Abstract
Klotho is a potential biomarker and therapeutic target in a model of acute kidney injury (AKI) induced in rats by ischemia-reperfusion injury. However, the sensitivity and specificity of serum Klotho for early detecting clinical AKI are unknown. This prospective study evaluated the significance of serum Klotho for early detection of postoperative AKI among adult patients undergoing cardiac valve replacement surgery. Moreover, we also compared the utilities of serum Klotho, serum creatinine and cystatin C in early detection of AKI. There was no marked difference between AKI and non-AKI groups in preoperative serum Klotho levels. Immediately after the operation, serum Klotho decreased significantly in patients with AKl. In spite of the poor specificity, its diagnostic sensitivity was excellent. On postoperative 1 d, with the rapid recovery toward the preoperative level, the ability of serum Klotho for early detecting AKI declined. Changes in serum Klotho levels at every time point among patients without AKI did not reveal any statistical significance. We showed that AKI is a state of transient Klotho deficiency in patients undergoing cardiac valve replacement surgery. Serum Klotho levels were drastically decreased beginning at 0h with ideal ROC-AUC, sensitivity but poor specificity, which didn’t exceed 4 h after operation, suggesting that serum Klotho could serve as a potential biomarker for CSA-AKI, especially during the short period after cardiac surgery. A larger multicentre cohort study of population in different ages undergoing on-pump cardiac surgery is required to identify the optimal timing of serum Klotho measurement and the optimal cut-off points for clinical use to further refine the optimal timing for early detection of AKI.
Keywords: Cardiac surgery, acute kidney injury, serum Klotho, cystatin C, serum creatinine
Introduction
Acute kidney injury (AKI) is a common and serious complication after cardiac surgery and is an independent risk factor for a series of adverse outcomes. The incidence of cardiac surgery associated acute kidney injury (CSA-AKI) is reported about 5%-47% [1,2]. In order to detect AKI at the early stage and to be able to provide interventions promptly, there have been several emerging biomarkers (NGAL, IL-18, KIM-1, L-FABP) that show reasonable sensitivity and specificity for the prediction of AKI after cardiopulmonary bypass (CPB) [3-5] and for the protection from CPB-associated AKI [6].
KL gene is closely related to senility and mainly expressed in kidney and brain choroid, especially in the renal tubular epithelial cells. The soluble form of Klotho functions as an endocrine substance that exerts multiple actions including the modulation of renal solute transport and the protection of the kidney from a variety of insults in experimental models [7]. Recently, it is reported that the expression of KL gene decreases in chronic kidney disease (CKD) patients and returns to normal with the improvement of renal function. What’s more, Hu et al found reduced Klotho in kidneys, urine, and blood of ischemia-reperfusion injury (IRI) rats, which returned to normal upon recovery [8]. In the clinical conditions, cardiac valve replacement surgery is one of the most typical IRI models, we therefore investigated whether serum Klotho in patients with CSA-AKI could display the similar change as in the CKD patients and IRI rats. It’s unknown if serum klotho could serve as a potential biomarker for early detecting CSA-AKI, the AUC-ROC was used to assess the sensitivity and specificity of serum Klotho for diagnosing CSA-AKI. Meanwhile, the abilities for early detecting CSA-AKI between traditional biomarkers (SCr, cystatinC) were compared in this prospective study.
Materials and methods
The procedures of our study were in accordance with the Helsinki Declaration and were approved by the ethics review committee of our hospital.
Patients selection and grouping criteria
Exclusion criteria mainly included preexisting renal insufficiency, serious cardiac insufficiency, perioperative nephrotoxic drug use, postoperative low cardiac output syndrome (LCOS). Finally, we enrolled 35 adult patients undergoing cardiac valve replacement surgery in Surgical Intensive Care Unit of the First Affiliated Hospital, Sun Yat-Sen University. Patients were divided into the AKI and non-AKI groups according to whether subjects developed AKI within 48 h after surgery. Postoperative AKI was defined according to AKIN criteria, which is an absolute increase in SCr of ≥26.4 μmol/L (0.3 mg/dl) from baseline or a relative increase in SCr of >1.5 fold from baseline within the first 48 h after cardiac surgery [9].
Blood samples and assays
From each patient, blood samples were collected on postoperative 0 h, 4 h, days 1 to 3 (at 06:00 a.m.) respectively to detect serum Klotho, SCr and cystatin C levels. Blood samples obtained on the day of hospital admission were used as baseline reference values. SCr and cystatin C were measured in the department of clinical laboratory, using standard assays. SCr values were obtained using an isotope dilution-mass spectrometry, traceable enzymatic assay. Cystatin C levels were measured using the particle-enhancing turbidimetry immune assay (PETIA). Serum Klotho concentrations were determined utilizing the human klotho ELISA Kit. Blood samples were centrifuged at 3000×g for 10 minutes, and the supernatants stored in aliquots at -80°C until measurement.
Statistical analysis
When data were normally distributed, the t test was used to test numerical data comparing patients who had AKI with those who did not. All results are presented as mean ± standard deviations or as median with interquartile range if appropriate. When data were not normally distributed, the Mann-Whitney U test was used for comparisons between groups, and the Wilcoxon rank sum test was used for continuous variables. Fisher’s exact test was used for categorical values as appropriate. Receiver-operating characteristic curves (ROCs) for clinical parameters were plotted to predict the onset of AKI. We then assessed the ability of serum Klotho to predict the onset of AKI by calculating the area under the ROC (AUC). An AUC of 0.90-1.0 indicated excellent, 0.80-0.89, good; 0.70-0.79, fair; 0.60-0.69, poor; and 0.50-0.59, no useful value. Statistical analysis was performed using SPSS version 13.0. P<0.05 was considered to be statistically significant.
Results
Patients’ characteristics
This study included 35 patients who met the inclusion criteria. 19 patients developed AKI within the first 48 h after cardiac surgery and were placed in the AKI group, while the remaining 16 patients did not and were placed in the non-AKI group. No significant differences were noted between the two groups with respect to gender, age, coexisting diseases, baseline renal function. Patients who developed AKI had significantly longer CPB and aortic cross-clamping duration compared with those who did not develop AKI (P=0.002, P=0.005) (Table 1).
Table 1.
Clinical characteristics of the included patients
| AKI (n=19) | N-AKI (n=16) | P value | |
|---|---|---|---|
| Gender (M/F) | 7/12 | 5/11 | 0.16 |
| Age (years) | 52.54±10.15 | 51.85±9.30 | 0.09 |
| Hypertension (N, %) | 4 (21.05%) | 2 (12.50%) | 0.10 |
| Diabetes mellitus (N, %) | 3 (15.79%) | 1 (6.25%) | 0.09 |
| NYHA >II (N, %) | 5 (26.30%) | 3 (18.75%) | 0.15 |
| eGFR (ml/min/1.73 m2) | 93.82±17.58 | 96.09±19.65 | 0.38 |
| CPB (min) | 100.54±28.08 | 88.20±25.23 | 0.002 |
| Aortic cross-clamping (min) | 70.06±22.18 | 58.55±24.11 | 0.005 |
Data are presented as means ± standard deviation (range), or number (%). M, male, F, female, NYHA, New York Heart Association heart failure classification, eGFR = 186× (SCr)-1.154×Age-0.203×(0.742 Female).
AKI secondary to cardiac surgery is an acute state of Klotho deficiency
Immediately after operation, serum Klotho decreased significantly in patients with AKl compared with baseline (101.97±16.93 vs 121.64±19.87, P<0.01), which briefly continued to the 4 h after CPB. On postoperative 1 d (14-16 h after operation), serum Klotho has already begun to recover markedly toward the preoperative levels. Finally, the serum concentrations of Klotho returned to baseline at the endpoint (121.64±19.87 vs 120.50±13.17, P= 0.635). The changes of serum Klotho levels at every time point in patients without AKI did not reveal any statistical significance compared with the preoperative levels. Immediate and 4 h postoperatively, the serum Klotho level in the AKI group was significantly lower than that in the non-AKI group (Figure 1; Table 2).
Figure 1.
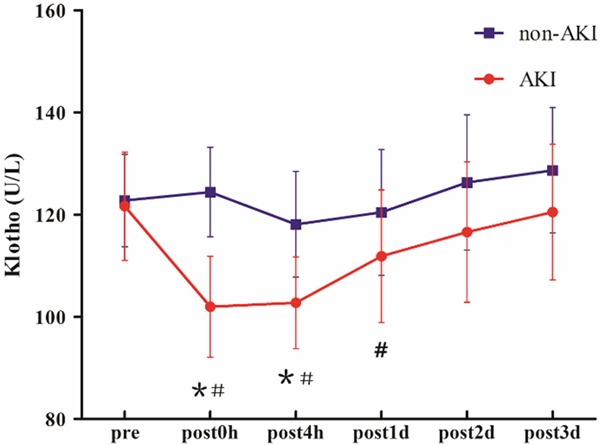
Changes in serum Klotho in the (red) acute kidney injury (AKI) and (blue) non-AKI groups. *P<0.05, compared with the non-AKI group; #P<0.05, compared with the preoperative level in AKI group.
Table 2.
Concentrations of Klotho, SCr/Kl, SCr and cys-C after operation
| Baseline | 0 h | 4 h | 1 d | 2 d | 3 d | ||
|---|---|---|---|---|---|---|---|
| Klotho | AKI | 121.64±19.87 | 101.97±16.93*,# | 102.77±14.44 | 111.85±11.78 | 116.58±12.73 | 120.50±13.17 |
| N-AKI | 122.76±20.18 | 124.40±20.66 | 118.10±18.74 | 120.43±17.55 | 126.29±17.78 | 128.67±18.84 | |
| SCr | AKI | 73.37±11.66 | 99.05±16.51*,# | 125.37±21.88★ | 128.37±43.56 | 131.58±62.57 | 120.42±63.53 |
| N-AKI | 73.19±16.67 | 74.56±12.43 | 86.13±20.73 | 71.94±20.58 | 71.88±22.00 | 71.19±.23.30 | |
| SCr/Kl | AKI | 0.621±0.151 | 0.997±0.238*,# | 1.256±0.328 | 1.166±0.427 | 1.148±0.550 | 1.018±0.557 |
| N-AKI | 0.606±0.174 | 0.600±0.169 | 0.749±0.246 | 0.606±0.212 | 0.583±0.209 | 0.569±0.206 | |
| Cys-C | AKI | 1.073±0.204 | 1.120±0.257*,# | 1.394±0.364 | 1.652±0.293 | 1.896±0.414 | 2.006±0.440 |
| N-AKI | 0.997±0.164 | 0.935±0.180 | 0.950±0.222 | 1.094±0.254 | 1.094±0.254 | 1.099±0.332 |
P<0.05, Biomarkers compared with the non-AKI group;
P<0.05, Biomarkers compared with baseline;
Reaching the AKIN criteria.
ROC analysis of serum Klotho for Early diagnosis of AKI after cardiac valve replacement surgery
For the early diagnosis of AKI, serum Klotho performed best at the time point of immediate post-operation with an AUC of 0.806 (95% confidence interval: 0.656 to 0.948, P = 0.003), and the diagnostic sensitivity and specificity were 0.895 and 0.572 respectively when the cutoff value was 119.145 U/L. On postoperative 4 h, AUC for AKI diagnosis was 0.753 (95% confidence interval: 0.587 to 0.916, P=0.013), and the diagnostic sensitivity and specificity were 0.898 and 0.534 respectively when the cutoff value was 122.68 U/L. At the subsequent time points after operation (1 d, 2 d, 3 d), the ability of serum Klotho for diagnosing AKI declined generally (Figure 2; Tables 3, 4).
Figure 2.
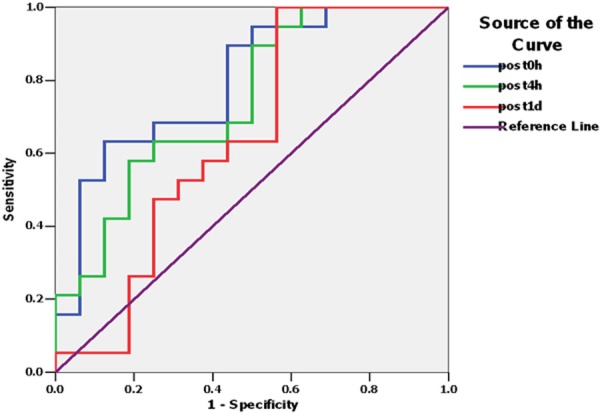
ROC of serum Klotho to detect AKI instant to day1 after operation.
Table 3.
ROC-AUC of Klotho, SCr, SCr/Kl and cys-C after cardiac surgery
| 0 h | 4 h | 1 d | 2 d | 3 d | |
|---|---|---|---|---|---|
| Klotho (95% CI) | 0.806 (0.656, 0.948) | 0.753 (0.587, 0.916) | 0.651 (0.474, 0.849) | 0.641 (0.461, 0.840) | 0.612 (0.412, 0.812) |
| P-value | 0.003 | 0.013 | 0.150 | 0.132 | 0.305 |
| SCr (95% CI) | 0.875 (0.759, 0.911) | 0.911 (0.812, 1.010) | 0.893 (0.791, 0.995) | 0.859 (0.737, 0.980) | 0.826 (0.685, 0.966) |
| P-value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| SCr/Kl (95% CI) | 0.924 (0.831, 1.018) | 0.888 (0.780, 0.997) | 0.901 (0.801, 1.002) | 0.845 (0.718, 0.973) | 0.822 (0.678, 0.967) |
| P-value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Cys-C (95% CI) | 0.742 (0.573, 0.911) | 0.862 (0.742, 0.982) | 0.969 (0.969, 1.019) | 0.972 (0.926, 1.018) | 0.944 (0.868, 1.021) |
| P-value | 0.015 | 0.01 | 0.01 | 0.01 | 0.01 |
Table 4.
Cutoff values, sensitivity and specificity of Klotho, SCr, SCr/Kl and cys-C
| 0 h | 4 h | 1 d | 2 d | 3 d | |
|---|---|---|---|---|---|
| Klotho (U/L) | 119.145 (0.895, 0.572) | 122.680 (0.898, 0.534) | 128.961 (0.915, 0.525) | 134.354 (0.945, 0.533) | 138.165 (0.898, 0.578) |
| SCr (μmol/L) | 86.50 (0.842, 0.812) | 106.00 (0.895, 0.875) | 88.50 (0.842, 0.750) | 96.50 (0.684, 0.937) | 79.50 (0.737, 0.750) |
| SCr/Kl | 0.695 (0.947, 0.875) | 0.895 (0.895, 0.875) | 0.735 (0.842, 0.750) | 0.597 (0.947, 0.625) | 0.575 (0.842, 0.687) |
| Cys-C (mg/L) | 0.965 (0.737, 0.750) | 1.150 (0.789, 0.750) | 1.275 (0.947, 0.875) | 1.405 (0.947, 0.875) | 1.380 (0.947, 0.812) |
The reevaluation of biomarkers (SCr, cystatin C) for early detecting AKI after cardiac valve replacement surgery
Serum creatinine has traditionally been used for the assessment of kidney function after cardiac surgery. However, compared with cystatin C, a gradually accepted biomarker for monitoring GFR [10], the usefulness of SCr for early detecting AKI has been questioned [11]. In this single centre study, we reappraised the performance of both SCr and cystatin C in diagnosing AKI after cardiac valve replacement surgery. Immediately after operation, both SCr and cystatin C were significantly higher in AKl groups. The AUC-ROC of SCr and cystatin C for AKI diagnosis was 0.875 versus 0.742. On postoperative 4 h, the average levels of SCr in AKI group has reached the AKIN criteria. SCr performed best at this time point with an AUC of 0.911(95% confidence interval: 0.812 to 1.010, P<0.01). Nevertheless, compared with SCr’s AUC that experienced a decline after reaching peak, the AUC-ROC of cystatin C demonstrated a sustained enhancement in AKI group during the study period (Table 3).
The SCr/Kl ratio may serve as an early biomarker for AKI after cardiac surgery
In consideration of the changes in opposite directions between SCr and serum Klotho values after AKI, we investigated the performance of SCr/Kl for early detecting AKI secondary to cardiac surgery. On postoperative 0h, SCr/Kl increased significantly in patients with AKl compared with baseline (0.997±0.238 vs 0.621±0.151, P<0.01), which continued to the endpoint. ROC analysis confirmed excellent accuracy of SCr/Kl in AKI diagnosis (AUC = 0.924, 95% confidence interval: 0.831 to 1.018), and the sensitivity and specificity were 0.947 and 0.875 respectively which was superior to that of SCr, Klotho and cystatin C. On postoperative 4 h to 3 d, SCr/Kl also showed ideal performances in diagnosis of AKI (Figures 3, 4; Tables 2, 3 and 4).
Figure 3.
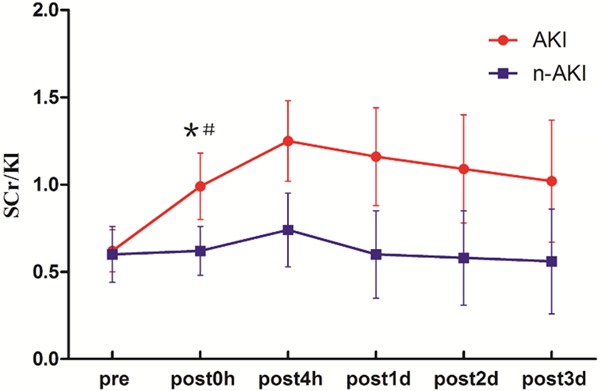
Changes in SCr/Kl in the (red) acute kidney injury (AKI) and (blue) non-AKI groups. *P<0.05, compared with the non-AKI group; #P<0.05, compared with the preoperative level in the AKI group.
Figure 4.
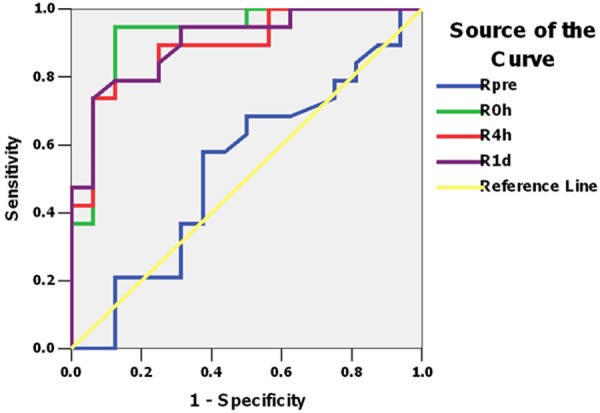
ROC of SCr/Kl to detect AKI instant to day1 after operation.
Discussion
Cardiac surgery is one of the major three etiologies of hospital-acquired AKI [12]. Recent international consensus guidelines have been published regarding the pathophysiology and management of this important entity, now termed “cardiac surgery-associated acute kidney injury” (CSA-AKI) [13,14]. The possible mechanisms of CSA-AKI are as follows: non-pulsatile perfusion, activation of proinflammatory mediators, the release of free hemoglobin and myohemoglobin, hemodilution, the formation of microemboli during the cardiopulmonary bypass (CPB) and so on. Despite recent advances, clinical outcomes after cardiac surgery, remain suboptimal in patients with renal dysfunction, regardless of clinical severity [15-17]. Increasingly, it has been recognized that even mild or subclinical deteriorations in renal function profoundly increase the risk for major adverse clinical outcomes after cardiac surgery. Therefore, there is an urgent need for more sensitive and specific biomarkers that can diagnose AKI earlier, possibly indicate the cause, and rapidly measure the response to therapy.
Klotho was originally identified as an aging suppressor gene [18,19] with pleiotropic functions. In mammalian kidney including mouse, rat and human, Klotho is prominently expressed in distal convoluted tubules [20]. The extracellular domain of Klotho is cleaved on the cell surface by membrane-anchored proteases, and released into blood, [21-23] urine and cerebrospinal fluid [23]. Secreted Klotho protein has multiple functions distinct from those of membrane Klotho, including anti-apoptosis, regulation of multiple ion channels [24-27] and oxidative stress [28]. Animal experiments clearly showed short-term renal Klotho deficiency in AKI from a variety of causes including ischemia-reperfusion injury (IRI) [29,30], indicating that renal Klotho downregulation in AKI is likely a general phenomenon. IRI-AKI model showed that both Klotho mRNA and protein started to fall on the first day and returned to near baseline around day 3 and 4, respectively [31]. While changes in kidney morphology are detectable after 5 h, renal Klotho protein levels were drastically and sustainably decreased beginning at 3 h, suggesting that renal Klotho protein may be one of the earliest biomarkers for kidney injury, at least in a rodent IRI-AKI model [31]. Unfortunately, there are limited human data to define the time course, specificity and sensitivity of urinary and serum Klotho.
Our prospective study is the first manuscript to evaluate the performance of serum Klotho for early detecting AKI in the clinical context. The purpose of this study was to determine whether Klotho is a potential biomarker for CSA-AKI. We showed that AKI is a state of acute Klotho deficiency in patients undergoing cardiac surgery. Patients with CSA-AKI revealed that serum Klotho started to fall on the time point of immediate post-operation and returned to near baseline on day 3 from the beginning of 4 h after operation. The changes of serum Klotho levels at every time point in patients without AKI did not reveal any statistical significance compared with the baseline. Serum Klotho levels were drastically decreased beginning at 0 h with ideal AUC and sensitivity but poor specificity, suggesting that serum Klotho could serve as a potential biomarker for CSA-AKI, especially during the short periods after cardiac surgery.
For further refinement of the definition of AKI, the AKIN was created, suggesting a modified version of the RIFLE classification representing the entire spectrum of AKI known as AKIN criteria [32]. Both of these definitions incorporate elevation in SCr as the primary criteria of AKI. However, serum concentration of creatinine is affected by age, gender, muscle mass, medication, and hydration status. Moreover, serum creatinine concentration may not change until 50% of kidney function has already been lost [33]. Cystatin C is a cysteine protease inhibitor that is synthesized by nucleated cells and subsequently released in the bloodstream, especially after renal injury [34]. Recently, some studies showed that serum cystatin C was superior to serum creatinine for the detection of CSA-AKI and significantly correlated with the risks of renal replacement therapy and in-hospital mortality [35-38]. Nevertheless, there were still other clinical trials indicated that cystatin C was equivalent or even inferior to SCr for early detection of CSA-AKI, especially on the postoperative day 1 [39-42]. Our study displayed that the ability of SCr for early detecting CSA-AKI (within 1 d) was superior to cystatin C while the performance for the latter to diagnose AKI was more stable and excellent during the tardy stage (1 d-3 d). It was noteworthy that the serum concentration of cystatin C maintained an upward trend in patients with AKI during the period of our observation, indicating that certain complications might still exert progressive influence on filtration since cystatin C is filtered almost completely by glomeruli.
In this manuscript, we used the combinational measurement of SCr/Kl ratio as a new biomarker in order to amplify the efficacy for detecting CSA-AKI. Consequently, the ideal diagnostic performance for SCr/Kl was continued to the endpoint compared with the transient significance of serum Klotho for detecting CSA-AKI. Furthermore, the SCr/Kl ratio possessed a better diagnostic sensitivity than that of SCr, particularly during the late period (2 d-3 d post-operation).
Recent studies demonstrated that Klotho may have a role in both reducing kidney damage and promoting kidney recovery. Klotho deficiency worsens ischemia reperfusion-induced injury in hindlimbs and overexpressing Klotho protein attenuates IRI-induced tissue damage through decreasing apoptosis [43,44] or promotes tissue regeneration through angiogenesis [45,46]. It is quite conceivable that Klotho may be a unique protein which does not only serve as a potentially useful biomarker for kidney disease, but also functions as a renoprotective protein to alleviate kidney injury.
This study has several limitations. First, it was a single-center, small sample size study, which implied the possible biases of our results. Second, the subjects enrolled were confined only to adult patients with rheumatic valvular heart disease undergoing cardiac valve replacement surgery. Our results will need to be validated in a larger population, including subjects of different ages undergoing various cardiac surgical operations. Third, ours was a cohort with relatively pristine kidney function, and it will be important to confirm our findings in documented high-risk settings such as preexisting kidney dysfunction, diabetes mellitus, volume depletion, concomitant nephrotoxic drug use, and the hemodynamically compromised patients.
Acknowledgements
This study was supported by grant from the National Natural Science Foundation of China (Grant No. 81201452).
Disclosure of conflict of interest
None.
References
- 1.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 2.Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. ANZ J Surg. 2003;73:144–153. doi: 10.1046/j.1445-2197.2003.02640.x. [DOI] [PubMed] [Google Scholar]
- 3.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinaseassociated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 4.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 5.Devarajan P, Nguyen M, Kathman T, Wang Z, Dent C, Parikh C. Validation of early AKI biomarkers discovered by proteomics. Free Commun J Am Soc Nephrol. 2008;19:92A. [Google Scholar]
- 6.Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis. 2009;53:584–595. doi: 10.1053/j.ajkd.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Hu MC, Makoto Kuro-o M, Moe OW. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant. 2012;27:2650–2657. doi: 10.1093/ndt/gfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlipak MG, Praught ML, Sarnak MJ. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens. 2006;15:270–275. doi: 10.1097/01.mnh.0000222694.07336.92. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–37. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 12.Garwood S. Cardiac surgery-associated acute renal injury: new paradigms and innovative therapies. J Cardiothorac Vasc Anesth. 2010;24:990–1001. doi: 10.1053/j.jvca.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Auriemma S, Fabbri A, D’Onofrio A, Katz N, McCullough PA, Ricci Z, Shaw A, Ronco C. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–178. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 14.Tolwani A, Paganini E, Joannidis M, Zamperetti N, Verbine A, Vidyasagar V, Clark W, Ronco C. Treatment of patients with cardiac surgery associated acute kidney injury. Int J Artif Organs. 2008;31:190–196. doi: 10.1177/039139880803100212. [DOI] [PubMed] [Google Scholar]
- 15.Van Straten AH, Soliman Hamad MA, van Zundert AA, Martens EJ, Schönberger JP, de Wolf AM. Preoperative renal dysfunction as a predictor of survival after coronary artery bypass grafting: Comparison with a matched general population. J Thorac Cardiovasc Surg. 2009;138:971–976. doi: 10.1016/j.jtcvs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Miceli A, Bruno VD, Capoun R, Romeo F, Angelini GD, Caputo M. Occult renal dysfunction: a mortality and morbidity risk in coronary artery bypass grafting surgery. J Thorac Cardiovasc Surg. 2011;141:771–776. doi: 10.1016/j.jtcvs.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Chonchol MB, Aboyans V, Lacroix P, Smits G, Berl T, Laskar M. Long-term outcomes after coronary artery bypass grafting: Preoperative kidney function is prognostic. J Thorac Cardiovasc Surg. 2007;134:683–689. doi: 10.1016/j.jtcvs.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- 21.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. Alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 22.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 23.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 24.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 25.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K (+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuro OM. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 29.Hu MC, Kuro-o M, Moe OW. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant. 2012;27:2650–2657. doi: 10.1093/ndt/gfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8:423–429. doi: 10.1038/nrneph.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 34.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:2151–2157. doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellomo R, Ronco C, Kellum JA, Mehta RA, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure definition, outcome measures, anaimal models, fluid therapy, and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander KP, Anstrom KJ, Muhlbaier LH, Grosswald RD, Smith PK, Jones RH, Peterson ED. Outcomes of cardiac surgery in patients: results from the National Cardiovascular Network. J Am Coll Cardiol. 2000;35:731–738. doi: 10.1016/s0735-1097(99)00606-3. [DOI] [PubMed] [Google Scholar]
- 37.Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Silvay G, Adams DH. Results and predictors of early and late outcomes of coronary artery bypass graft surgery in octogenarians. J Cardiothorac Vasc Anesth. 2007;21:784–792. doi: 10.1053/j.jvca.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Yin R, Wu H, Yi J, Luo L, Dong G, Jing H. Cystatin C as a reliable marker of renal function following heart valve replacement surgery with cardiopulmonary bypass. Clin Chim Acta. 2006;374:116–121. doi: 10.1016/j.cca.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Ristikankare A, Pöyhiä R, Kuitunen A, Skrifvars M, Hämmäinen P, Salmenperä M, Suojaranta-Ylinen R. Serum cystatin C in elderly cardiac surgery patients. Ann Thorac Surg. 2010;89:689–694. doi: 10.1016/j.athoracsur.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery-a prospective cohort study. Crit Care Med. 2009;37:553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 41.Magro MC, Vattimo MD. Impact of cystatin C and RIFLE on renal function assessment after cardiac surgery. Biol Res Nurs. 2013;15:451–8. doi: 10.1177/1099800412446742. [DOI] [PubMed] [Google Scholar]
- 42.Ristikankare A, Pöyhiä R, Kuitunen A, Skrifvars M, Hämmäinen P, Salmenperä M, Suojaranta-Ylinen R. Serum cystatin C in elderly cardiac surgery patients. Ann Thorac Surg. 2010;89:689–695. doi: 10.1016/j.athoracsur.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Song JH, Lee MY, Kim YJ, Park SR, Kim J, Ryu SY, Jung JY. Developmental immunolocalization of the Klotho protein in mouse kidney epithelial cells. Eur J Histochem. 2014;58:2256. doi: 10.4081/ejh.2014.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014;85:855–870. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukino K, Suzuki T, Saito Y, Shindo T, Amaki T, Kurabayashi M, Nagai R. Regulation of angiogenesis by the aging suppressor gene klotho. Biochem Biophys Res Commun. 2002;293:332–337. doi: 10.1016/S0006-291X(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 46.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]


