Abstract
Objective: The purpose of this study was to explore the effects of Xueshuan Xinmai tablets (XXMT) for the treatment of cognition, brain activation in the rehabilitation period of ischemic stroke patients. Methods: 28 adults patients, aged 50-80 years, in the rehabilitation period of ischemic stroke were divided into XXMT treatment group and placebo control group. Patients received 3 months treatment (oral 0.8 g, 3 times per day). Before and after treatment, all patients were evaluated by a series of neuropsychological tests followed by resting-state functional magnetic resonance imaging (fMRI). Results: In the XXMT treatment group, the patients’ episodic memory showed significant improvement. The resting-state fMRI analysis indicated that a significant decline in the fractional amplitude of low-frequency fluctuation value was observed in the bilateral middle cingulate gyrus. Conclusions: Yiqi Huoxue effect under XXMT administration has a favorable mediation on episodic memory, consequently suppresses the activation of the cingulate gyrus in the rehabilitation period of ischemic stroke patients.
Keywords: Episodic memory, brain activation, post-stroke rehabilitation patients, Chinese medicinal formula, resting-state fMRI
Introduction
Ischemic stroke is the second leading cause of death worldwide and an important contributor to vascular dementia and senile dementia, of which Lacunar infarct is one of the most common neuropathological causes in the chronic phase [1]. Post-stroke cognitive impairments including defects in perceptual speed, semantics, episodic memory are common and decisive prognostic factors [2,3]. Cognitive impairment generally emerges at different degrees and has a negative impact on quality of life in the rehabilitation period of ischemic stroke patients. Furthermore, the presence of cognitive impairment increases the risk of dementia. Therefore, the early diagnosis and treatment of ischemic stroke patients in the rehabilitation period are vital in the prevention of subsequent stroke and dementia and the protection of brain cognitive functions.
Although aspirin is widely accepted as a standard antiplatelet therapy against ischemic stroke, some patients show clinic resistance [4,5]. Meanwhile, studies also described that the combination of antiplatelet therapy does not significantly reduce the risk of recurrent stroke, but considerably increase the risk of bleeding and death [6,7]. Thereby, it draws more attention to explore other effective treatment strategy in clinic. Interestingly, many studies have confirmed the beneficial effects of Traditional Chinese Medicine (TCM) on the treatment of post-stroke. Xueshuan Xinmai tablets (XXMT), is one of well-known Chinese patent medicine which is functionally characterized as benefiting qi via activating blood circulation (Yiqi Huoxue). Similar as the classic prescription in TCM, XXMT is composed of Radix Salviae Miltiorrhizae and ginsenosides, which have been widely used in TCM clinical practice in China. Up to date, the research on animal model and related clinical practices with XXMT which are composed by Radix Salviae Miltiorrhizae and its extracts demonstrate that it can benefit the learning and memory through multiple factors including anti-inflammatory, anti-oxidant, blood rheology-improving, blood lipid-regulating and neuroprotective effects.
fMRI, a functional neuroimaging procedure using MRI technology to measure changes in blood flow, has been widely used to detect abnormal functional brain activity during task performance in various patient populations [8]. With many advantages as reflecting the availability, relatively high spatial, temporal resolution and noninvasive safety image technique, it allows for multiple repeated scans over the course of a longitudinal study [8-10]. Moreover, while a subject is not performing an explicit task, the resting-state fMRI can be further used to investigate the regional interactions. Therefore, resting-state fMRI has recently been applied for a wide range of pharmacological treatment assessments [11,12].
In the present study, a placebo controlled fMRI trial has been conducted to assess the effects of XXMT treatment on post-stroke patients in the rehabilitation period using neuropsychological tests accompanying with fMRI analysis. The current study aims to provide further insight into the possible functional mechanisms under TCM Yiqi Huoxue administration on post-stroke patients in the rehabilitation period.
Materials and methods
Experimental design
A randomized, double-blind controlled, 3-month parallel trial was conducted. All patients were assigned to MRI scan and characterized by the presence of a focal hyperintensity lesion (2 to 15 mm in diameter), and T2-weighted and fluid-attenuated inversion recovery (FLAIR) images [13,14], were included in this study [3]. All procedures involving human subjects were in accordance with the ethical standards established by the institution and/or national research committee in consistent with the Helsinki declaration of 1975. The informed consent was obtained from all individual participants included in the study.
To ensure the quality of the cognitive testing, investigators were trained before the start of the study. Two independent experienced neurologists reviewed all the medical histories and fMRI results for patients to ensure their eligibility. The research protocol here was approved by the Ethics Committee of Beijing Hospital, (number 2011010), and written informed consent was obtained from each patient. The authors confirm that all ongoing and related trials for this drug are registered in The Chinese clinical trial registry center (ChiCTR) and the World Health Organization international clinical trials registered organization registered platform (registration number: ChiCTR-TRC-12003074).
Patients
The patients were recruited from eight communities from January 1st, 2012 to June 1st, 2013. The participants recruition criteria for the study were set as follows: (1) between 50 and 80 years old; (2) diagnosed as ischemic stroke according to clinical standards in China; (3) clinical favorable candidates from the TCM standards for diagnosis of apoplexy; (4) in the rehabilitation period according to TCM criteria (between 15 days and 6 months after the onset of symptoms); and (5) informed consent signed. The exclusion criteria were as follows: (1) transient ischemic attacks (2) major cognitive impairment (Mini-Mental Status Examination (MMSE) score ≤24); (2) a diagnosis of coronary disease, diabetes or intracranial tumor; (3) a diagnosis of psychiatric disease; and (4) a history of psychoactive medication use or drug addiction.
A total of 28 eligible patients were included in this study. Among those, 14 patients were randomly allocated to the XXMT treatment group and the remaining patients were allocated to the placebo control group. The treatment group received XXMT (lot number Z20030145) oral 0.8 g, 3 times per day for 3 months according to the instructions; the placebo control group received a placebo tablets at the same dose for 3 months. The appearance, smell and taste of the placebo tablets were designed to be identical to the treatment tablets. A computer program was used to generate the random allocation sequence. Numbered drug containers were used to implement the random allocation process. None of investigators or patients was aware of treatment allocation in the treatment and placebo group until study completion. The duration of intervention lasted for 3 months. All patients were revisited at the end of the trial. For the safety assessment, we monitored vital signs, conducted physical examinations and evaluated adverse events.
Neuropsychological tests
During both visits, a set of neuropsychological tests were set for patient including general cognitive status and other domains, such as memory, attention, spatial processing, executive function and language ability. The following tests were also conducted: (1) memory: Auditory Verbal Learning Test (AVLT) [15], Rey-Osterrieth Complex Figure Test (ROCF) (recall) [16] and Digit Span [17]; (2) spatial processing: ROCF (copy) [16] and Clock Drawing Test (CDT) [18] (3) attention: Stroop Color and Word Test (SCWT)-A and Symbol Digit Modalities Test (SDMT); (4) executive function: SCWT-B and SCWT-C [19]; and (5) language ability: Boston Naming Test (BNT) and the Category Verbal Fluency Test (CVFT).
MRI data acquisition and collection
The MRI data were acquired using the procedures described above on a SIEMENS TRIO 3T scanner in the Imaging Center for Brain Research, Beijing Normal University, including high-resolution T1 scan, T2-FLAIR and resting-state fMRI data. T1-weighted, sagittal 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequences were acquired, covering the entire brain [176 slices, repetition time (TR) = 1900 ms, echo time (TE) = 3.44 ms, slice thickness = 1 mm, flip angle = 9°, inversion time = 900 ms, field of view (FOV) = 256 × 256 mm2, and acquisition matrix = 256 × 256]. The resting-state data were collected using a gradient echo EPI sequence [TE = 30 ms, TR = 2000 ms, flip angle = 90°, 33 slices, slice thickness = 4 mm, and matrix = 64 × 64].
Imaging preprocessing
All resting-state fMRI data were preprocessed using the Data Processing Assistant for Resting-State fMRI (DPARSF) [20]. Because of signal equilibrium, the first 10 volumes of the functional images were discarded. The remaining functional images were corrected intra-volume for time delay between the slices, and it was realigned the inter-volume for head motion. Subsequently, all images were spatially normalized to the Montreal Neurological Institute (MNI) space using EPI templates to the time and motion corrected images. The resulting normalized functional images were spatially smoothed with 6 mm full width and half maximum (FWHM) Gaussian kernel and subtracted linear trends. All images were temporally filtered (0.01-0.1 HZ) to eliminate high-frequency noise and low-frequency drifts.
For a given voxel, the time series was first converted to the frequency domain using a Fast Fourier Transformation. The square root of the power spectrum was computed and averaged across a 0.01-0.1 HZ frequency interval. The fractional amplitude of low frequency fluctuation (fALFF), calculated as the fraction of ALFF in a given band to the ALFF over the entire frequency range detectable in the given signal, was analyzed [21].
Statistical analysis
The demographic and behavioral data were analyzed using SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for all variables; continuous variables were summarized as the means and standard deviations, and the categorical variables were summarized as frequencies and proportions. To determine differences in the demographic data between groups, a two-sample t-test or the chi-square test was used where appropriate. To determine differences in the results of the neuropsychological tests between groups after three months of treatment, a one-way ANCOVA was conducted with the baseline as a covariate.
The resting-state fMRI data were analyzed using REST software (http://www.restfmri.net). To evaluate differences in the fALFF value between the treatment and control groups, one-way ANCOVA was conducted, considering the resting-state fMRI data in the first interview as a covariate. The results were visualized using the REST Slice viewer (http://www.restfmri.net).
Results
Nocio-demographic characteristics
One patient from the treatment group was out of follow up and excluded from this study. In total, 27 patients were counted for final analysis in this study, including 13 patients in treatment group and 14 patients in control group. The demographic characteristics showed no significant difference between the treatment and control groups in gender, age, years of education, hypertension status, alcohol addiction status and smoking status (P>0.05, Table 1).
Table 1.
Socio-demographic characteristics and neuropsychological testing
| Control Group (n = 14) | Treatment Group (n = 13) | Baseline | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Baseline | 3-months | Baseline | 3-months | T-value (χ2) | P-value | |
| Gender (M/F) | 4/10 | 8/5 | 2.967 | 0.128 | ||
| Age (years) | 64.93±6.75 | 67.92±8.38 | -1.007 | 0.324 | ||
| Education (years) | 10.15±3.95 | 12.09±3.47 | -1.262 | 0.220 | ||
| Hypertension (%) | 8 (57.1%) | 9 (69.2%) | 0.422 | 0.516 | ||
| Alcohol Addiction | - | - | - | - | ||
| Smoking | - | - | - | - | ||
| non/past/current | 12/0/2 | 9/0/4 | 1.060 | 0.303 | ||
| General mental status | ||||||
| MMSE | 26.57±2.44 | 26.21±2.45 | 27.31±2.56 | 27.46±1.71 | 1.34 | 0.194 |
| Memory | ||||||
| AVLT delayed recall | 4.29±2.30 | 4.93±1.94 | 5.38±2.56 | 5.77±2.35 | 0.43 | 0.668 |
| AVLT-T | 28.14±7.22 | 25.57±6.39 | 28.15±10.47 | 31.85±9.19 | 2.54 | 0.018* |
| ROCF delayed recall | 10.00±5.21 | 10.54±4.63 | 12.69±6.18 | 17.62±6.87 | 2.70 | 0.013* |
| Digit Span | 10.43±1.78 | 10.00±1.75 | 11.38±2.18 | 10.62±2.46 | 0.35 | 0.729 |
| Spatial Processing | ||||||
| ROCF-copy | 28.46±10.32 | 30.62±7.78 | 34.31±1.49 | 34.08±1.75 | 0.27 | 0.791 |
| CDT | 22.36±4.51 | 24.21±3.70 | 26.33±2.64 | 26.08±9.17 | -0.33 | 0.745 |
| Attention | ||||||
| SCWT-A-time | 49.07±23.96 | 37.93±13.67 | 32.88±8.99 | 34.00±10.55 | 0.31 | 0.756 |
| SDMT | 24.50±8.14 | 21.69±8.28 | 28.46±12.50 | 30.25±15.07 | 1.80 | 0.087 |
| Executive Function | ||||||
| SCWT-B-time | 51.93±16.48 | 47.57±18.05 | 43.38±17.48 | 44.15±18.28 | 0.38 | 0.709 |
| SCWT-C-time | 83.71±35.20 | 89.50±25.08 | 89.85±23.06 | 89.00±26.14 | -0.45 | 0.659 |
| SCWT-C-Right | 44.93±11.08 | 40.36±7.85 | 40.77±9.52 | 43.31±7.13 | 1.19 | 0.245 |
| Language ability | ||||||
| BNT | 22.57±3.41 | 23.07±3.33 | 23.07±3.08 | 23.42±3.57 | -0.15 | 0.885 |
| CVFT | 39.57±11.56 | 37.71±10.57 | 45.54±13.75 | 41.62±15.26 | -0.11 | 0.917 |
Note: Continuous variables were summarized as means and standard deviations and categorical variables were summarized as frequencies and proportions. The demographic data were analyzed using two-sample t-test or chi-square test. The neuropsychological characteristics were analyzed using one-way ANCOVA, considering the baseline value as the covariate,
P<0.05 was considered statistically significant.
Neuropsychological characteristics
The auditory verbal learning test (AVLT, P = 0.018) and ROCF delayed recall (P = 0.013) manifested a highly significant increase in the treatment group compared to the control group. The data were analyzed using one-way ANCOVA after adjusting the baseline value (Table 1; Figure 1). A borderline significant improvement was also observed in the symbol digit modalities test in the treatment group (SDMT, P = 0.089, Table 1). The infarct focus locations are shown in Table 2.
Figure 1.
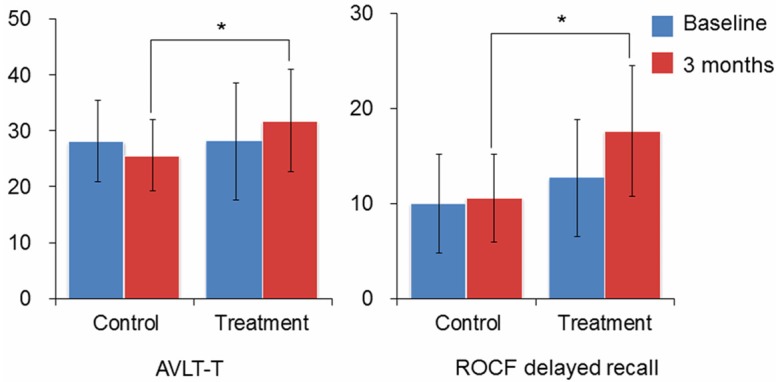
ANCOVA analysis of AVLT-T and ROCF delayed recall. Significant differences are observed in the AVLT-T and ROCF-delayed recall tests using ANCOVA analysis after correcting the baseline performance, *P<0.05. AVLT-T: Auditory Verbal Learning Test-Total; ROCF-delay: Rey-Osterrieth Complex Figure Test (delayed recall).
Table 2.
Number and percentage of the infarcts distribution in two groups
| Control (n = 14) | Treatment (n = 13) | |
|---|---|---|
| Basal ganglion | 14 (100%) | 13 (100%) |
| Brainstem | 3 (21.4%) | 2 (15.4%) |
| Centrum semiovale | 1 (7.1%) | 0 (0%) |
| Corona radiate | 3 (21.4) | 3 (23.1%) |
| Mesencephalon | 1 (7.1%) | 1 (7.7%) |
Brain activation
One-way ANCOVA analysis was applied for brain activation definition, and the statistical threshold was set at P<0.01. Compared to the control group, a significant decline in the fALFF value was observed in the bilateral middle cingulate gyrus in cluster 1 in the treatment group (cluster corrected P = 0.006). The right middle frontal gyrus also had a lower fALFF value in cluster 2, while the left calcarine and the left lingual gyrus had a higher fALFF value in cluster 3 in the treatment group, although no significant difference was observed after correction at the cluster level (Table 3; Figure 2). After extracting the ROI signals of the bilateral middle cingulate gyrus, a significant decline in the fALFF value was observed in the right middle cingulate gyrus (P = 0.007) in the treatment group compared with the control group (Figure 3).
Table 3.
The fALFF differences shown in brain regions
| Cluster | Cluster size | Brain regions | Brodmann | Vol (mm3) | MNI coordinates (mm) | Maximum Z | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| 1* | 143 | Cingulum_Mid_L | 24 | 1161 | 0 | 3 | 33 | 4.10 |
| Cingulum_Mid_R | 32 | 945 | 6 | 9 | 42 | 3.88 | ||
| 2 | 79 | Frontal_Mid_R | 8 | 729 | 42 | 24 | 48 | 4.01 |
| 3 | 65 | Calcarine_L | N/A | 594 | -21 | -64 | 9 | -2.35 |
| Lingual_L | 19 | 459 | -12 | -66 | -3 | -2.33 | ||
Note: x, y, z, coordinates of primary peak locations in the MNI space; Z, statistical value of peak voxel showing fALFF differences. N/A, not applicable.
Cluster level corrected P = 0.006.
Figure 2.
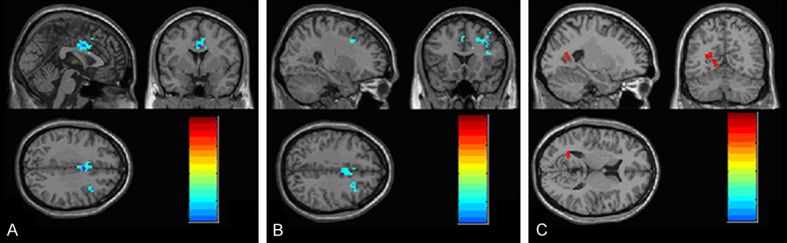
fALFF maps of brain activation between the treatment and control groups. The hot color represents higher fALFF values from the treatment group compared to the control group, whereas the blue color represents lower fALFF values. The results are obtained from ANCOVA analysis. The statistical threshold is set at Z > 2.8 (P<0.01), with a cluster size > 1350 mm3. R, right; L, left; P, posterior; A, anterior. A. bilateral middle cingulated gyrus; B. right frontal gyrus; C. calcarine and lingual gyrus.
Figure 3.
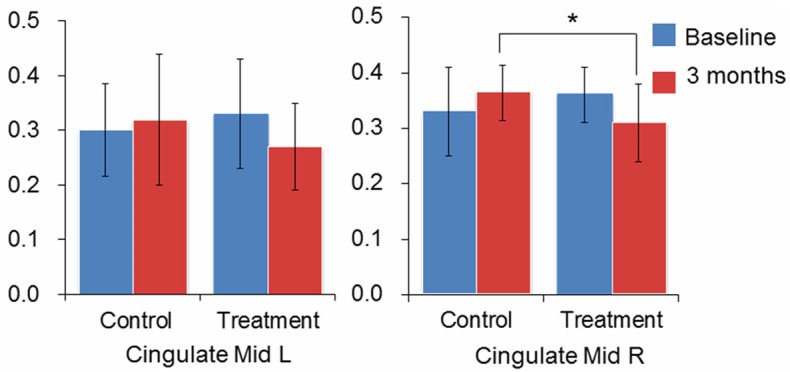
ANCOVA analysis of fALFF value of bilateral cingulate gyrus. ANCOVA analysis of extracted ROI fALFF value with the baseline value as the covariate, *P<0.05.
Discussion
In the present study, we conducted an continuous study of the effect of Chinese medicine Yiqi Huoxue treatment on post-stroke patients in the rehabilitation period within the realm of behavioral performance and brain region activation via neuropsychological tests combining with resting-state fMRI scans, respectively. It’s notable that we found significant positive effect of Yiqi Huoxue treatment on post-stroke rehabilitation patients in several cognitive domains and the different brain activation mode of treatment group compared to the control group. Yiqi Huoxue treatment under XXMT administration promoted cognitive functions in patients which were defined as significant improvements in AVLT and ROCF delayed recall, and a marginally improvement in SDMT. Moreover, Yiqi Huoxue treatment improved brain activations which were spatially characterized as the significant decline in the fALFF value in the bilateral middle cingulate gyrus, more specifically the right middle cingulate gyrus. To the best of our knowledge, this study is the first to explore the effects of a Chinese medicinal formula on episodic memory and brain region activation in post-stroke rehabilitation patients, which provides new insight into future treatments for the rehabilitation period of post-stroke patients.
TCM is a medical mediation with over 3000 years of continuous practice experience and refinement through treatment observations [22]. TCM has been used for a long time and formed a theory of diagnosis and treatment of chronic ischemic stroke. In TCM philosophy, qi deficiency and blood stasis were the main pathogenesis of chronic ischemic stroke. Rooted on this theory, Yiqi Huoxue method has been widely used in clinical practice of treating chronic ischemic stroke [23,24]. Its formula had been confirmed to be effective in treatment for chronic ischemic stroke in both bench and clinic bed side. The proposed molecular mechanisms of this action were involved in decreasing the cell apoptosis, anti-lipid peroxidation, preventing the overload of calcium, anti-inflammatory, inhibiting the expression levels of interleukin-8 and tumor necrosis factor, thrombus precursor protein and plasma fibrinogen [25-27]. XXMT is one representative Yiqi Huoxue formula for the treatment of chronic ischemic stroke in TCM. It had been shown to suppress platelet aggregation, dilate blood vessels, enhance the tolerance of ischemic brain tissue to hypoxia and protect against ischemic reperfusion injury in ischemic stroke patients [28-31]. All these may collectively contribute to improvement of episodic memory and brain region activation in ischemic post-stroke patients.
In terms of the neuropsychological result, AVLT is a neuropsychological tool that is widely used in cognitive function assessments, such as memory, attention and learning abilities [32]. The significant improvement in the AVLT scores in this study also suggested that Yiqihuoxue treatment could effectively attenuate the cognitive deficits in post-stroke patients in the rehabilitation period. Additionally, ROCF is also a widely used neuropsychological test that requires the analysis and reproduction of an unfamiliar, non-meaningful figure. Gomez-Gil et al., reported an activating androgens effects on visual memory in patients undergoing androgen treatment for at least 6 months compared to those who were not treated in the ROCF test and other visual memory tasks [33]. Similarly, our date also suggested that Yiqi Huoxue treatment could effectively promote visual memory of post-stroke patients in the rehabilitation period.
We also identified changes in the fALFF value in both groups in the bilateral middle cingulate cortex, right frontal gyrus, left calcarine and lingual gyrus. Compared to the control group, the XXMT treatment group showed a significant decrease in brain activation in the bilateral middle cingulate cortex (BA 24 and 32), potentially reflecting the outputs of the anterior cingulate gyrus, which is associated with a wide variety of autonomic and rational cognitive functions. The left middle cingulate cortex (BA 24), is an integral part of the limbic system and is associated with learning and memory [34,35]. The right middle cingulate gyrus (BA 32), called the dorsal part of the anterior cingulate cortex (ACC), projects to the rostral superior temporal gyrus, midorbitofrontal cortex and lateral prefrontal cortex. Eisenberger et al showed that self-reported distress is positively correlated with a more active ACC [36]. In internet addiction disorder (IAD) studies, IAD subjects showed increased activation in this region and decreased activation in the orbitofrontal cortex after error response [37]. Additional executive function research has also shown error-related activity in the same region [38]. Therefore, the decreased brain activation in the right middle cingulate cortex was considered beneficial, indicating the positive treatment effect from Yiqihuoxue adminstration on post-stroke patients in the rehabilitation period.
The middle frontal gyrus is functionally associated with attention, short-term memory and other cognitive functions. Calcarine is the brain region that is primarily associated with primary visual cortex concentration [39]. The lingual gyrus is a brain structure that is suggested to play an important role in visual memory, visual-limbic connection and dreaming [40]. Other related studies have shown that activation in the lingual gyrus might be associated with image encoding and selective attention [41,42]. Therefore, the higher activation in the lingual gyrus in the treatment group here suggests that Yiqi Huoxue administration can sufficiently improve the brain function of post-stroke patients in the rehabilitation period.
There are also some limitations in the present study. First, some patients had a history of hypertension that might have influenced their brain function. However, the hypertension status was highly comparable between the treatment and control groups, diminishing the potential confounding effects of hypertension and ensuring the credibility of the final results. Second, improvement in brain function is a long and complicated process that might not be fully reflected after a 3-month treatment. Therefore, more extensive neuropsychological trials over a longer follow-up period are needed.
Conclusion
In the present study, we demonstrated that Yiqi Huoxue treatment significantly alleviates cognitive impairment, alters brain activation in the rehabilitation period of post-stroke patients over a 3-month treatment period. These results are providing some insight into future treatments for post-stroke rehabilitation patients and should contribute to the prevention of stroke recurrence. Subsequent studies with a larger sample size and longer treatment duration should explore the curative effect and underlying mechanism of action of Yiqi Huoxue treatment on post-stroke patients in the rehabilitation period.
Acknowledgements
This study was funded by the State Key Program of National Natural Science of China (Grant No. 81430100), Beijing New Medical Discipline Based Group (Grant No. 100270569), the Natural Science Foundation of China (Grant No. 30873458 and 81173460), project of Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences (Grant No. Z0175 and Z0288), project of Jilin Science and Technology Development (Grant No. 20130301005ZY), program for New Century Excellent Talents in University (Grant No. NCET-10-0249) and China Postdoctoral Science Foundation Grant (Grant No. 2013M541156).
Disclosure of conflict of interest
None.
References
- 1.Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3:391–393. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YJ, Wang J, Zhang JY, Zhang T, Chen KW, Fleisher A, Wang YY, Zhang ZJ. Aberrant Functional Networks Connectivity and Structural Atrophy in Silent Lacunar Infarcts: Relationship with Cognitive Impairments. J Alzheimers Dis. 2014;42:841–850. doi: 10.3233/JAD-140948. [DOI] [PubMed] [Google Scholar]
- 4.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 5.Hankey GJ, Eikelboom JW. Aspirin resistance. The Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 6.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usman MHU, Notaro LA, Nagarakanti R, Brahin E, Dessain S, Gracely E, Ezekowitz MD. Combination antiplatelet therapy for secondary stroke prevention: enhanced efficacy or double trouble? Am J Cardiol. 2009;103:1107–1112. doi: 10.1016/j.amjcard.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Dickerson BC. Advances in functional magnetic resonance imaging: technology and clinical applications. Neurotherapeutics. 2007;4:360–370. doi: 10.1016/j.nurt.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci. 2000;21:314–318. doi: 10.1016/s0165-6147(00)01507-8. [DOI] [PubMed] [Google Scholar]
- 10.Honey G, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25:366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Flodin P, Gospic K, Petrovic P, Fransson P. Effects of L-Dopa and Oxazepam on Resting-State Functional Magnetic Resonance Imaging Connectivity: A Randomized, Cross-Sectional Placebo Study. Brain Connect. 2012;2:246–253. doi: 10.1089/brain.2012.0081. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Gao B, Hua J, Liu W, Deng Y, Zhang L, Jiang B, Zang Y. Effects of methylphenidate on resting-state brain activity in normal adults: an fMRI study. Neurosci Bull. 2013;29:16–27. doi: 10.1007/s12264-013-1306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. 1998;50:841–841-a. doi: 10.1212/wnl.50.4.841-a. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh ER. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M. A Handbook. Los Angeles: Western Psychological Services; 1996. Rey Auditory Verbal Learning Test. [Google Scholar]
- 16.Rey A. L-examen psychologique dans lescas d’encephalopathie traumatique. Arch Psychologie. 1941;28:286–340. [Google Scholar]
- 17.Gong Y. Wechsler Adult Intelligence Scale-Revised in China Version. Changsha, Hunan/China: Hunan Medical College; 1992. [Google Scholar]
- 18.Sunderland T, Hill JL, Mellow AM, Lawlor BA. Clock drawing in Alzheimer’s disease: a novel measure of dementia severity. J Am Geriatrics Soc. 1989;37:725–9. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 19.Golden C. Stroop Color and Word Test. Chiacago: Stoelting Company; 1978. [Google Scholar]
- 20.Yan CG, Zang YF. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou QH, Zhu CZ, Yang YH, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu A, Jiang M, Zhang C, Chan K. An integrative approach of linking traditional Chinese medicine pattern classification and biomedicine diagnosis. J Ethnopharmacol. 2012;141:549–556. doi: 10.1016/j.jep.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Tang JJ, Han H, Bao YC, Hu CP, Wang SY. [Progress in research of Yiqi Huoxue method on ischemic stroke] . Zhong Guo Zhong Yi Ji Zheng. 2010;19:1578–1580. [Google Scholar]
- 24.Li Y, Huang Z, Ji XX, Li WB, Zhu H, Shen YY. [Progress in research of Yiqi Huoxue method on ischemic stroke] . Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2012;10:1112–1114. [Google Scholar]
- 25.Chen CL, Young SH, Gan HH, Singh R, Lao AY, Baroque AC 2nd, Chang HM, Hiyadan JH, Chua CL, Advincula JM, Muengtaweepongsa S, Chan BP, de Silva HA, Towanabut S, Suwanwela NC, Poungvarin N, Chankrachang S, Wong KS, Eow GB, Navarro JC, Venketasubramanian N, Lee CF, Bousser MG. Chinese medicine neuroaid efficacy on stroke recovery: a double-blind, placebo-controlled, randomized study. Stroke. 2013;44:2093–2100. doi: 10.1161/STROKEAHA.113.002055. [DOI] [PubMed] [Google Scholar]
- 26.Venketasubramanian N, Young S, Tay SS, Chang HM, Umapathi T, Chan B, de Silva A, Wong L, Navarro J, Zhao YD, Tan SB, Chen C. Chinese medicine NeuroAiD efficacy stroke recovery-extension study (CHIMES-E study): an observational multicenter study to investigate the longer-term efficacy of NeuroAiD in stroke recovery. Cerebrovasc Dis. 2013;35(Suppl 1):18–22. doi: 10.1159/000346233. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Wang J, Hu JP. [Progress in experimental research of Yiqi Huoxue method on ischemic stroke] . Clinical Journal of Traditional Chinese Medicine. 2013;25:260–262. [Google Scholar]
- 28.Zhang X, Zhang C, Sai J, Li F, Liu J, Li Y, Wang F. Xueshuan Xinmaining Tablet Treats Blood Stasis through Regulating the Expression of F13a1, Car1, and Tbxa2r. Evid Based Complement Alternat Med. 2015;2015:704390. doi: 10.1155/2015/704390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du XL, Du C, Liu Y, Yi SH. [The effects of Xueshuan Xinmaining tablets on hemorheology and blood lipid levels in the rehabilitation period of ischemic stroke patients] . Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2007;5:902–903. [Google Scholar]
- 30.Zhao L, Sun XH, Li FY. [The effects of Xueshuan Xinmainig tablets on ischemic stroke patients in the rehabilitation period in community] . Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2009;7:1253–1254. [Google Scholar]
- 31.Jia YL, Jia WF, Chen C, Ruan Z. [A Systematic review of controlled clinical trial of Xueshuan Xinmaining tablets on ischemic stroke patients] . Chinese Journal of Practical Nervous Disease. 2012;15:78–80. [Google Scholar]
- 32.Salgado JV, Malloy-Diniz LF, Abrantes SSC, Moreira L, Schlottfeldt CG, Guimarães W, Freitas DMU, Oliveira J, Fuentes D. Applicability of the Rey Auditory-Verbal Learning Test to an adult sample in Brazil. Revista Brasileira de Psiquiatria. 2011;33:234–237. doi: 10.1590/s1516-44462011005000007. [DOI] [PubMed] [Google Scholar]
- 33.Gómez-Gil E, Cañizares S, Torres A, de la Torre F, Halperin I, Salamero M. Androgen treatment effects on memory in female-to-male transsexuals. Psychoneuroendocrinology. 2009;34:110–117. doi: 10.1016/j.psyneuen.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 35.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 36.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 37.Dong G, Shen Y, Huang J, Du X. Impaired Error-Monitoring Function in People with Internet Addiction Disorder: An Event-Related fMRI Study. Eur Addict Res. 2013;19:269–275. doi: 10.1159/000346783. [DOI] [PubMed] [Google Scholar]
- 38.Carter CS, MacDonald AW, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiat. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 39.Hasnain MK, Fox PT, Woldorff MG. Intersubject variability of functional areas in the human visual cortex. Hum Brain Mapp. 1998;6:301–315. doi: 10.1002/(SICI)1097-0193(1998)6:4<301::AID-HBM8>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50:607–614. doi: 10.1136/jnnp.50.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machielsen W, Rombouts SA, Barkhof F, Scheltens P, Witter MP. FMRI of visual encoding: reproducibility of activation. Hum Brain Mapp. 2000;9:156–164. doi: 10.1002/(SICI)1097-0193(200003)9:3<156::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangun GR, Buonocore MH, Girelli M, Jha AP. ERP and fMRI measures of visual spatial selective attention. Hum Brain Mapp. 1998;6:383–389. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<383::AID-HBM10>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]


