Abstract
There are limited reports with respect to the study on the epithelium-mesenchymal transformation (EMT) mediated by Snail in the ovarian cancer. This study detected the expression of Snail and related EMT markers in the ovarian cancer tissues, and explored the possible molecular mechanism of EMT mediated by Snail in the metastasis of ovarian cancer. The patients diagnosed with ovarian cancer according to the pathology were recruited in this study during 2010-2014. The carcinoma tissue and normal tissue adjacent to carcinoma were surgically obtained from patients. The genes of E-cadherin, β-catenin, Fibronectin and N-cadherin were detected using the RT-PCR. The 64 patients were recruited and diagnosed as ovarian cancer by pathological examination. The expression levels of Snail, Fibronectin and N-cadherin in the stage III and IV were higher than those in the stage I and II, respectively (all P < 0.05). However, the expression levels of E-cadherin and β-catenin decreased along with the stage developed (trend test, both P < 0.05), respectively. The expression of Snail was positively correlated with the expression of Fibronectin, N-cadherin, but negatively correlated with the expression of E-cadherin and β-catenin. The number of A2780 cells entering into the lower compartment in the group of carcinoma tissue were significantly higher than that in the group of normal tissue after transfected with Snail expression vector. While, the invasion ability of A2780 significantly reduced after RNAi-Snail. The correlation between Snail and invasion and metastasis of ovarian cancer and epithelial-mesenchymal transition based on tissue and cell levels, and to some extent explored the molecular mechanism of the EMT process mediated by Snail.
Keywords: Snail, epithelium-mesenchymal transformation, molecular mechanism, ovarian cancer
Introduction
As the top of the death rate among the gynecology malignant tumors, ovarian cancer also has a high mortality, third only to cervical cancer and uterine cancer. Inhibition of invasion and metastasis for ovarian cancer cells is the important direction in the clinical treatment of ovarian cancer [1,2], but the occurrence, development and infiltration as well as proliferation mechanism of the ovarian cancer are still unclear.
In recent years, it has been found that the epithelium-mesenchymal transformation (EMT) is closely related to the metastasis of the tumors. EMT makes the cancer cells invasion and diffusion, and EMT plays an important role in the invasion and metastasis of the epithelial cancer. EMT is featured by up-regulation of epithelial markers (such as E-cadherin and β-catenin) and down-regulation of the interstitial markers (such as N-cadherin and Fibronectin). Snail is an important regulatory factor in EMT process, and its abnormal expression leads to activation of EMT event by activating multiple signaling pathways, thus promoting the invasion and metastasis of tumor cells [3-5]. At present, there are limited reports with respect to the study on EMT mediated by Snail in the ovarian cancer.
Therefore, this study detected the expression of Snail and related EMT markers in the ovarian cancer tissues, and explored the possible molecular mechanism of EMT mediated by Snail in the metastasis of ovarian cancer.
Materials and methods
Patients and cells
The patients diagnosed with ovarian cancer according to the pathology were recruited in this study from January 2010 to September 2014 in the affiliated hospital of Qiqihar Medical University. The carcinoma tissue and normal tissue adjacent to carcinoma were surgically obtained from patients. The research protocol was approved by the human ethics committee of the hospital, and all participants provided written informed consent.
The A2780 cell lines were purchased from ATCC and maintained in liquid nitrogen. The cells were incubated in 5% CO2 at 37°C using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Real-time PCR (RT-PCR)
RNA were extracted from samples using the RNeasy Plus Mini Kit (QIAGEN) and the cDNA sample was synthesized by using iScript cDNA Synthesis Kit (Bio-Rad). The genes of E-cadherin, β-catenin, Fibronectin and N-cadherin were detected using the RT-PCR by SsoAdvanced SYBR Green Super mix (Bio-Rad) with the primers listed in Supplemental Table 1. The expression level of target genes was calculated as the previous reported [6].
Cell transfection
Snail gene were amplified (F: 5’-CGGGATCCTTCTTCTGCGCTACTGCTGCG-3’; R: 5’-CGGAATTCGGGCAGGTATGGAGAGGAAGA-3’), recycled and purified. Xho I, EcoR I restriction enzyme were used to cut amplification products and pcDNA3.1 plasmid, respectively. They connected at 4°C with T4 ligase overnight and confirmed by sequencing the clones, and then transformed into E. coli. The pcDNA3.1-q eukaryotic expression plasmid were extracted.
Cells were incubated in plates. A total of 500 μL complete medium was added each well with 70-80% cell density in transfection. 1 μL Lipofectamine 3000 was diluted in 50 μL serum-free medium, which mixed with dilution plasmids, following incubation for 20 minutes at room temperature. After 4 to 6 hours of culture, the transfection medium was replaced with fresh medium.
Western blot analysis
The samples were grinded in liquid nitrogen and solubilized in lysis buffer of ReadyPrep protein extraction kit with the addition of protease inhibitors cocktail. After 30 minutes on ice, cells were cracked by ultrasonic and centrifuged at 13,000 r/min at 4°C for 20 min. Concentration of protein was determined with Protein Assay Kit.
The extracts were separated by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis and then electroblotted onto a nitrocellulose membrane. The electroblotted membrane was soaked in a blocking solution (5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20, TBST) for 1 h at room temperature. The membrane was then incubated with antibodies for 2 h at room temperature. The membrane was washed for 3 times lasting 5-10 minutes every time and incubated with secondary antibody (diluted 1:10,000) for 1 h. The blots were finally detected by enhanced chemiluminescence. Experiments were performed in triplicate. Grey value was analyzed using Quantity one v4.62 software by stripe trajectory quantitative method drawing optical density curve. According to the optical density value of different electrophoresis banding, the optical density area under the curve was calculated for analysis.
Cell invasion assay
Matrigel was diluted to 1 mg/ml by 4°C precooled serum-free DMEM medium. 100 μL diluted Matrigel were added to the middle of the bottom of the 24-Transwell chamber and incubated for 5 h at 37°C, in which 200 μL DMEM medium was added later. Cells were digested by pancreatic enzyme, washed with PBS and seeded into the upper compartment of the Transwell insert (5000 cells/ml) in serum-free culture medium. Medium with 10% FBS was added to the lower chamber as a chemoattractant factor. Cells were cultured at 37°C, then the non-invading cells on the upper surface of the membrane were gently removed with a cotton swab. After incubating for the indicated times, the cells were fixed with 4% methanol and stained with 0.1% crystal violet solution for 30 min at 37°C. The fixed cells were then observed under a microscope and the number of cells was counted under 3 random visual fields.
Statistical analysis
Descriptive statistics were calculated for all variables; continuous variables were summarized as means and standard deviations (SD) or as medians and ranges, and categorical variables were summarized as frequencies and proportions. To determine the difference between groups, a χ2 test, a Fisher exact test, or a nonparametric test was used where appropriate. A 2-sided P value of < 0.05 was considered to be statistically significant. All analyses were performed using SPSS software, version 17.0.
Results
Basic information
The 64 patients were recruited and diagnosed as ovarian cancer by pathological examination, including 25 ovarian serous adenocarcinoma, 17 ovarian borderline serous tumor and 22 ovarian serous glands tumor from January 2010 to September 2014. The mean of age were 41.3, and 38 (59.4%) were equal or older than 60 years. There were 13 in stage I, 9 in stage II, 26 in stage III and 16 stage IV according to FIGO staging criteria. A total of 64 carcinoma tissue and 57 normal tissue adjacent to carcinoma of patients with ovarian cancer were surgically obtained.
Expression level of the snail and EMT related molecular markers
The expression level of the Snail and EMT related molecular markers in different stages in the group of carcinoma tissue were shown in Figure 1 (detailed in Supplemental Table 2). The results showed the expression levels of Snail, Fibronectin and N-cadherin in the stage III and IV were higher than those in the stage I and II, respectively (all P < 0.05). However, the expression levels of E-cadherin and β-catenin decreased along with the stage developed (trend test, both P < 0.05), respectively.
Figure 1.
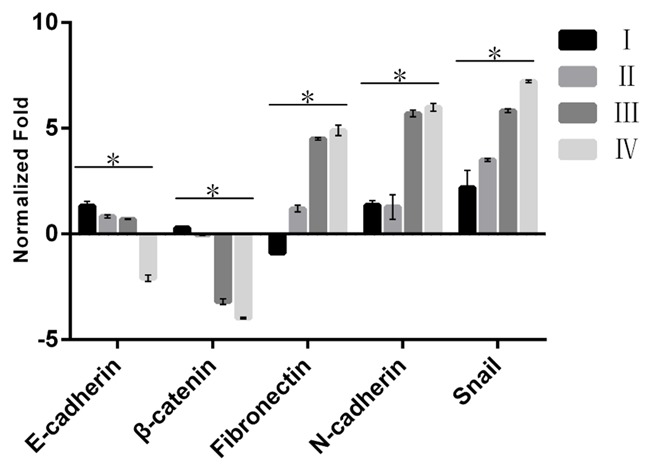
The expression level of the Snail and EMT related molecular markers in different stages in the group of carcinoma tissue. The different clinical stages include Stage I, Stage II, Stage III and Stage IV. The higher column indicates higher expression level.
Compared to the expression levels of molecular markers in the group of normal tissue adjacent to carcinoma tissue, the up-regulated expression of Snail, Fibronectin and N-cadherin were higher in the group of carcinoma tissue, respectively (all P < 0.05) while the expression of E-cadherin and β-catenin were lower (both P < 0.05) in Figure 2.
Figure 2.
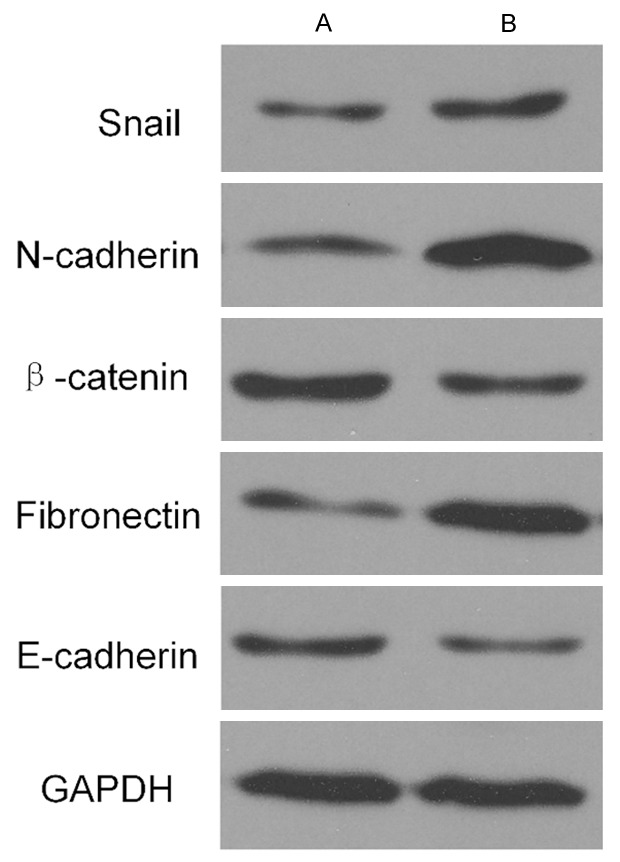
The expression level of the Snail and EMT related molecular markers in different stages in the two groups of carcinoma tissue and normal tissue adjacent to carcinoma tissue. A. Normal tissue adjacent to carcinoma tissue. B. Carcinoma tissue. Thicker stripe indicates the higher expression level.
Effect of snail on the EMT related molecular markers
The expression of Snail was positively correlated with the expression of Fibronectin, N-cadherin, but negatively correlated with the expression of E-cadherin and β-catenin in Figure 3.
Figure 3.
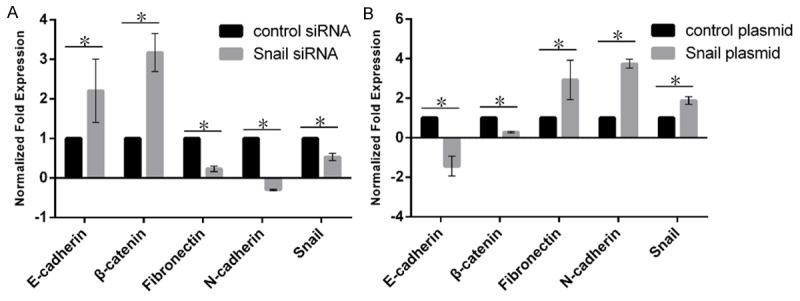
The effect of the expression level of Snail on EMT related molecular markers. A. The effect of Snail on EMT molecular markers in siRNA. B. The effect of Snail on EMT molecular markers in plasmid. The black represents the control group; the gray represents the Snail group. The higher column indicates higher expression level.
Effect of snail on invasion and metastasis ability of A2780 cell lines
As shown in Figure 4, the number of A2780 cells entering into the lower compartment in the group of carcinoma tissue were significantly higher than that in the group of normal tissue after transfected with Snail expression vector. While, the invasion ability of A2780 significantly reduced after RNAi-Snail.
Figure 4.
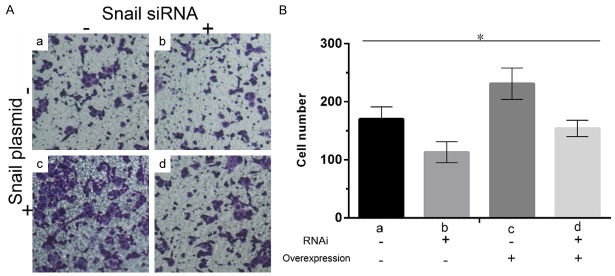
The effect of the expression level of Snail on the invasion and metastasis ability of A2780 cell lines. (A) The stained cell under the microscope. (B) The cell counting in the four groups. a, Snail siRNA (-) and overexpression (-); b, Snail siRNA (+) and overexpression (-); c, Snail siRNA (-) and overexpression (+); d, Snail siRNA (+) and overexpression (+). The darker color (A) and higher column (B) indicates more cells.
Discussion
Ovarian cancer cells can easily invade and diffuse in the pelvic cavity, which is a crucial reason for the high mortality of ovarian cancer which is the top one among a variety of gynecological tumors [7]. The EMT process is an early event in the course of invasion and metastasis of tumor cells [8,9], in which Snail acts as a vital regulator. Recently, Snail has become a focus of the tumor research. Therefore, it’s clinically significant for studying the mechanism of the invasion and metastasis of ovarian cancer cells to explore whether the EMT process exists in ovarian cancer tissues and the expression of Snail in carcinoma and adjacent tissues.
The concept of EMT was firstly proposed by GarryG and Elisabeth in 1982, referring to the phenomenon that epithelial cells differentiated into interstitial cells in certain stimulation of physiological or pathological conditions [10], such as wound healing, embryonic development and early stage of tumor metastasis. The common cytologic features of the EMT process are the gradual loss of cell adhesion and the enhancement of cells migration ability [11,12]. EMT induces epithelial cells to lose polarity and cell adhesion, and filamentous actin tonofilaments of epithelial cells rearrange and thus express lamellipodia and filopodia, contributing to the acquirement of cell migration and invasion ability and eventual formation of interstitial cells [13-15]. The occurrence of EMT is not activated by a single factor but by internal and external factors of tumor cells. This process in which a variety of molecules of multiple signaling pathways have participated is commonly regulated by the crosstalk of signaling pathways. The regulation of transcription factors is one of important regulation approaches, in which the zinc finger transcription factor Snail is a key regulator and has a significant role in the occurrence of EMT. The main structural basis of the EMT process Snail participated in is the interaction between highly conserved specific sequences of carboxyl terminal of Snail family and the DNA promoter containing E-box (CAGGTG).
This study determined the expression levels of EMT markers of ovarian cancer tissues in different FIGO stages. The results demonstrated that the expression levels of Snail, Fibronectin and N-cadherin in late FIGO stages (stage III and IV) were higher than that in early FIGO stages (stage I and II), while the expression of E-cadherin and β-catenin in stage III and IV were lower than that in stage I and II. The capability of ovarian cancer cells invasion and metastasis is relatively weaker in stage I and II when there are no lymph node and peritoneal metastasis. Ovarian cancer cells highly differentiate in stage III and IV when tumor metastasis generally has been detected [16,17]. The results definitely revealed that Snail played a vital role in mediating tumor cells metastasis. In addition, the EMT process was related to FIGO stages. With later FIGO stages, the EMT process was more obvious, and cells lost polarity and eventually became interstitial cells. Therefore, it would be a potential target for gene therapy of ovarian cancer to inhibit Snail expression and suppress the invasion and metastasis of ovarian cancer cells.
In the study, we also detected the impact of Snail on the gene expression of EMT markers. The results indicated that the expression of Snail was positively associated with that of Fibronectin and N-cadherin. Fibronectin and N-cadherin levels were up-regulated with the over expression of Snail while down-regulated after silencing Snail genes compared to the control group, suggesting that Fibronectin and N-cadherin were the downriver effector molecules in the EMT process mediated by Snail. N-cadherin is an adhesion molecule mediating intercellular aggregation, which could resist the hydrolysis of protease in the presence of Ca2+ and plays an important role in tumor cells metastasis. E-cadherin is a homologous protein of N-cadherin which is one of Ca2+ adhesion molecules, having a great effect on the maintenance of epithelial cells structure and cell polarity, and participating in the regulation of cell differentiation. N-cadherin could promote the EMT process, whose effect is opposite to that of E-cadherin [18-20]. Further, we studied the influence of Snail over expression or silencing on the invasion and metastasis of A2780 cell line. After transfected the Snail expression vector, the number of cells entering into the lower chamber through Transwell was significantly higher than that of the control group. After RNAi-Snail, the invasive capacity of A2780 was significantly decreased, implying that the occurrence of EMT led to decreased cell polarity, cell dedifferentiation, changes of connections between cells and strengthened cells invasive ability.
In conclusion, the present study revealed the correlation between Snail and invasion and metastasis of ovarian cancer and epithelial-mesenchymal transition based on tissue and cell levels, and to some extent explored the molecular mechanism of the EMT process mediated by Snail. However, there are several limitations in the study. Further researches on different types of patients with ovarian cancer and expansion of the clinical sample size are needed to conduct a more detailed study on the metastasis of ovarian cancer, which would be the focus of our future studies.
Acknowledgements
This work was supported by Heilongjiang Province Education Department Science and Technology Research Project (No. 12541935).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, Fiering S, Conejo-Garcia JR. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye H, Karim AA, Loh XJ. Current treatment options and drug delivery systems as potential therapeutic agents for ovarian cancer: a review. Mater Sci Eng C Mater Biol Appl. 2014;45:609–619. doi: 10.1016/j.msec.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZS, Ling DJ, Zhang YD, Feng JX, Zhang XY, Shi TS. Octamer-binding protein 4 affects the cell biology and phenotypic transition of lung cancer cells involving beta-catenin/ E-cadherin complex degradation. Mol Med Rep. 2015;11:1851–1858. doi: 10.3892/mmr.2014.2992. [DOI] [PubMed] [Google Scholar]
- 5.Stewart CJ, McCluggage WG. Epithelial-mesenchymal transition in carcinomas of the female genital tract. Histopathology. 2013;62:31–43. doi: 10.1111/his.12057. [DOI] [PubMed] [Google Scholar]
- 6.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Liang C, He J. Large malignant ovarian tumors during pregnancy: two cases. Onco Targets Ther. 2014;7:2121–2125. doi: 10.2147/OTT.S69799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du F, Wu X, Liu Y, Wang T, Qi X, Mao Y, Jiang L, Zhu Y, Chen Y, Zhu R, Han X, Jin J, Ma X, Hua D. Acquisition of paclitaxel resistance via PI3Kdependent epithelialmesenchymal transition in A2780 human ovarian cancer cells. Oncol Rep. 2013;30:1113–1118. doi: 10.3892/or.2013.2567. [DOI] [PubMed] [Google Scholar]
- 9.Lili LN, Matyunina LV, Walker LD, Wells SL, Benigno BB, McDonald JF. Molecular profiling supports the role of epithelial-to-mesenchymal transition (EMT) in ovarian cancer metastasis. J Ovarian Res. 2013;6:49. doi: 10.1186/1757-2215-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay ED. The extracellular matrix in development and regeneration. An interview with Elizabeth D. Hay. Int J Dev Biol. 2004;48:687–694. doi: 10.1387/ijdb.041857rt. [DOI] [PubMed] [Google Scholar]
- 11.Acloque H, Thiery JP, Nieto MA. The physiology and pathology of the EMT. Meeting on the epithelial-mesenchymal transition. EMBO Rep. 2008;9:322–326. doi: 10.1038/embor.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 13.Shi JW, Liu W, Zhang TT, Wang SC, Lin XL, Li J, Jia JS, Sheng HF, Yao ZF, Zhao WT, Zhao ZL, Xie RY, Yang S, Gao F, Fan QR, Zhang MY, Yue M, Yuan J, Gu WW, Yao KT, Xiao D. The enforced expression of c-Myc in pig fibroblasts triggers mesenchymal-epithelial transition (MET) via F-actin reorganization and RhoA/Rock pathway inactivation. Cell Cycle. 2013;12:1119–1127. doi: 10.4161/cc.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 16.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2011:CD005340. doi: 10.1002/14651858.CD005340.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfisterer J, Harter P, Canzler U, Richter B, Jackisch C, Hahmann M, Hasenburg A, Burges A, Loibl S, Gropp M, Huober J, Fink D, Bois A AGO Ovarian Committee; AGO Ovarian Cancer Study Group (AGO-OVAR) The role of surgery in recurrent ovarian cancer. Int J Gynecol Cancer. 2005;15(Suppl 3):195–198. doi: 10.1111/j.1525-1438.2005.00430.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Das A, Sen S. Extracellular matrix density promotes EMT by weakening cell-cell adhesions. Mol Biosyst. 2014;10:838–850. doi: 10.1039/c3mb70431a. [DOI] [PubMed] [Google Scholar]
- 19.Lu WH, Wang G, Li Y, Li S, Song XY, Wang XY, Chuai M, Lee KK, Cao L, Yang X. Autophagy functions on EMT in gastrulation of avian embryo. Cell Cycle. 2014;13:2752–2764. doi: 10.4161/15384101.2015.945850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma-Nicolas JP, Lopez-Colome AM. Thrombin induces slug-mediated E-cadherin transcriptional repression and the parallel up-regulation of N-cadherin by a transcription-independent mechanism in RPE cells. J Cell Physiol. 2013;228:581–589. doi: 10.1002/jcp.24165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


