garka KE, Sims JE. Four new members expand the interleukin-1 superfamily. J Biol Chem. 2000;275:1169–1175. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- 10.McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ, Lv L, Wang DD, Zheng HX, Jiang SS, Zhang XF, Xia JC. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep. 2014;4:5177. doi: 10.1038/srep05177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Cai X, Liu S, Wang S, Nold-Petry CA, Nold MF, Bufler P, Norris D, Dinarello CA, Fujita M. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci U S A. 2014;111:15178–15183. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S, van de Veerdonk FL, Dinarello CA, Romani L. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10:e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381:503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, Sonoda H, Shimizu T, Fujiyama Y, Andoh A. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410–416. doi: 10.1111/cei.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, Liang Z, Zhao F, Peng L, Chen Z. Modulation of IL-37 expression by triptolide and triptonide in THP-1 cells. Cell Mol Immunol. 2014 doi: 10.1038/cmi.2014.92. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagawa Y, Onoe K. Enhanced IL-10 production by TLR4- and TLR2-primed dendritic cells upon TLR restimulation. J Immunol. 2007;178:6173–6180. doi: 10.4049/jimmunol.178.10.6173. [DOI] [PubMed] [Google Scholar]
- 19.Qian C, Jiang X, An H, Yu Y, Guo Z, Liu S, Xu H, Cao X. TLR agonists promote ERK-mediated preferential IL-10 production of regulatory dendritic cells (diffDCs), leading to NK-cell activation. Blood. 2006;108:2307–2315. doi: 10.1182/blood-2006-03-005595. [DOI] [PubMed] [Google Scholar]
- 20.Elass E, Coddeville B, Kremer L, Mortuaire M, Mazurier J, Guerardel Y. Mycobacterial lipomannan induces MAP kinase phosphatase-1 expression in macrophages. FEBS Lett. 2008;582:445–450. doi: 10.1016/j.febslet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek J, Ignatowicz L, Kallenius G, Svenson SB, Pawlowski A, Hamasur B. Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells. PLoS One. 2012;7:e42515. doi: 10.1371/journal.pone.0042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 2002;4:937–944. doi: 10.1016/s1286-4579(02)01611-8. [DOI] [PubMed] [Google Scholar]
- 23.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor ga- mma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DW. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways. Infect Immun. 2001;69:2001–2010. doi: 10.1128/IAI.69.4.2001-2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Q, Wang Q, Sun X, Xia X, Wu S, Luo F, Zhang XL. Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis infection in mice and rhesus monkeys. Mol Ther. 2014;22:940–951. doi: 10.1038/mt.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 27.Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P, Boekschoten MV, Muller M, Kersten S, Li S, Kim S, Eini H, Lewis EC, Joosten LA, Tilg H, Netea MG, Tack CJ, Dinarello CA, Stienstra R. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:4711. doi: 10.1038/ncomms5711. [DOI] [PubMed] [Google Scholar]
- 28.Guo XG, Ji TX, Xia Y, Ma YY. Autophagy protects type II alveolar epithelial cells from Mycobacterium tuberculosis infection. Biochem Biophys Res Commun. 2013;432:308–313. doi: 10.1016/j.bbrc.2013.01.111. [DOI] [PubMed] [Google Scholar]
- 29.Devaraj S, Jialal I. Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine. 2009;47:6–10. doi: 10.1016/j.cyto.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79:12658–12666. doi: 10.1128/JVI.79.20.12658-12666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leopold K, Fischer W. Molecular analysis of the lipoglycans of Mycobacterium tuberculosis. Anal Biochem. 1993;208:57–64. doi: 10.1006/abio.1993.1008. [DOI] [PubMed] [Google Scholar]
- 32.Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C, Posselt G, Italiani P, Nold MF, Nold-Petry CA, Bufler P, Dinarello CA. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127–147. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 33.Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003;9:264–268. doi: 10.1179/096805103225001477. [DOI] [PubMed] [Google Scholar]
- 34.Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev. 2011;35:1126–1157. doi: 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, Capper EA, Tal-Singer R, Wells GI, Doyle ML, Young PR. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275:10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 36.Fujita H, Inoue Y, Seto K, Komitsu N, Aihara M. Interleukin-37 is elevated in subjects with atopic dermatitis. J Dermatol Sci. 2013;69:173–175. doi: 10.1016/j.jdermsci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu T, Chen B, Zhang J, Ding L, Du J, Huang Z. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. J Transl Med. 2014;12:69. doi: 10.1186/1479-5876-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adlakha N, Vir P, Verma I. Effect of mycobacterial secretory proteins on the cellular integrity and cytokine profile of type II alveolar epithelial cells. Lung India. 2012;29:313–318. doi: 10.4103/0970-2113.102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatterjee D, Roberts AD, Lowell K, Brennan PJ, Orme IM. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992;60:1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 41.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ, Ernst JD. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 42.Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang K, Huang Z. Increased expression of IL-37 in patients with Graves’ disease and its contribution to suppression of proinflammatory cytokines production in peripheral blood mononuclear cells. PLoS One. 2014;9:e107183. doi: 10.1371/journal.pone.0107183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fratti RA, Chua J, Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J Biol Chem. 2003;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
- 45.Vergne I, Chua J, Singh SB, Deretic V. Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 46.Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, Larrouy-Maumus G, Gurcha SS, Movahedzadeh F, Geurtsen J, Brown EJ, Eysink Smeets MM, Besra GS, Willemsen PT, Lowary TL, van Kooyk Y, Maaskant JJ, Stoker NG, van der Ley P, Puzo G, Vandenbroucke-Grauls CM, Wieland CW, van der Poll T, Geijtenbeek TB, van der Sar AM, Bitter W. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang CY, Bai N, Zhang ZH, Liang N, Dong L, Xiang R, Liu CH. TLR2 signaling subpathways regulate TLR9 signaling for the effective induction of IL-12 upon stimulation by heat-killed Brucella abortus. Cell Mol Immunol. 2012;9:324–333. doi: 10.1038/cmi.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarnicki AG, Conroy H, Brereton C, Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J, Lavelle EC, Dunne P, Mills KH. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180:3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]


