Abstract
Objective: To investigate correlations among A-kinase anchor protein95 (AKAP95), Connexin43 (Cx43), CyclinE1 and CyclinD1 in esophageal squamous cell cancer tissues, and their relationship with clinical and pathological parameters. Methods: The protein levels of AKAP95, Cx43, CyclinE1 and CyclinD1 in 54 cases of esophageal squamous cell cancer tissues were determined by immunohistochemistry. Results: The expression of AKAP95, CyclinE1 and CyclinD1 in esophageal squamous cell cancer tissues (53.70%, 88.89%, 72.22%, respectively) was significantly increased when compared to pericarcinoma tissues (20.00%, P < 0.05; 6.67%, P < 0.01; and 20.00%, P < 0.05; respectively). By contrast, Cx43 expression in esophageal squamous cell cancer tissues (22.22%) was lower than that in pericarcinoma tissues (60.00%, P < 0.05). The expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in the tissues of esophageal squamous cell carcinoma was unrelated to lymph node metastasis and the degree of differentiation. The expression of Cx43, CyclinE1, CyclinD1 in the tissues of esophageal squamous cell carcinoma was significantly correlated with AKAP95, respectively (P < 0.05). Conclusion: Expression levels of AKAP95, CyclinE1 and CyclinD1 were higher, and that of Cx43 lower in esophageal squamous cell carcinoma tissues as compared pericarcinoma tissues, which suggests their importance in the incidence and development of esophageal squamous cell carcinoma. The expression of Cx43, CyclinE1, CyclinD1 in the tissues of esophageal squamous cell carcinoma was correlated with AKAP95, respectively. The expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in the tissues of esophageal squamous cell carcinoma was unrelated to the degree of differentiation and lymph node metastasis.
Keywords: Esophageal cancer, AKAP95, Cx43, CyclinE1, CyclinD1, correlation analyses
Introduction
Esophageal cancer is one of the most common human malignancies, and the incidence of esophageal squamous cell carcinoma is the most common. CyclinD1 and CyclinE1 are members of the CyclinD and E family of proteins that play a key role in promoting cell cycle. The expression of both CyclinD1 and CyclinE1 is increased in a variety of tumor tissues [1-4].
Tatjana et al. found that A-kinase anchor protein95 (AKAP95) forms a complex with CyclinD/E and PKA in Chinese hamster ovary (CHO) cells [5]. Further studies showed that Connexin43 (Cx43) is down-regulated in a variety of tumors [6-8]. The phosphorylation sites, Ser365, Ser368, Ser369 and Ser373, of Cx43 are phosphorylated by PKA in rat granulosa cells, which in turn increases the formation of the cell gap junction, and enhances cellular communication [9].
Moorby et al. found that the G2/M phase of the cell cycle was increased when Cx43-deficient mutants were expressed in 3T3 cells [10]. In addition, we have previously shown that the expression of AKAP95 is correlated to Cx43 expression in lung cancer tissues [11].
In the current study, we used an immunohistochemical approach to investigate the protein expression levels of AKAP95, Cx43, CyclinD1 and CyclinE1 in the tissues of 54 cases of esophageal squamous cell carcinoma, and analyzed the correlations among them.
Materials and methods
Tissue collection
A total of 54 cases of esophageal carcinoma, comprising 1 female and 53 males subjects, with a mean age of 59.8 years (range 38-82), were diagnosed at the First Affiliated Hospital of Liaoning Academy of Medical Sciences from 2010 to 2011 (and confirmed by pathology diagnosis). Among them, 25 cases presented with lymph node metastasis, 28 cases lacked lymph node metastasis and one case could not be confirmed. Further, 19, 29 and 6 cases displayed high, moderate and low differentiation, respectively. Control group specimens were collected from tissues that were located 3 cm from esophageal carcinoma tissues, and included 15 cases of pericarcinoma that were identified by pathology and confirmed that the tissue specimens lacked detectable cancer cells. The study was approved by the ethics committee of Xiamen university (Xiamen, China), and the written informed patient consents were obtained from the patients or the patients’ family.
Reagents and methods
All the specimens were fixed in 10% formalin, embedded in paraffin, and cut in serial sections (4 µm). The staining was performed using an SP immunohistochemical kit (Kit-9710) according to the manufacturer’s instructions (Fuzhou Maxim Biotech Inc., China), and then color-developed with DAB reagent and counterstained with hematoxylin. The antibodies included mouse anti-human CyclinE1 (sc-247, 1:300), AKAP95 (sc-100643, 1:150), Cx43 (sc-13558, 1:300) and CyclinD1 (sc-20044, 1:300) monoclonal antibodies (Santa Cruz Biotechnology, Inc., Dallas, Texas, USA). The negative control was PBS instead of antibodies.
Positive judgment standard
The staining of tissue specimens with a brownish-yellow color and without a brownish- yellow color was considered the positive and negative assay end-points, respectively. Ten different fields of view of each section were randomly selected, and 200 tumor cells in each field were counted. The frequency of positive cells was considered as the judgment parameter, and the judgment standard was set as follows:“-” (0 ≤ the percentage of positive cells < 10%), “+-” (10 ≤ the percentage of positive cells < 25%), “+” (25 ≤ the percentage of positive cells < 50%), “++” (50 ≤ the percentage of positive cells < 75%) and “+++” (the percentage of positive cells ≥ 75%). In addition, “-” and “+-” were regarded as negative staining, “+”, “++” and “+++” were regarded as positive staining.
Statistical analysis
Statistical analysis of the data was carried out using SPSS13.0 software (SPSS Inc., Chicago, IL, USA) by the X2 test. Correlation analyses of differential expressed proteins was analyzed by Spearman’s rank correlation coefficient. An alpha value of P < 0.05 was considered statistically significant.
Results
The expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in esophageal carcinoma and pericarcinoma tissues
Table 1 summarizes the data. In addition, the percentage of AKAP95 positive expression in esophageal carcinoma tissues was 53.70% (29/54 samples), which significantly decreased to 20.00% (3/15 samples) in pericarcinoma tissues (P < 0.05). AKAP95 protein expression was predominantly localized to the nucleus, with minimal expression found in the cytoplasm (Figure 1).
Table 1.
The expression AKAP95, CyclinE1, CyclinD1 and Cx43 in esophageal carcinoma tissues
| Protein | Results | Tumor | Pericarcinoma tissues | X2 | P |
|---|---|---|---|---|---|
| AKAP95 | Positive | 29 | 3 | 5.362 | 0.021 |
| Negative | 25 | 12 | |||
| CyclinE1 | Positive | 48 | 1 | 34.664 | <0.0001 |
| Negative | 6 | 14 | |||
| CyclinD1 | Positive | 39 | 6 | 5.373 | 0.02 |
| Negative | 15 | 9 | |||
| Cx43 | Positive | 12 | 9 | 6.229 | 0.013 |
| Negative | 42 | 6 |
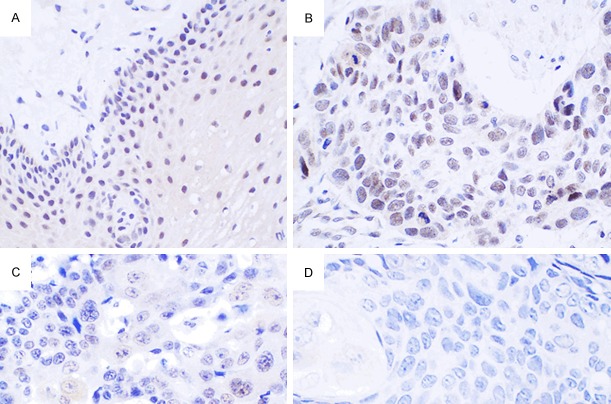
Figure 1.
Expression of AKAP95 in pericarcinoma and esophageal carcinoma tissues. (A) AKAP95 is mainly expressed in the nucleus with minimal expression in the cytoplasm of pericarcinoma tissues. (B-D) In esophageal carcinoma tissues, AKAP95 is expressed in the nucleus of moderately differentiated tumor tissues (B), is weakly expressed in the cytoplasm, and minimally expressed in the nucleus of poorly differentiated tumor tissues (C), and is not expressed in highly differentiated tumor tissues (D). (Magnification, × 400).
In addition, 12 out of 54 (22.22%) cases of esophageal carcinoma tissues showed positive Cx43 expression. Moreover, we found that the positive expression rate of Cx43 had significantly increased to 60.00% (9 out of 15 samples) in pericarcinoma, and showed marked difference when compared to esophageal carcinoma tissues (P < 0.05). Cx43 protein was predominantly localized to the cytoplasm, and minimal nuclear expression (Figure 2).
Figure 2.
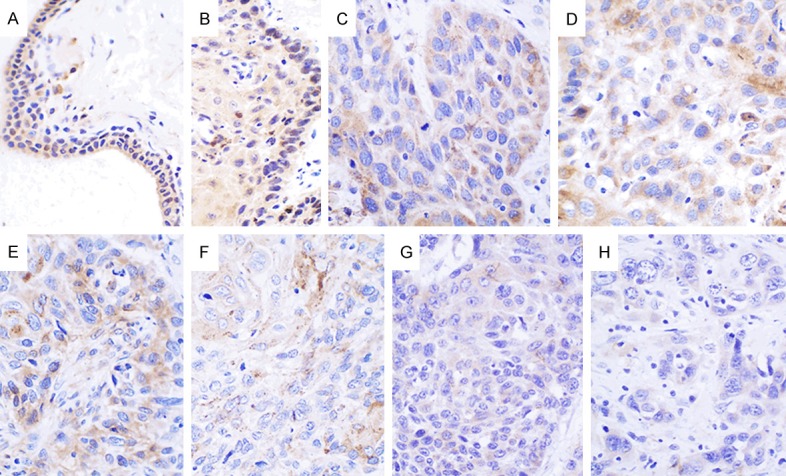
Expression of Cx43 in pericarcinoma and esophageal carcinoma tissues. Cx43 is expressed in the cytoplasm and minimally expressed in the nucleus of pericarcinoma tissues (A, B). Cx43 is also expressed in the cytoplasm of highly differentiated tumor tissues (C, D). Cx43 is weakly expressed in the cytoplasm of moderately differentiated tumor tissues (E, F). Cx43 is weakly expressed in the cytoplasm of poorly differentiated tumor tissues (G, H). (Magnification, × 400).
We found that 48 out of 54 (88.89%) samples of esophageal carcinoma tissues showed positive CyclinE1 expression. However, only one of 15 (6.67%) cases of pericarcinoma tissues samples was positive for CyclinE1, which was markedly lower than that found in esophageal carcinoma tissues (P < 0.05). CyclinE1 protein expression was mainly localized to the cytoplasm, with minimal nuclear expression in both esophageal carcinoma and pericarcinoma tissues (Figure 3).
Figure 3.
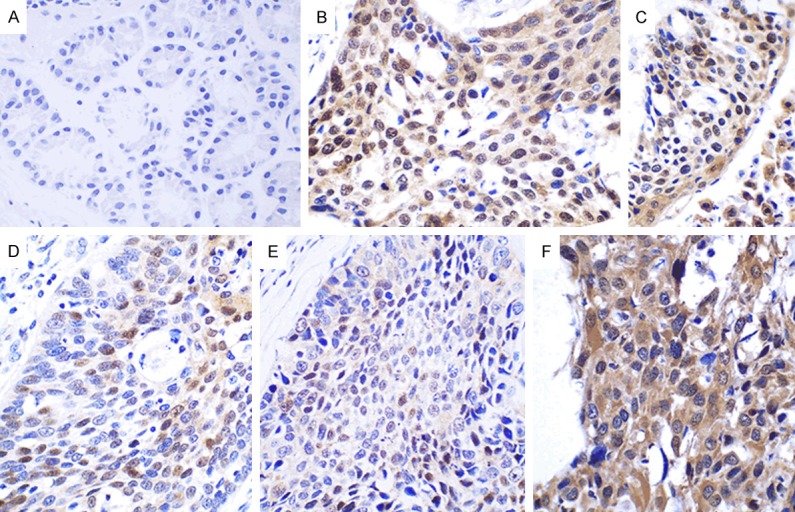
Expression of CyclinE1 in pericarcinoma and esophageal carcinoma tissues. A. Pericarcinoma tissues lack CyclinE1 expression. B. Nuclear CyclinE1 expression of highly differentiated tumors. C. Cytoplasmic and nuclear co-expression of CyclinE1 in highly differentiated tumors. D. CyclinE1 is expressed in the nucleus and weakly expressed in the cytoplasm of moderately differentiated tissues. E. CyclinE1 is weakly expressed in the nucleus and cytoplasm of moderately differentiated tumors. F. CyclinE1 is co-expressed in the cytoplasm and nucleus of poorly differentiated tumors. (Magnification, × 400).
There were 39 out of 54 (72.22%) cases of esophageal carcinoma tissues that showed positive CyclinD1 expression. However, this expression level was dramatically decreased in pericarcinoma tissues (P < 0.05), where only 6 out of 15 (40.00%) cases of pericarcinoma tissues were positive for CyclinD1 expression. CyclinD1 protein expression localized to the cytoplasm predominantly, and minimal expression levels were nuclear localized (Figure 4).
Figure 4.
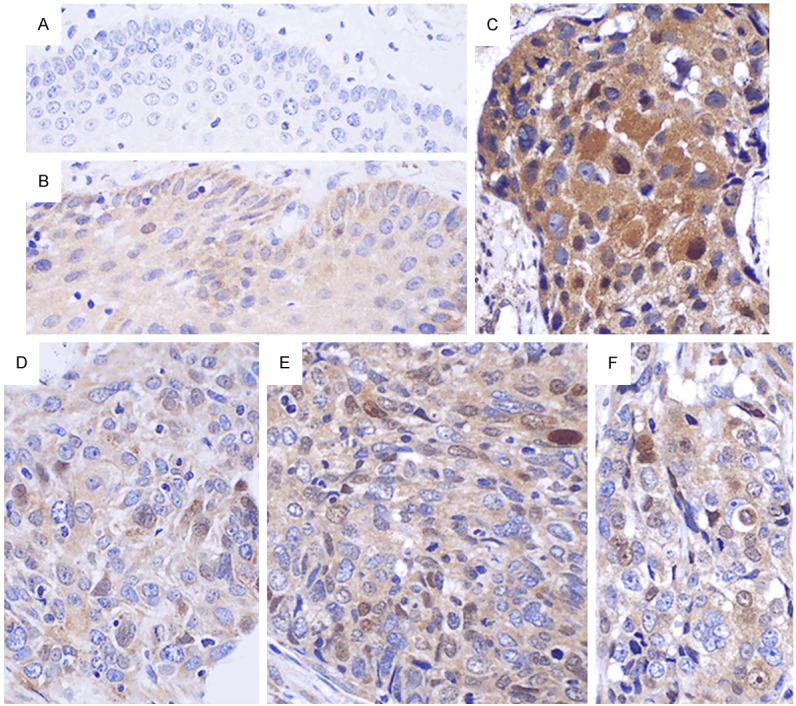
Expression of CyclinD1 in pericarcinoma and esophageal carcinoma tissues. A. Pericarcinoma tissues express CyclinD1 negatively. B. CyclinD1 is expressed in the cytoplasm of pericarcinoma tissues. C. CyclinD1 is expressed in the cytoplasm and poorly expressed in the nucleus of highly differentiated tumors. D. CyclinD1 is mainly expressed in the cytoplasm of highly differentiated tumors. E. CyclinD1 is co-expressed in the cytoplasm and nucleus of moderately differentiated tumors. F. CyclinD1 is mainly expressed in the cytoplasm and minimally expressed in the nucleus of poorly differentiated tumors. (Magnification, × 400).
The expression of AKAP95, CyclinE1, CyclinD1 and Cx43, and their association with clinical and pathological parameters
As shown in Table 2, the expression of AKAP95, CycinE1, CycinD1 and Cx43, was not associated with the extent of tumor differentiation, or lymph node metastasis (P > 0.05).
Table 2.
The expression of AKAP95, CyclinE1, CyclinD1 and Cx43 in esophageal carcinoma, and their correlation with clinical and pathological parameters
| Characteristics | N | AKAP95 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Differentiation | |||||
| High | 19 | 9 | 10 | 0.470 | 0.856 |
| Moderate | 29 | 14 | 15 | ||
| Low | 6 | 2 | 4 | ||
| Lymph node | |||||
| Postive | 25 | 11 | 14 | 0.191 | 0.662 |
| Negative | 28 | 14 | 14 | ||
|
| |||||
| Characteristics | N | CyclinE1 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Differentiation | |||||
| High | 19 | 18 | 1 | 3.261 | 0.216 |
| Moderate | 29 | 26 | 3 | ||
| Low | 6 | 4 | 2 | ||
| Lymph node | |||||
| Positive | 25 | 22 | 3 | 0.018 | 0.894 |
| Negative | 28 | 26 | 2 | ||
|
| |||||
| Characteristics | N | CyclinD1 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Differentiation | |||||
| High | 19 | 11 | 8 | 2.971 | 0.226 |
| Moderate | 29 | 23 | 6 | ||
| Low | 6 | 5 | 1 | ||
| Lymph node | |||||
| Positive | 25 | 18 | 7 | 0.002 | 0.963 |
| Negative | 28 | 20 | 8 | ||
|
| |||||
| Characteristics | N | Cx43 | |||
|
| |||||
| Positive | Negative | X2 | P | ||
|
| |||||
| Differentiation | |||||
| High | 19 | 3 | 16 | 2.747 | 0.253 |
| Moderate | 29 | 6 | 23 | ||
| Low | 6 | 3 | 3 | ||
| Lymph node | |||||
| Positive | 25 | 5 | 20 | 0.189 | 0.664 |
| Negative | 28 | 7 | 21 | ||
The correlation among AKAP95, CyclinE1, CyclinD1 and Cx43 in esophageal carcinoma
There were significant correlations between the expression of AKAP95 and CyclinE1, AKAP95 and CyclinD1, as well as AKAP95 and Cx43 (P < 0.05, Tables 3, 4 and 5). By contrast, there was an insignificant correlation between CyclinE1 and CyclinD1, CyclinE1 and Cx43, as well as CyclinD1 and Cx43 (P > 0.05, Tables 6, 7 and 8).
Table 3.
Correlation analysis of AKAP95 and CyclinE1 in esophageal carcinoma tissues
| AKAP95 | CyclinE1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 2 | 3 | 1 | 3 | 5 | 0.294 | 0.031 |
| +- | 1 | 0 | 2 | 5 | 7 | ||
| + | 0 | 0 | 2 | 3 | 15 | ||
| ++ | 0 | 0 | 1 | 2 | 2 | ||
| +++ | 0 | 0 | 0 | 0 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Table 4.
Correlation analysis of AKAP95 and CyclinD1 in esophageal carcinoma tissues
| AKAP95 | CyclinD1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 5 | 1 | 3 | 3 | 2 | 0.227 | 0.042 |
| +- | 1 | 4 | 5 | 5 | 0 | ||
| + | 1 | 3 | 8 | 4 | 4 | ||
| ++ | 0 | 0 | 1 | 3 | 1 | ||
| +++ | 0 | 0 | 0 | 0 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Table 5.
Correlation analysis of AKAP95 and Cx43 in esophageal carcinoma tissues
| AKAP95 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 9 | 5 | 0 | 0 | 0 | 0.360 | 0.007 |
| +- | 7 | 5 | 3 | 0 | 0 | ||
| + | 5 | 8 | 6 | 1 | 0 | ||
| ++ | 2 | 1 | 1 | 0 | 1 | ||
| +++ | 0 | 0 | 0 | 0 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Table 6.
Correlation analysis of CyclinE1 and CyclinD1 in esophageal carcinoma tissues
| CyclinE1 | CyclinD1 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 2 | 0 | 0 | 0 | 1 | 0.138 | 0.321 |
| +- | 1 | 0 | 1 | 1 | 0 | ||
| + | 0 | 1 | 3 | 2 | 0 | ||
| ++ | 2 | 1 | 5 | 4 | 1 | ||
| +++ | 2 | 6 | 8 | 8 | 5 | ||
rs: Spearman’s rank correlation coefficient.
Table 7.
Correlation analysis of CyclinE1 and Cx43 in esophageal carcinoma tissues
| CyclinE1 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 2 | 1 | 0 | 0 | 0 | 0.030 | 0.827 |
| +- | 2 | 1 | 0 | 0 | 0 | ||
| + | 0 | 5 | 1 | 0 | 0 | ||
| ++ | 6 | 3 | 4 | 0 | 0 | ||
| +++ | 13 | 9 | 5 | 1 | 1 | ||
rs: Spearman’s rank correlation coefficient.
Table 8.
Correlation analysis of CyclinD1 and Cx43 in esophageal carcinoma tissues
| CyclinD1 | Cx43 | rs | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | +- | + | ++ | +++ | |||
| - | 5 | 2 | 0 | 0 | 0 | 0.212 | 0.124 |
| +- | 4 | 3 | 1 | 0 | 0 | ||
| + | 6 | 5 | 6 | 0 | 0 | ||
| ++ | 5 | 8 | 1 | 0 | 1 | ||
| +++ | 3 | 1 | 2 | 1 | 0 | ||
rs: Spearman’s rank correlation coefficient.
Discussion
Esophageal cancer is one of the most common malignancies. Therefore, studies that focus on the molecular mechanism that underlies the incidence and development of this cancer are very important. As a protein kinase A (PKA) carrier protein, AKAP95 phosphorylates targeted proteins by anchoring the RII subunit of PKA, which is mainly expressed in the nucleus. Moreover, AKAP95 is involved in cAMP-mediated transduction of multiple signaling pathways [12,13]. The zinc finger structural motifs (ZF1 and ZF2) of AKAP95 are involved in chromosome condensation during cellular mitosis [14], and AKAP95 provides a scaffolding role for mini-chromosome maintenance protein 2 (MCM2) during DNA replication [15]. The studies conducted by Kamada et al. showed that AKAP95 exhibits a role in apoptosis by binding Caspase 3 [16].
Both CyclinD1 and CyclinE1 regulate the G1/S phase transition of the cell cycle and are over-expressed in many cancer tissues. The protein Cx43 is a tumor suppressor, and is down-regulated in a variety of tumors. In the current study, we found that the expression of AKAP95, CyclinD1 and CyclinE1 were increased, and the expression of Cx43 was reduced in esophageal cancer tissues, which were consistent with previous findings [1,3,7,11]. The studies by Arsenijevic et al. had shown that AKAP95 could bind with CyclinD/E to form a CyclinD/E-AKAP95-PKA complex in CHO cells [5]. Moreover, AKAP95 can competitively replace the binding of CDK4 and CyclinD3, and the binding of CDK2 and CyclinE1, which suggests that AKAP95 might regulate the cell cycle through CyclinD/E [5]. The studies conducted by Moorby et al. found that the expression of Cx43-deficient mutants in NIH 3T3 cells, prolonged the G2/M phase of the cell cycle [10], suggesting that Cx43 is involved in the regulation of the cell cycle.
In this study, we found that the expression of AKAP95 was correlated with CyclinD1, CyclinE1 and Cx43 in esophageal carcinoma tissues, which is consistent with our previous findings in the tissues of lung cancer [11,17]. However, AKAP95 is correlated with CyclinE1, but not with CyclinD1 and Cx43 in colorectal cancer tissues (data not shown). This contrasting phenomenon may be caused by histological differences. Combined with previous findings, our study suggests that the pro-tumorigenic effect of AKAP95 might be related to CyclinD1 and CyclinE1. However, the correlation between Cx43 and AKAP95 in esophageal carcinoma tissues suggested that Cx43 might play a negative role in the pro-tumorigenic effect of AKAP95.
Previously, it was shown that the phosphorylation of pRb by CyclinD is a precondition for the transcription of CyclinE that is activated by transcription factor E2F [18]. Caldon et al. found that in the model of estrogen-induced proliferation, c-Myc and CyclinD1 activate the CyclinE2-CDK2 complex by blocking and/or down-regulating the expression of the CDK inhibitor p21Waf1/Cip1. Moreover, the inducible expression of CyclinD1 can upregulate levels of CyclinE2, and CyclinE2-CDK2 activation by estrogen occurs via E2F- and CHD8-mediated transcription of cyclinE2 downstream of cyclinD1 [19]. These findings suggest that there is a potential correlation between CyclinD and E, which is also supported by our previous studies in colorectal cancer tissues, where we found that the expression of both CyclinD and E was correlated (data not shown). However, this correlation was not found in esophageal squamous cell carcinoma tissues and lung cancer tissues (data not shown), which is consistent with the studies conducted by Anayama et al. wherein it was shown that there was no correlation in esophageal squamous cell carcinoma tissues [20]. There differential results that were seen in different tissues may be associated with earlier expression of CyclinD1 relative to CyclinE1 in the G1 phase of the cell cycle [21], which contribute to the differential timing of the cell cycle. Similar findings were conducted by Keenan et al. who showed that the ability of CyclinE to promote the G1/S transition of the cell cycle was unaffected by the CyclinD-CDK4 complex [22].
In this study, we did not find any correlations between Cx43 and CyclinD1, or Cx43 and CyclinE1 in esophageal cancer tissues. However, our previous studies demonstrated that Cx43 was correlated with CyclinD1 and CyclinE1 in colorectal cancer tissues (data not shown). This controversial outcome might be related to the differential expression of Cx43, CyclinD1 and CyclinE1 in different tissues, or because of the various cell cycle phases of tumor cells. In addition, the expression of AKAP95, CyclinD1, CyclinE1 and Cx43 in esophageal cancer tissues was unrelated to the degree of differentiation and lymph node metastasis.
Acknowledgements
This work is supported by National Natural Science Foundation (No. 81071927), Fujian Creative Project (No. 2012-CXB-25), Graduate Creative Project (No. 2014X0478) and Xiamen University Creative Grant (No. CXB2013024).
Disclosure of conflict of interest
None.
References
- 1.Lodén M, Stighall M, Nielsen NH, Roos G, Emdin SO, Ostlund H, Landberg G. The CyclinD1 high and CyclinB high subgroups of breast cancer: Separate pathways in tumorogenesis based on pattern of genetic aberrations and inactivation of the pRb node. Oncogene. 2002;21:4680–4690. doi: 10.1038/sj.onc.1205578. [DOI] [PubMed] [Google Scholar]
- 2.Geng Y, Yu Q, Whoriskey W, Dick F, Tsai KY, Ford HL, Biswas DK, Pardee AB, Amati B, Jacks T, Richardson A, Dyson N, Sicinski P. Expression of CyclinsE1 and E2 during mouse development and in neoplasia. PNAS. 2001;98:13138–13143. doi: 10.1073/pnas.231487798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldon CE, Musqrove EA. Distinct and redundant functions of Cyclin E1 and Cyclin E2 in development and cancer. Cell Division. 2010;5:2. doi: 10.1186/1747-1028-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, Cho H, Hampton GM, Theodorescu D. Cdc6 and cyclin E2 are PTEN-regulated genes associated with human prostate cancer metastasis. Neoplasia. 2009;11:66–76. doi: 10.1593/neo.81048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arsenijevic T, Degraef C, Dumont JE. G1/S Cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle. 2006;5:1217–1222. doi: 10.4161/cc.5.11.2802. [DOI] [PubMed] [Google Scholar]
- 6.Arsenijevic T, Degraef C, Dumont JE, Roger PP, Pirson I. Downregulation of connexin 43 in nasopharyngeal carcinoma cells is related to promoter methylation. Oral Oncol. 2007;43:898–904. doi: 10.1016/j.oraloncology.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Tang B, Peng ZH, Yu PW, Yu G, Qian F. Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Med Oncol. 2011;28:502–508. doi: 10.1007/s12032-010-9492-5. [DOI] [PubMed] [Google Scholar]
- 8.Sirnes S, Bruun J, Kolberg M. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer. 2012;131:570–581. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- 9.Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, Rivedal E. PKA implicated in the phosphorylation of Cx43 induced by stimulation with FSH in rat granulosa cells. J Reprod Dev. 2006;52:321–328. doi: 10.1262/jrd.17107. [DOI] [PubMed] [Google Scholar]
- 10.Moorby CD. A connexin 43 mutant lacking the carboxyl cytoplasmic domain inhibits both growth and motility of mouse 3T3 fibroblasts. Molecular Carcinogenesis. 2000;28:23–30. [PubMed] [Google Scholar]
- 11.Chen YD, Chen XX, Shen LN, Liang FC, Ding Y, Yu XY, Xue MQ, Zhang YX. Expression of A-kinase anchor protein 95, cyclinE2, and connexin43 in lung cancer tissue, clinical significance of their expression, and their expression correlation. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012;30:725–729. [PubMed] [Google Scholar]
- 12.Eide T, Coghlan V, Orstavik S, Holsve C, Solberg R, Skâlhegg BS, Lamb NJ, Langeberg L, Fernandez A, Scott JD, Jahnsen T, Taskén K. Molecular cloning, chromosomal localization, and cell cycle-dependent subcellular distribution of the A-kinase anchoring protein, AKAP95. Exp Cell Res. 1998;238:305–316. doi: 10.1006/excr.1997.3855. [DOI] [PubMed] [Google Scholar]
- 13.Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eide T, Carlson C, Taskén KA, Hirano T, Taskén K, Collas P. Distinct but overlapping domains of AKAP95 are implicated in chromosome condensation and condensin targeting. EMBO Rep. 2002;3:426–432. doi: 10.1093/embo-reports/kvf089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eide T, Taskén KA, Carlson C, Williams G, Jahnsen T, Taskén K, Collas P. Protein kinase A-anchoring protein AKAP95 interacts with MCM2, a regulator of DNA replication. J Biol Chem. 2003;278:26750–26756. doi: 10.1074/jbc.M300765200. [DOI] [PubMed] [Google Scholar]
- 16.Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. A-kinase-anchoring protein 95 functions as a potential carrier for the nuclear translocation of active caspase 3 through an enzyme-substrate-like association. Mol Cell Biol. 2005;25:9469–9477. doi: 10.1128/MCB.25.21.9469-9477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu SX, Kong XY, Yuan YY, Teng BG, Zhi XH, Zhuang WX, Yu XY, Liu WZ, Zhang YX. Relationship between AKAP95, cyclin E1, cyclin D1, and clinical and pathological parameters in lung cancer tissue. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013;31:890–894. [PubMed] [Google Scholar]
- 18.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 19.Caldon CE, Sergio CM, Schütte J, Boersma MN, Sutherland RL, Carroll JS, Musgrove EA. Estrogen regulation of cyclin E2 requires cyclin D1 but not c-Myc. Mol Cell Biol. 2009;29:4623–4639. doi: 10.1128/MCB.00269-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anayama T, Furihata M, Ishikawa T, Ohtsuki Y, Ogoshi S. Positive correlation between p27Kip1 expression and progression of human esophageal squamous cell carcinoma. Int J Cancer. 1998;79:439–443. doi: 10.1002/(sici)1097-0215(19980821)79:4<439::aid-ijc22>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Gene. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 22.Keenan SM, Lents NH, Baldassare JJ. Expression of cyclin E renders cyclin D-CDK4 dispensable for inactivation of the retinoblastoma tumor suppressor protein, activation of E2F, and G1-S phase progression. J Biol Chem. 2004;279:5387–5396. doi: 10.1074/jbc.M310383200. [DOI] [PubMed] [Google Scholar]