Abstract
Objective: To explore the biological effects of ray cartilage extract (RCE) on human breast cancer cell line MCF-7 and its mechanism. Methods: MCF-7 cells were treated with RCE of different concentrations for different durations, and then MCF-7 cell proliferation was evaluated with MTT test, cell cycle was detected with flow cytometer and the protein levels of cyclin D1 and p21 were determined with Western blot. Results: MTT test indicated that MCF-7 cell proliferation was inhibited by RCE with an optimal inhibiting concentration of 10 μmol/L and an optimal action time of 48 h. Flow cytometer displayed that with the time prolongation of RCE action, the cells in S phase were significantly increased, but the cells in G2/M phase were significantly decreased; and MCF-7 apoptosis significantly increased as compared with blank control group (all P<0.05). Western blot found that with the time prolongation of RCE action, the level of cyclin D1 was significantly decreased, but the level of p21 was significantly increased as compared with blank control group (all P<0.05). Conclusion: RCE inhibits MCF-7 cell proliferation via arresting MCF-7 cell transformation from S phase to G2 phase. This may be associated with regulating the expressions of cyclin D1 and p21. RCE may be used as a drug for treatment of breast cancer in the future.
Keywords: Breast cancer, ray cartilage extract, MCF-7 cell line, cyclin
Introduction
Breast cancer, a common malignant tumor, is caused by abnormal proliferation of breast-ductal epithelial cells under the action of carcinogenic factors. Breast cancer occurs mainly in women, but rarely in men. The incidence of breast cancer in women is nearly 100 times as much as that in men. About 1.2 million women have breast cancer and about 500 thousand women died of it in the world every year.
Breast cancer is characterized by breast lump with invasion and slow progression. The therapeutic methods for breast cancer include surgery, radiotherapy, chemotherapy and endocrine therapy. However, most patients cannot tolerate severe side effects of postoperative chemotherapy including hair loss, nausea and vomiting, bone marrow suppression and secondary infection, so some patients give up treatment. Therefore, it is necessary to find a new effective drug with less toxicity.
Tumorigenesis is strongly associated with abnormal cell proliferation and differentiation [1], so cell proliferation-related cyclins have become a hotspot in investigating tumorigenesis. It has been reported that ray cartilage extract (RCE) can inhibit CEM cell proliferation in vitro and the growth of sarcoma-180 in mice, showing that RCE has anticancer effects [2]. In this study, human breast cancer MCF-7 cells were treated with RCE, and then the effects of RCE on MCF-7 cells and expressions of cyclins D1 and p21 were observed and its anticancer mechanism was explored. This study provides in vitro data on RCE’s anti-cancer activity and sheds some light on the possible mechanisms.
Materials and methods
All study methods were approved by ethics committee of the First Affiliated Hospital, Liaoning Medical University.
Materials
RCE, a single polysaccharide with a molecular weight of 9.7×104, is extracted from sting ray’s cartilage. Human breast cell line MCF-7 was provided by Cell bank of Shanghai. RPMI-1640 AND Trypsin were purchased from Gibo (Grand Island, Newyork, USA). Fetal bovine serum (FBS) was from Sijiqing Biological Engineering Materials Co., Ltd (Hangzhou, China). MTT, DMSO, Annexin V-FITC, mouse anti human monoclonal antibodies of cyclins D1 and p21, and alkaline phosphatase-labeled secondary antibody were purchased from Sigma (St. louis. MO, Missouri, USA).
Cell culture
Human breast cancer MCF-7 cells were incubated in RPMI medium 1640 containing 10% FBS, 100 U/ml of penicillin and streptomycin, respectively, in an atmosphere of 5% CO2 at 37°C. The medium was changed every 2-3 days and passage was performed with trypsin every 4-7 days.
MTT assay
The MCF-7 cells in logarithmic growth phase were digested with 0.25% of trypsin, and then were inoculated into 96-well plate at a rate of 1×104/ml (100 μl). After adherence, 2.5, 5, 10, 20 and 40 μmol/L of RCE (100 μl) was added into five wells for each group, respectively. And then, 24 h, 48h and 72 h later, MTT (0.5 mg/ml, 100 μl) was added. Following 4 h, the supernatant was removed, then 150 μl of DMSO was added and mixed for 15 min followed by determining absorbance value (A value) at 490 nm with ELSA monitor. At the same time, blank group was also set. The formula to calculate inhibition rate was as follows: inhibition rate = 1-absorbance in RCE groups/absorbance in control group ×100%.
Flow cytometry
After MCF-7 cells (1×106/ml) were treated with 10 μmol/L of RCE for 24 h and 48 h respectively. A total of 100 μl (1×106/ml) was washed with PBS twice, centrifuged at 1000 r/min for 5 min and fixed with cold alcohol followed by PI staining for analysis of cell cycle. The MCF-7 cells in logarithmic growth phase were treated with 10 μmol/L of RCE for 24 h and 48 h respectively, were washed with PDS twice at 4°C and centrifuged at 2000 rpm for 5 min. After resuspension with 250 μl of buffer, A total of 100 μl (1×106/ml) was placed into a tube, and then 5 μl of Annexitin V-FITC/PI and 10 μl of PI (20 mg/ml) were added at room temperature in the dark for 15 min. Finally, 400 μl of PBS was added for analysis of apoptosis.
Western blot
MCF-7 cells were inoculated in a 6-well plate at 1.5×106/well overnight, and then treated with 10 μmol/L of RCE for 24 h and 48 h, respectively. Lysate (165 μl) containing 1 mmol/L of PMSF was added in MCF-7 cells for clearage on ice followed by centrifugation at 12000 r/min at 4°C for 10 min. After removal of supernatant, protein was extracted followed by quantitation. Protein in each group underwent degeneration in water bath (100°C), 10% SDS-PAGE electrophoresis and semi-dry transmembrane. The membrane was washed with TTBS, sealed with 10% of dried skim milk followed by addition of mouse anti-human monoclonal antibody of cyclins D1, p21 and β-actin, respectively, overnight. After washed with TTBS once, the membrane was placed in alkaline phosphatase-labeled secondary antibody (1:10000) for one hour, and then visualized with work liquid prepared according to the instructions of Bio-Rad kit.
Statistical analysis
Statistical treatment was performed with SPSS16.0 software. Measurement data were expressed as (x̅±s). Two-way analysis of variance was used in the analysis of MTT assay. One-factor analysis of variance was used in the analysis of action time and concentration of drugs. q test was used in the comparisons between groups. Statistical significance was established at P<0.05.
Results
Inhibitory effects of RCE on MCF-7 cell proliferation
As shown in Figure 1 and Table 1, with the increases in the concentration and action time of RCE, the inhibitory effects of RCE on MCF-7 cell proliferation were not always increased, and the optimal inhibiting concentration was 10 μmol/L and the optimal action time was 48 h. There were significant differences in inhibition rate between different-concentration groups at the same time point and between different time points at the same concentration (P<0.05). The concentrations were irrelevant to action time without interaction (P>0.05).
Figure 1.
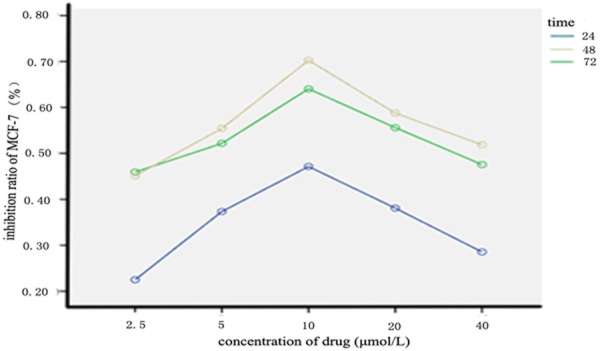
Inhibition rates of MCF-7 cells caused by different-concentration RCE. Note: RCE: Ray cartilage extract.
Table 1.
Inhibition Rates of MCF-7 Cells Caused by RCE (%)
| Groups | 24 h | 48 h | 72 h |
|---|---|---|---|
| 2.5 μmol/l | 22.50±1.60 | 45.93±4.99 | 45.11±3.20 |
| 5.0 μmol/l | 37.34±6.68 | 52.17±2.55 | 49.45±3.89 |
| 10 μmol/l | 47.15±4.71 | 64.04±3.06 | 61.27±0.90 |
| 20 μmol/l | 38.08±3.76 | 55.60±7.37 | 53.77±4.73 |
| 40 μmol/l | 28.54±3.22 | 47.57±4.16 | 41.87±2.45 |
Notes: There are significant differences between different-concentration groups at the same time point (F=63.79, P<0.05). There are significant differences between different-time points at the same concentration (F=194.82, P<0.05). There are no significant differences between concentrations and action time (F=1.05, P>0.05). C: Concentration; T: Time; RCE: Ray cartilage extract.
Flow cytometry
With the time prolongation of RCE action, MCF-7 cells in S phase were increased, but MCF-7 cells in G2/M phase were decreased (Figure 2). Compared with blank control group, more MCF-7 cells were arrested in S phase in RCE groups, suggesting that RCE action was associated with MCF-7 cell transformation from S phase to G2 phase, namely that the inhibition point of RCE was S/G2. With the time prolongation of RCE action, MCF-7 cell apoptosis was increased. Compared blank control group, apoptosis rates in 24 h- and 48 h-treated groups were significantly increased (all P<0.05) (Figure 3).
Figure 2.
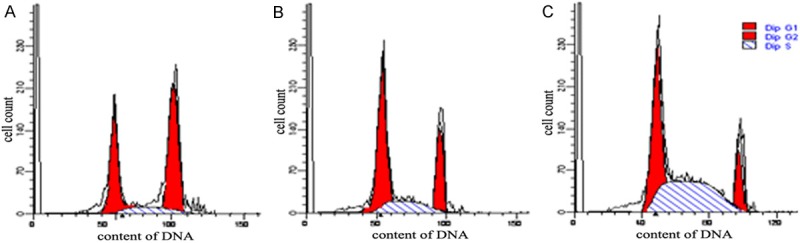
Effects of RCE on MCF-7 cell cycle determined with flow cytometer. A: Blank control group: 36.76% of MCF-7 cells in G1 phase, 12.94% of MCF-7 cells in S phase, and 50.30% of MCF-7 cells in G2 phase. B: RCE (24 h-treated) group: 49.66% of MCF-7 cells in G1 phase, 20.34% of MCF-7 cells in S phase, and 30.00% of MCF-7 cells in G2 phase. C: RCE (48 h-treated) group: 25.91% of MCF-7 cells in G1 phase, 44.08% of MCF-7 cells in S phase, and 20.01% of MCF-7 cells in G2 phase. Note: RCE: Ray cartilage extract.
Figure 3.
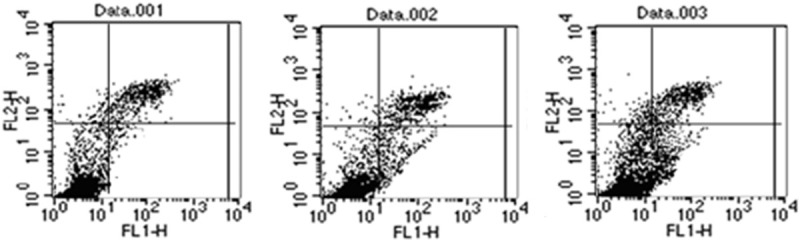
Effects of RCE on MCF-7 cell apoptosis determined with flow cytometer. Data. 001: Blank control group: Apoptosis rate is 1.57%. Data. 002: RCE (24 h-treated) group: Apoptosis rate is 6.25%. Data. 003: RCE (48 h-treated) group: Apoptosis rate is 16.98%. Note: RCE: Ray cartilage extract.
Western blot
Effects of RCE on the expressions of cyclins D1 and p21 are shown in Figure 4. Compared with blank control group, cyclin D1 expression was significantly decreased in RCE groups (P<0.05). Cyclin D1 expression in 24 h-treated group was (34.11±1.17)% of cyclin D1 expression in blank control group and in 48 h-treated group was (21.31±0.78)%. Compared with blank control group, cyclin p21 expression was significantly increased in RCE groups (P<0.05). Cyclin p21 expression in 24 h-treated group was (1.22±0.24) times cyclin P21 expression in blank control group, and in 48 h-treated group was (1.45±0.29) times.
Figure 4.
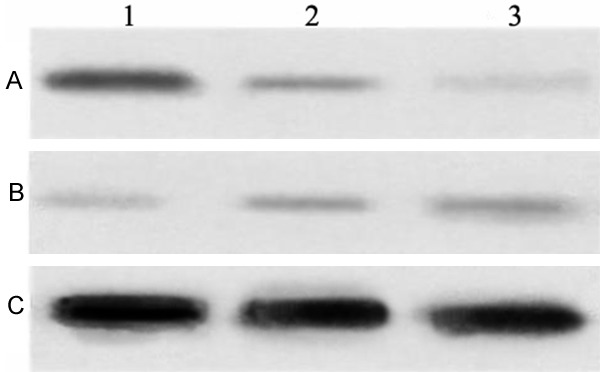
Expressions of cyclins D1 and p21 in each group detected with Western blot. Notes: Lane 1: Blank control group, Lane 2: 24 h-treated group and Lane 3: 48 h-treated group. A: Cyclin D1, B: Cyclin p21; RCE: Ray cartilage extract.
Discussion
RCE, a single polysaccharide, is extracted from sting ray’s cartilage [2]. Tumor cells are characterized by uncontrolled proliferation. Cell proliferation and differentiation are regulated by both oncogenes and anti-oncogenes [3].
Cyclin D1, an important member of cyclin G family, is regarded as oncogene. It is located on 11q13, and contains 5 exons and 4 introns with a length of 15 kb. Cyclin D1-encoding protein contains 295 amino acid residues with a molecular weight of 36 kDa [4]. Cyclin D1 plays its biological role during G1 phase through activating CDK4 or CDK6, and its over-expression can promote cell transformation from G1 phase to S phase [5]. Cyclin D1 as a candidate cancer gene was first found in B-Cell lymphoma and parathyroidoma, afterwards was confirmed to be involved in molecular mechanisms of some solid tumors, especially breast cancer [6].
P21, an important negative regulator of cell cycle and an arrestin of cyclin dependent kinase, is involved in cell growth and differentiation, and DNA repair. p21 plays an important role in regulating cell cycle, and cell differentiation and apoptosis through mediating many transcription factors [7]. The changes in cell cycle are complex and many proteins are involved in regulation of cell cycle. Cyclin D1, a promotor in cell cycle and a protooncogene, can promote cell transformation along with many oncogenes p21, a regulatory factor of cell cycle, can inhibit the activity of cyclin D1, leading to cell cycle arrest and cell proliferation suppression p21 plays a negative role in cell proliferation [8].
In this study, after human breast cancer MCF-7 cells were treated with RCE, MTT, flow cytometry and Western blot were performed. Our study suggests that RCE can inhibit MCF-7 cell proliferation and induce MCF-7 cell apoptosis via down-regulation of cyclin D1 and up-regulation of cyclin p21. This study provides in vitro data on RCE’s anti-cancer activity and sheds some light on the possible mechanisms.
Disclosure of conflict of interest
None.
References
- 1.Wang HB, Gong JP, Qiu FZ, Wu ZD, Tao DD. Cyclin expressions andmechanisms of their actions in human tumor cell differentiation. Chinese Journal of Experimental Surgery. 2003;20:39–40. [Google Scholar]
- 2.Guo B, Fan HY, Jia YH. Effects of ray cartilage extract on immune function in tumor-bearing mice. Liaoning Journal of Traditional Chinese Medicine. 2002;29:104. [Google Scholar]
- 3.Zhao JJ, Zhu Min, Huang TS. Relation between genetic polymorphism of p21WAF1 and the risk of breast cancer. Jiangsu Medical Journal. 2007;33:1110–1112. [Google Scholar]
- 4.Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. CyclinD1, EMS1 and 11q13 amplication in breast cancer. Breast Cancer Res Treat. 2003;78:323–335. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- 5.Zhang SQ, Li DC. Expression and significance of and cyclin D1 in hepatocellular carcinoma. Jiangsu Medical Journal. 2004;30:26–28. [Google Scholar]
- 6.Elsheikh S, Green AR, Aleskandarany MA, Grainge M, Paish CE, Lambros MB, Reis-Filho JS, Ellis IO. CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res Treat. 2008;109:325–335. doi: 10.1007/s10549-007-9659-8. [DOI] [PubMed] [Google Scholar]
- 7.Altieri DC. Survivin, versatile modulation of cell division andapoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 8.Lebeau A, Unholzer A, Amann G, Kronawitter M, Bauerfeind I, Sendelhofert A, Iff A, Lohrs U. EGFR, HER-2/neu, cyclinD1, p21 and p53 in correation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2003;79:187–198. doi: 10.1023/a:1023958324448. [DOI] [PubMed] [Google Scholar]


