Abstract
POEMS syndrome is a rare plasmacyte-associated disease, one of the major diagnostic criteria of which is sclerotic bone lesion. To detect bone lesions in POEMS syndrome, which imaging method should be routinely applied and what characteristics they display are still unconfirmed. We analyzed clinical data and imaging characteristics of bone lesions in 22 patients with POEMS using multimodal methods, including conventional X-ray, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT). Images on X-ray and CT exhibited plaque-like high-density for osteosclerotic lesions and punched-out low-density appearance for osteolytic ones. X-ray had advantage in detecting bone lesions in skull, extremity long bones, clavicle, and scapula, while CT could display sharp outline of lesions and was more sensitive than X-ray in detecting the small lesions. Osteosclerotic lesions on MRI demonstrated decreased signal intensity on both T1 and T2-weighted sequences, while osteolytic lesions or osteolytic part of mixed lesions showed high signal intensity on T2-weighted sequences. MRI had same sensitivity as CT, but with superiority in distinguishing the active osteolytic lesions from the osteosclerotic ones. PET-CT showed 18F-FDG uptake was normal in the majority of osteosclerotic lesions, and slightly increased in mixed ones, but obviously elevated in osteolytic ones. PET/CT was less sensitive in detecting osteosclerotic lesions than in detecting osteolytic ones. In conclusion, to detect bone lesions in POEMS, conventional X-ray scan should be first performed, further followed by more sensitive CT or MRI. PET-CT is optional when the osteolytic lesions are suspected.
Keywords: Bone lesions, POEMS syndrome, X-ray, CT, MRI, PET/CT
Introduction
Polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) syndrome, also called osteosclerotic myeloma, Crow-Fukase syndrome, or Takatsuki syndrome [1,2], is a rare plasmacyte-associated disorder. Osteosclerosis is a unique abnormality and one of the major diagnostic criteria of this disease [3-5]. Moreover, whether the lesion is solitary or multiple determines whether the patient should receive systematic chemotherapy or focal irradiation [5,6]. So it is very important to confirm the presence and number of bone lesions in order to establish diagnosis and plan appropriate treatment. Imaging methods play a vital role in detecting the bone lesions, but the appliance of different imaging approaches varies throughout the literature [7-10]. Which method should be routinely applied and what characteristics these imaging methods display in detecting bone lesions of POEMS are still unconfirmed. The reports about the role of CT in detecting bone lesion in POEMS syndrome are only a few [10,11]. MRI descriptions for the bone lesions in POEMS syndrome are rare [8,12,13]. Although the utility of FDG PET/CT has been described in multiple myeloma and solitary plasmocytoma, its usefulness in POEMS has yet to be clearly defined [9,14-17]. Multimodal imaging comparison for these bone lesions is rarer. In current study we used the multimodal imaging techniques, including conventional X-ray, CT, MRI, and PET/CT, for the assessment of bone involvement in 22 patients with POEMS syndrome. The clinical characteristics of these bone lesions also were analyzed.
Patients and methods
Patients
This study included 22 patients with POEMS syndrome between the year of 2003 and 2011. All the patients met both two major criteria and one minor criterion for the diagnosis of POEMS syndrome [18]. In brief, the diagnostic criteria in 2003 included two major criteria (polyneuropathy and monoclonal plasma proliferation) and seven minor criteria (sclerotic bone lesions, Castleman’s disease, organomegaly, extravascular volume overload, endocrinopathy, skin changes, and papilledema). The institution review board approved the present retrospective study, and the requirement for informed consent was waived.
Imaging scan and interpretation
Twenty one patients received chest/abdomen/pelvic CT and eight underwent conventional X-ray examination. CT scan was made from the upper margin of sternum to the sacral vertebrae. The scope of X-ray survey involved skull and long bones, which were out of field for CT, as well as pelvis, ribs, vertebrae, clavicles and scapula. MRI scans of thoracic and lumbar vertebrae were further performed selectively in four patients with suspicious lesions by CT or X-ray. Six patients received whole-body PET/CT examination. All images were reviewed again independently by two senior radiologists with 15 and 12 years of experience in radiological diagnosis, respectively.
The clinical data, including histories, symptoms, physical examinations and laboratory tests also were analyzed in these patients.
Results
Clinical characteristics
The mean age of the 22 patients with POEMS syndrome was 49.5±11.6 years (ranging from 32 to 76). Sex ratio was 9:2 (18 men versus 4 women). The symptoms of these patients were related to multiple organs and multiple systems, including polyradiculoneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell proliferation, skin change, extravascular volume overload, and thrombocytosis, as well as the bone lesions which were found in 45% patients (Table 1). In the ten patients with bone lesions no fracture was found, and bone pain was found only in two patients whose skeletal change were confirmed as osteolytic or mixed lesions by X-ray or CT (shown below). The detailed clinical data and imaging features of the ten patients were shown in Table 2.
Table 1.
Detailed clinical manifestations of 22 patients with POEMS
| Characteristics | % (n=22) or Mean ± SD |
|---|---|
| Polyradiculoneuropathy | 100 (22/22) |
| Organomegaly | |
| Splenomegaly | 73 (16/22) |
| Hepatomegaly | 23 (5/22) |
| Lymphadenopathy | 54 (12/22) |
| Endocrinopathy | |
| Abnormal ACTH | 58 (7/12) |
| Increased prolactin | 91 (10/11) |
| Decreased FT3 | 55 (10/18) |
| Decreased FT4 | 39 (7/18) |
| Increased TSH | 72 (13/18) |
| Monoclonal plasma cell proliferation | |
| M protein on immunofixation electrophoresis | 95 (20/21) |
| Increased plasma cells in BM | 55 (10/18) |
| value | 2.7±5.9% |
| Skin change | 86 (19/22) |
| Extravascular volume overload | |
| Edema | 73 (16/22) |
| Pleural effusion | 82 (18/22) |
| Pericardial effusion, | 64 (14/22) |
| Ascites | 59 (13/22) |
| Bone lesions | 45 (10/22) |
| Thrombocytosis | 45 (10/22) |
ACTH: adrenocorticotropic hormone; FT3: free triiodothyronine; FT4: free unbound thyroxine; TSH: thyroid stimulating hormone; BM:bone marrow smear.
Table 2.
Summary of clinical and imaging features of the ten patients with bone lesions
| Cases | Age (years) | Sex | Location | Bone pain | Number | Nature | X-ray (density) | CT (density) | MRI (T2 signal) | PET/CT (SUV) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | F | skull, vertebrae, pelvis, ribs, scapula, femur, sternum | N | multiple | sclerotic | high | hyper | hypo | normal |
| 2 | 54 | M | vertebrae, pelvis | Y | multiple | mixed | mixed | mixed | mixed | increased |
| 3 | 33 | M | vertebrae | N | multiple | sclerotic | hyper | hypo | ||
| 4 | 64 | M | vertebrae, ribs | N | multiple | sclerotic | hyper | normal | ||
| 5 | 32 | M | ribs | Y | solitary | lytic | hypo | |||
| 6 | 58 | M | femur | N | solitary | sclerotic | hyper | normal | ||
| 7 | 49 | M | vertebrae | N | multiple | sclerotic | hyper | |||
| 8 | 54 | M | clavicle, humerus | N | multiple | sclerotic | hyper | normal | ||
| 9 | 62 | M | vertebrae | N | multiple | sclerotic | hyper | |||
| 10 | 76 | M | ribs | N | multiple | mixed | mixed | mixed |
M: male; F: female; N: no; Y: yes.
Multimodal imaging characteristics of bone lesions in POEMS syndrome
Bone lesions were detected in 62.5% (5/8) patients by X-ray, 38.1% (8/21) by CT and 71.4% (5/7) by both of them. Bone lesions on X-ray or CT were classified into three groups: “osteosclerotic” defined as a lesion with high density surrounded by normal bone marrow or fused with bone cortex, “osteolytic” defined as a lesion with low density in the bone cortex, and “mixed”. Osteosclerotic lesions were found in seven patients, osteolytic in one, and mixed in two patients, respectively. Osteosclerotic lesions on X-ray exhibited multiple, scattered, variably sized, and thickening plaque-like appearance (Figures 1A-C, 2C, 2D). Most of the osteosclerotic lesions were less than 10 mm in diameter. The long diameter of largest lesion measured in a patient’s left ischial ramus reached approximately 40 mm (Figure 1C). Some of the lesions were shown to possess peripheral irregularities and spiculations, characterized as a mulberry-like appearance (Figure 1C). Some osteosclerotic lesions were sporadic while others were confluent, which made the margins of the lesions ranging from appearing well defined to having fluffy sclerosis (Figure 1C). X-ray frequently showed bone lesions in skull, long bones, clavicle, and scapula, where some lesions were not detected by the chest/abdomen/pelvic CT (Table 2, cases 6 and 8). However, as compared to X-ray (Figure 1A-C), the CT images clearly showed sharp outline of the lesions, even including those whose diameters were less than 2 mm (Figures 1D-G, 3A), suggesting that CT was superior to X-ray in detecting small bone lesions. Osteolytic lesions on X-ray and CT exhibited punched-out low-density features (Figure 2F, 2H).
Figure 1.
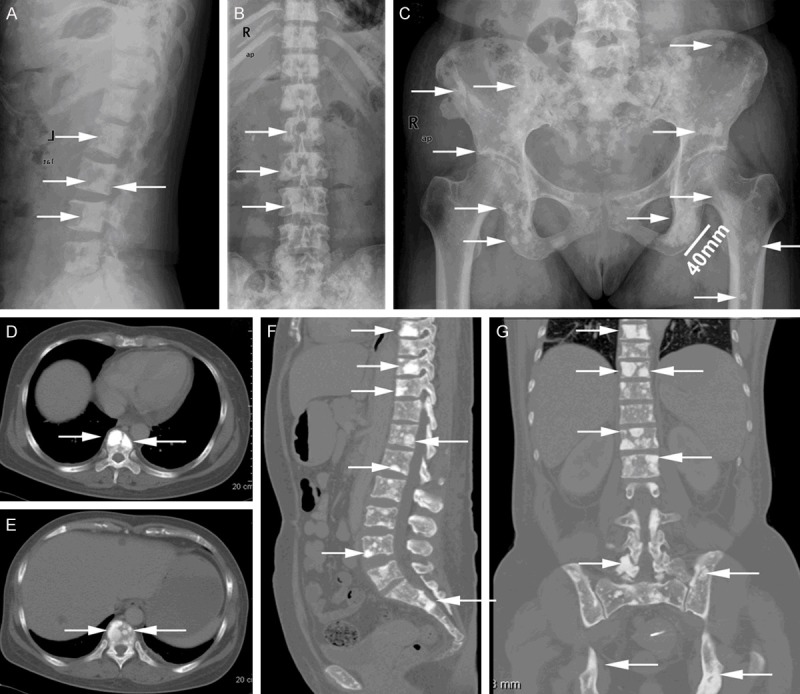
A 47-year-old woman with multiple osteosclerotic lesions (case 1). (A-C) Osteosclerotic lesions on X-ray exhibit multiple, scattered, variably sized, and disseminated “thickening plaque-like” appearance. The long diameter of the lesion in the left ischial ramus reached approximately 40 mm (C). (D and E) Axial CT images clearly show sharp outline of osteosclerotic lesions. (F and G) Sagittal and coronal multiplanar images reconstructed from the axial CT data display various disseminated osteosclerosis in vertebrae and pelvis. Similar lesions were also present in the skull, ribs, and scapula. Note the difference between X-ray and CT images.
Figure 2.

A 54-year-old man with mixed bone lesions (case 2). (A and B) Bone lesions in thoracic vertebrae (T) 12 and lumbar vertebrae (L) 5 are of low signal intensity on both MRI T1 (A) and T2 (B) -weighted sequences, but lesions in L3 demonstrated high-low mixed signal intensity on T2-weighted sequence. (C and D) X-ray shows bone lesions in T9, and L3. Note that the small lesions in T12 and L5 were missed. (E) Coronal T2-weighted MRI image demonstrates the high-low mixed signal intensity in L3 and significant increase signal in right ischial ramus. (F) X-ray shows punched-out low-density osteolysis in right ischial ramus. Lesion in L3 is not clearly shown by X-ray. (G-J) 18F-FDG PET/CT reveals slightly increased metabolism of osteosclerotic lesion in L3 (G) and sternum (I), while very elevated metabolism of osteolytic lesion in right ischial ramus (H and J). It also shows the high 18F-FDG uptake of right inguinal (H) and bilateral axillary (I) lymph nodes.
Figure 3.

A 33-year-old man with multiple osteosclerotic lesions (case 3). (A) Serial axial CT images from thoracic to lumbar vertebrae show multiple small “thickening” osteosclerotic lesions. (B and C) MRI T1 (B) and T2 (C)-weighted sequences of sagittal MRI images show several variably sized lesions with low signal intensity in whole lumbar vertebrae. Note the signal features and the detecting sensitivity between CT and MRI.
In four patients who had MRI examination, three were detected with bone lesions. The majority of osteosclerotic lesions confirmed by CT (Figure 3A) or X-ray were of homogeneously decreased signal intensity on both T1 and T2-weighted sequences (Figure 3B, 3C). While the osteolytic lesion in the right ischial ramus of case 2 with punched-out low-density on X-ray (Figure 2F) displayed high signal intensity on MRI T2-weighted sequence (Figure 2E). A mixed bone lesion in lumbar vertebrae (L) 3 of case 2, which was not clearly displayed by X-ray (Figure 2C, 2D, 2F) and CT (Figure 2G), was detected by MRI showing a small area of increased T2 signal surrounded by low-signal area (Figure 2A, 2B, 2E). MRI has the same sensitivity as CT in showing sharp outline of the lesions (Figure 3). And thus MRI also could detect small lesions in vertebrae, including those missed by X-ray. For example, the small osteosclerotic lesions in thoracic vertebrae (T) 12 and L 5 with low signal intensity on both MRI T1 and T2-weighted sequences were detected by MRI (Figure 2A, 2B), but missed by X-ray (Figure 2C, 2D).
In six patients receiving PET/CT scans, three had multiple bone lesions confirmed by X-ray, CT, or MRI (including case 1), but only one patient had elevated 18F-FDG uptake in bones (case 2). This patient had four osteosclerotic lesions in T9, T12, L5, and sternum (Figure 2A-D, 2I), one mixed lesions in L3 (Figure 2A, 2B, 2E, 2G), and a large osteolytic lesion in right ischial ramus (Figure 2E, 2F, 2H, 2J). The 18F-FDG metabolism was normal in T9, T12 and L5, and only slightly increased in sternum (Figure 2I) (maximum standardized uptake value (SUVmax=3.5) and L3 (Figure 2G) (SUVmax=3.0), while significantly elevated (SUVmax=22) in the osteolytic site of right ischial ramus (Figure 2H, 2J). In this patient, the right inguinal (Figure 2H) and bilateral axillary (Figure 2I) lymph nodes, the biopsy of which was diagnostic for the hyaline-vascular of Castleman’s disease, also were displayed with hypermetabolism.
By comprehensive utilization of multiple imaging methods above, the majority of the bone lesions were found to be sclerotic, multiple, and frequently present in axial bone, such as vertebrae, ribs, and pelvis (Table 2).
Discussion
As POEMS syndrome is a rare and complicated disease, making the diagnosis of it is a big challenge. One of the major diagnostic criteria of POEMS syndrome is the skeletal damage with typical osteosclerotic or mixed lesions. The definitive diagnosis could be confirmed by the finding of an osteosclerotic lesion if the other two major criteria are also fulfilled. Moreover, the number of bone lesions influences decisions regarding therapy. Thus, it is very important to confirm the presence and number of them. Recognizing imaging characteristics of these bone lesions is the first step in effectively detecting them. This study made a delineation of multimodal imaging characteristics of the bone lesions and an evaluation of the role of these imaging methods in detecting bone lesions in POEMS syndrome.
On X-ray and CT, osteosclerotic lesions took a high-density appearance, consistent with the previous reports [3,8,10,19], while osteolytic lesions with a sclerotic rim or lesions with a mixed soap-bubble appearance described by previous literature [3] were not found in the current study. Because conventional X-ray can cover the wider area, it has an advantage in detecting the bone lesions in skull, extremity long bones, clavicle, and scapula, where CT is not suitable for. However, as compared with X-ray, CT can display sharp outline of the lesions and specially be sensitive to reveal the small lesions. Shibuya K et al. [10] also reported CT is particularly useful to detect small bone lesions, as compared to bone scintigraphy. Here we provided the novel direct evidence on the superiority of CT over X-ray in detection of bone lesions.
The T2 signal of MRI can reflect the situation of edema and activity in the lesion sites [8]. In current study, the decreased T2 signal within the vast majority of osteosclerotic lesions likely reflects the absence of reactive edema and may indicate the relative inactivity and chronic nature of them. The increased T2 signal in mixed bone lesions in L3 of case 2, which was undetectable on X-ray and CT scan, may represent slight activity and edema. While the significantly elevated T2 signal in the osteolytic lesions in the right ischial ramus of case 2 probably reflects high activity in this site. There have been just a few reports on the contribution of MRI in the diagnosis of POEMS syndrome [8,12,13] and here we provided new proofs. MRI has the same sensitivity as CT in detecting small bone lesions, but has its superiority in distinguishing the active osteolytic lesions from the inactive osteosclerotic ones.
It is not practical to examine all locations by any of the above imaging methods. Many researchers prefer to utilize whole-body PET/CT to scan bone lesions because of its wider whole body fields of view. Previous reports showed focal osteolytic lesions of multiple myeloma could be well seen with 18F-FDG PET/CT [20]. It was also reported that 18F-FDG PET/CT had its superiority in defining the activity as well as the extent and localization of bone lesions in POEMS [7,9,16]. Our study showed that in six patients receiving PET/CT scan, including three patients with confirmed bone lesions by CT, only one had abnormal 18F-FDG uptake. In all of the bone lesion sites, only one fourth of osteosclerotic sites, as well as one mixed site, had slightly increased 18F-FDG uptake, but the only osteolytic site had very high 18F-FDG uptake, correlated with the high MRI T2 signal mentioned above. These findings were consistent with the fact that plasma cells in the bone marrow of patients with POEMS are less than those with multiple myeloma [3,4,21] and the course of it is chronic. The median percent of plasma cells observed is less than 5%, according to the previous reports [3,4]. In current study, the average percent of plasma cells in the bone marrow were only about 2.7%. The case 1 who had disseminated osteosclerotic lesions but a normal 18F-FDG uptake, received CT-guided biopsy from the sclerotic bone site and the biopsy only showed a slight increase of plasmacytes about 1-5%, which was reported in detail by us previously [22]. Some previous reports have stated local increased 18F-FDG uptake in the sites of bone lesions. But if you carefully read, you will find that the so called hot spots were in low-density osteolytic sites, not in high-density osteosclerotic sites [3,5,20]. Even a few of osteosclerotic sites displayed hypermetabolic characteristics, the extent of which was slight [9,16]. Alberti et al. reported a patient in whom only one of three lesions was found with hyper-metabolism by PET/CT [9]. It suggested that metabolism in osteosclerotic sites is lower than that in osteolytic ones and PET/CT is less sensitive in detecting osteosclerotic lesions than in detecting osteolytic lesions. It was also reported that sclerotic bone metastases frequently show no or only a low degree of FDG uptake on PET/CT, in comparison with lytic or mixed bone metastases [23-25]. Thus, PET/CT has limited value for detecting osteosclerotic lesions, which predominate in POEMS, but is useful in detecting osteolytic or mixed ones. In addition, PET/CT may be beneficial for detecting soft tissue plasmacytoma [26] and monitoring the responses after treatment [17,26,27], as well as estimating other manifestation of POEMS syndrome, such as Castleman’s disease-relevant lymphadenopathies which are seen in 11-30% of patients with POEMS [9,18,28].
The previous studies reported that lesions were usually found in vertebrae and pelvis [10,11], and our study confirmed it. The majority of bone lesions were found multiple, suggesting that systematic chemotherapy should be used more frequently than focal irradiation in these patients. Focal solitary sclerotic lesions were also present in the minority of the patients, but must be differentiated from other disorders [3]. The best way to distinguish POEMS syndrome from other diseases is to determine whether or not there are other unique clinical symptoms or signs of POEMS syndrome described in Table 1. The overwhelming majority of bone lesions are sclerotic or mixed, while only a few are lytic, consistent with the literature [3,7]. Pain and fractures associated with the osteosclerotic lesions in POEMS syndrome are rare. Patients with POEMS syndrome are usually younger (49.5±11.6 year-old) than those with multiple myeloma, which has a peak incidence in the 7th and 8th decades [3].
In this study only 45% patients were found with bone lesions, while the reports from the Western countries showed bone lesions occur in approximately 95% of patients [3]. The disparities in the rates may originate from race difference between the Eastern and Western, but also probably from the limitation of imaging methods utilized [10,21,29]. Two reports from China showed bone lesions were detected in 27% patients by X-ray examination of only skull, vertebrae, pelvis, femur, and humerus [21] and 41% by systematic X-ray survey [29]. In our study, bone lesions were detected in 62.5% patients by X-ray and 38.1% by CT, but 71.4% by both of them. The detection rate was increased through the use of the both techniques. The false negative results are possible using single imaging method. Comprehensive utilization of multimodal imaging methods can elevate the detection rate of bone lesions in POEMS syndrome. Additionally the heterogeneity and different manifestation of POEMS syndrome also foreshadow the necessity of a complex evaluation of different imaging methods in mutual concordance.
Because POEMS syndrome is rare and bone lesions are present only in a portion of patients, the statistical analysis was unavailable. Additionally, the multimodal imaging methods were not obtained in all patients, because the physicians whom the patients first visited specializing in different disciplines often paid more attentions to their own fields and neglected other fields, thus failed to order imaging examination in some patients. However, the direct comparison between the counterparts of multiple imaging methods in representative patients can lead us to draw the following conclusions: X-ray has an advantage in detecting the bone lesions in skull, extremity long bones, clavicle, and scapula; CT can display sharp outline of the lesions and is sensitive in detecting the small lesions; MRI has the same sensitivity as CT but with superiority in distinguishing the active osteolytic lesions from the osteosclerotic ones; PET/CT is less sensitive in detecting osteosclerotic lesions than in detecting osteolytic lesions.
Conclusion
To detect the bone lesions in POEMS syndrome, conventional whole body X-ray scan should be first performed, further followed by more sensitive CT or MRI. PET-CT is optional for osteolytic ones. We present typical multimodal imaging characteristics to increase awareness of bone lesions in POEMS syndrome for clinicians and provide the objective evaluation of different imaging methods in detecting them.
Acknowledgements
This work was supported in part by grants from the Science and Technology Commission of Zhenjiang Municipality (SH2012029), National Natural Science Foundation of China (81270594), and China Postdoctoral Science Foundation (2013M541528).
Disclosure of conflict of interest
None.
References
- 1.Takatsuki K, Sanada I. Plasma cell dyscrasia with polyneuropathy and endocrine disorder: clinical and laboratory features of 109 reported cases. Jpn J Clin Oncol. 1983;13:543–555. [PubMed] [Google Scholar]
- 2.Nakanishi T, Sobue I, Toyokura Y, Nishitani H, Kuroiwa Y, Satoyoshi E, Tsubaki T, Igata A, Ozaki Y. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology. 1984;34:712–720. doi: 10.1212/wnl.34.6.712. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A. POEMS syndrome. Blood Rev. 2007;21:285–299. doi: 10.1016/j.blre.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:591–601. doi: 10.1002/ajh.22050. [DOI] [PubMed] [Google Scholar]
- 5.Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119:5650–5658. doi: 10.1182/blood-2012-03-378992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwabara S, Dispenzieri A, Arimura K, Misawa S, Nakaseko C. Treatment for POEMS (polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes) syndrome. Cochrane Database Syst Rev. 2012;6:CD006828. doi: 10.1002/14651858.CD006828.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minarik J, Scudla V, Bacovsky J, Pika T, Ctvrtlik F, Metelkova I, Myslivecek M. Comparison of imaging methods in POEMS syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:52–57. doi: 10.5507/bp.2011.053. [DOI] [PubMed] [Google Scholar]
- 8.Chong ST, Beasley HS, Daffner RH. POEMS syndrome: radiographic appearance with MRI correlation. Skeletal Radiol. 2006;35:690–695. doi: 10.1007/s00256-005-0941-8. [DOI] [PubMed] [Google Scholar]
- 9.Alberti MA, Martinez-Yelamos S, Fernandez A, Vidaller A, Narvaez JA, Cano LM, Gamez C, Martinez-Matos JA. 18F-FDG PET/CT in the evaluation of POEMS syndrome. Eur J Radiol. 2010;76:180–182. doi: 10.1016/j.ejrad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Shibuya K, Misawa S, Horikoshi T, Kanai K, Isose S, Nasu S, Sekiguchi Y, Noto Y, Fujimaki Y, Nakaseko C, Kuwabara S. Detection of bone lesions by CT in POEMS syndrome. Intern Med. 2011;50:1393–1396. doi: 10.2169/internalmedicine.50.5263. [DOI] [PubMed] [Google Scholar]
- 11.Narvaez JA, Majos C, Narvaez J, Valls C, Fernandez-Cabrera L. POEMS syndrome: unusual radiographic, scintigraphic and CT features. Eur Radiol. 1998;8:134–136. doi: 10.1007/s003300050353. [DOI] [PubMed] [Google Scholar]
- 12.Michel JL, Gaucher-Hugel AS, Reynier C, Lhoste A, Philippe P, Aumaitre O, Piette JC, Soubrier M. [POEMS syndrome: imaging of skeletal manifestations, a study of 8 cases] . J Radiol. 2003;84:393–397. [PubMed] [Google Scholar]
- 13.Kim JW, Lee SK, Ha KM, Kim KH, Joh GY, Kim HJ, Yang SO, Hong SH. POEMS syndrome--a case report. J Korean Med Sci. 1992;7:79–84. doi: 10.3346/jkms.1992.7.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonti R, Salvatore B, Quarantelli M, Sirignano C, Segreto S, Petruzziello F, Catalano L, Liuzzi R, Rotoli B, Del Vecchio S, Pace L, Salvatore M. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in evaluation of patients with multiple myeloma. J Nucl Med. 2008;49:195–200. doi: 10.2967/jnumed.107.045641. [DOI] [PubMed] [Google Scholar]
- 15.Dispenzieri A, Moreno-Aspitia A, Suarez GA, Lacy MQ, Colon-Otero G, Tefferi A, Litzow MR, Roy V, Hogan WJ, Kyle RA, Gertz MA. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. Blood. 2004;104:3400–3407. doi: 10.1182/blood-2004-05-2046. [DOI] [PubMed] [Google Scholar]
- 16.Montoriol PF, Cachin F, Michel JL, Soubrier M. Two more cases of evaluation of POEMS syndrome using 18-FDG PET/CT. Eur J Radiol. 2011;80:861–864. doi: 10.1016/j.ejrad.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Stefanelli A, Treglia G, Leccisotti L, Laurenti L, Luigetti M, Sabatelli M, Giordano A. Usefulness of F-18 FDG PET/CT in the follow-up of POEMS syndrome after autologous peripheral blood stem cell transplantation. Clin Nucl Med. 2012;37:181–183. doi: 10.1097/RLU.0b013e31823ea154. [DOI] [PubMed] [Google Scholar]
- 18.Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, Greipp PR, Witzig TE, Basu R, Suarez GA, Fonseca R, Lust JA, Gertz MA. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 19.Brandon C, Martel W, Weatherbee L, Capek P. Case report 572. Osteosclerotic myeloma (POEMS) syndrome. Skeletal Radiol. 1989;18:542–546. doi: 10.1007/BF00351758. [DOI] [PubMed] [Google Scholar]
- 20.Walker RC, Brown TL, Jones-Jackson LB, De Blanche L, Bartel T. Imaging of multiple myeloma and related plasma cell dyscrasias. J Nucl Med. 2012;53:1091–1101. doi: 10.2967/jnumed.111.098830. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhou DB, Huang Z, Jiao L, Duan MH, Zhang W, Zhao YQ, Shen T. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011;90:819–826. doi: 10.1007/s00277-010-1149-0. [DOI] [PubMed] [Google Scholar]
- 22.Shi X, Yang Z, Wu L, Hu S, Jiang Q, Ba R, Zhuang Q, Yu X, Wang L, Xi X, Zhang Y, Zhu Y. Disseminated sclerotic bone lesions with normal F-FDG uptake in POEMS syndrome. Leuk Lymphoma. 2014 doi: 10.3109/10428194.2014.987766. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J. Clin. Oncol. 1998;16:3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 24.Abe K, Sasaki M, Kuwabara Y, Koga H, Baba S, Hayashi K, Takahashi N, Honda H. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19:573–579. doi: 10.1007/BF02985050. [DOI] [PubMed] [Google Scholar]
- 25.Nakai T, Okuyama C, Kubota T, Yamada K, Ushijima Y, Taniike K, Suzuki T, Nishimura T. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging. 2005;32:1253–1258. doi: 10.1007/s00259-005-1842-8. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza A, Lacy M, Gertz M, Kumar S, Buadi F, Hayman S, Dingli D, Zeldenrust S, Kyle R, Ansell S, Inwards D, Johnston P, Micallef I, Porrata L, Litzow M, Gastineau D, Hogan W, Dispenzieri A. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120:56–62. doi: 10.1182/blood-2012-04-423178. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Zhou DB. New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol. 2013;161:303–315. doi: 10.1111/bjh.12236. [DOI] [PubMed] [Google Scholar]
- 28.Murphy SP, Nathan MA, Karwal MW. FDG-PET appearance of pelvic Castleman’s disease. J Nucl Med. 1997;38:1211–1212. [PubMed] [Google Scholar]
- 29.Zhang B, Song X, Liang B, Hou Q, Pu S, Ying JR, Gao C. The clinical study of POEMS syndrome in China. Neuro Endocrinol Lett. 2010;31:229–237. [PubMed] [Google Scholar]


