Abstract
Mechanical stimulation is highly associated with pathogenesis of human hypertrophic scar. Although much work has focused on the influence of mechanical stress on fibroblast populations from various tissues and organs in the human body, their effects on cultured dermal fibroblasts by the area of the body have not been as well studied. In this study, cultures of skin fibroblasts from two different body sites were subjected to cyclic mechanical stimulation with a 10% stretching amplitude at a frequency of 0.1 Hz for 24, 36 and 48 hours, respectively, and thereafter harvested for experimental assays. Fibroblasts from scapular upper back skin, subjected to mechanical loads for 36 and 48 hours, respectively, were observed to proliferate at a higher rate and reach confluent more rapidly during in vitro culturing, had higher expression levels of mRNA and protein production of integrin β1, p130Cas and TGF β1 versus those from medial side of upper arm. These data indicate that skin fibroblasts, with regard to originated body sites studied in the experiments, display a diversity of mechanotransduction properties and biochemical reactions in response to applied mechanical stress, which contributes to the increased susceptibility to hypertrophic scars formation at certain areas of human body characterized by higher skin and muscle tension.
Keywords: Fibroblasts, body sites, hypertrophic scar, normal skin, mechanical stress
Introduction
Although a complete molecular picture is still lacking, mechanical stimulation is believed to play a major role in the pathogenesis of human hypertrophic scar (HTS), which is a dermal form of fibroproliferative disorder [1]. Key cells involved in HTS are dermal fibroblasts, whose heterogeneity might contribute to the occurrence of HTS [2]. Therefore, an exploration of mechanotransduction mechanisms by which skin fibroblasts convert mechanical signals into cellular biological events is warranted. In human body, especially for Asian patients, certain specific areas are prone to scarring, of which include the anterior chest, shoulder, scapular, and suprapubic regions. These sites are frequently subjected to mechanical forces, for example, upper limb movements and body bending motions at the shoulder, and scapula skin. In contrast, scar formation is rare to arise on the scalp, medial side of upper arm and anterior lower leg at which sites the skin is unusually exposed to skin stretching and a frequently moved joint. The site specificity of scarring suggests that the mechanical stress may be a primary contributing factor for an increased susceptibility to hypertrophic scar formation with diverse incidence rates and subsequent processes [3,4,12]. Studies have shown that fibroblast populations from various tissues and organs in the human body are intrinsically different in response to mechanical stimuli. In this study, we hypothesized that a difference exists in the response of skin fibroblasts from different body sites, namely scapular part of upper back and medial side of upper arm, to the mechanical forces. Moreover, the underlying biomechanical mechanism for hypertrophic scar formation is also being discussed.
Material and methods
Laboratory apparatus and reagents
Flexercell® Tension Plus™ System FX-4000 and 6-well flexible-bottomed culture plates were purchased from Flex-cell International Incorporation (Hillsborough, NC, USA). Alpha-MEM culture medium, FBS and EDTA-trypsin were all obtained from HyClone Laboratories (Logan, Utah, USA).
Isolation of skin fibroblasts
Healthy skin tissue samples were obtained during skin surgery from scapular part of upper back and medial side of upper arm of each patient (1 male and 2 females, age range 20-40 years). The surgical protocol was viewed and approved by the Institutional Review Board of Qindao University School of Medicine. All skin tissues were taken after informed consent was obtained from patients or guardian. Human skin fibroblasts were isolated with an explant outgrowth culture system and used at passages 5-8 for all subsequent experiments. Each experiment was performed in triplicate.
Application of cyclic mechanical strains
Briefly, skin fibroblasts were seeded at a density of 3×104 cells/cm2 into 6 well flexible-bottomed culture plates and sub-cultured at 37°C under 5% CO2 in α-MEM medium containing 10% fetal bovine serum, such that they will be 70-90% confluent at the time of the experiment. After replenishing the complete medium, the 6-well culture plates were transferred onto multi-channel tension-loading bioreactor and subjected to the uniaxial cyclic mechanical stress for 24, 36 and 48 hours, respectively, with a 10% stretching amplitude at a frequency of 0.1 Hz. Cells were subsequently harvested for experimental assays. For control experiments, cells were treated in the same bioreactor under the same conditions but not given any mechanical stimulation.
Cell proliferation assay
At each time-point after cyclic mechanical loads, the medium was aspirated and stretched fibroblasts in the 6-well culture plates were washed twice with phosphate-buffered saline. After that, the CCK-8 assay reagents from the Cell Counting Kit-8 (Dojindo Molecular Technologies, Japan) were added and incubated for additional 3 hours. The supernatant from 6-well culture plates were then collected and used for reading optical density (OD) at wavelength of 450 nm, with an Absorbance Microplate Reader (Tecan Trading AG, Switzerland).
Messenger RNA expressions of target genes with quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from individual cultures using a TRIzol® reagent (Invitrogen, Carlsbad, CA), according to manufacturer’s recommendations. The extracted RNA was quantified spectrophotometrically at 260 nm wavelength. After DNase treatment, 1 μg of total RNA from each sample was transcribed into cDNA with Oligo-dT primers using an AMV RNA PCR Kit (TaKaRa, Tokyo, Japan).
A highly sensitive quantitative PCR method was performed on an ABI-7500 quantitative PCR System (Life Technologies, Grand Island, NY) using the SYBR® Premix TaqTM II detection kit (TaKaRa, Tokyo, Japan). The primers used for amplifying target genes are shown in Table 1 (designed and synthesized by Shanghai Sangon Biotech, China). The 20 μl reaction mix contained 2×SYBR® Premix TaqTM II, 2 μL template cDNA and 0.4 µM of each primer. The thermal protocol consisted of 10 min polymerase activation at 95°C, followed by 40 cycles of denaturation at 95°C for 5 sec, primer annealing at 60°C for 20 sec and extension at 72°C for 30 sec. Human GAPDH was used as an endogenous reference gene. During expression analysis, each sample was amplified in triplicate, the average Ct value was calculated and a dissociation curve was generated by plotting each of PCR products against its specific melting temperature (Tm) for verification. The mRNA expression levels were normalized to those of endogenous reference gene GAPDH and reported as relative values (ΔΔCT) to those obtained from each control groups.
Table 1.
Primer pairs for target and housekeeping genes used in qRT-PCR assays
| Gene | Sequences (5’-3’) | Sizes | GeneBank Accession# |
|---|---|---|---|
| Integrin β1 | F: ACAATGGAGAGTGCGTCTGC | 231 bp | NM_002211.3 |
| R: CGTTGCTGGCTTCACAAGTA | |||
| TGF β1 | F: ATTCCTGGCGATACCTCAG | 128 bp | NM_000660.4 |
| R: AAGGCGAAAGCCCTCAAT | |||
| p130Cas | F: CAGCAAGTTCGTCATCCTCA | 210 bp | NM_001170715.1 |
| F: TTGACCCTCTCCACCATGTC | |||
| COL1A1 | F: CCTGGAAAGAATGGAGATGATG | 147 bp | NM_000088.3 |
| R: ATCCAAACCACTGAAACCTCTG | |||
| GAPDH | F: TCACCATCTTCCAGGAGCGA | 239 bp | NM_002046.4 |
| R: CACAATGCCGAAGTGGTCGT |
Abbreviation: TGF β1, Transforming Growth Factor beta 1; COL1A1, type I collagen, alpha 1 chain; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis with cells lysate
Cultures of dermal fibroblasts were harvested at each designated time point, and lysed with cell lysis buffer containing l mmol/L PMSF. Cell lysates were resolved by standard SDS-PAGE and electroblotted onto a PVDF membrane and incubated with rabbit anti human integrin β1 (Abcam, Cambridge, MA) and rabbit anti human p130Cas antibodies (Cell Signaling Technology, Inc., Danvers, MA) and with a secondary anti-rabbit horseradish peroxidase (HRP)-conjugate antibody (CWbiotech Inc., Beijing, China). Chemiluminescent detection of proteins was carried out with ECL detection reagent Kit (GE Healthcare Amersham, Pittsburgh, PA) according to the supplier’s instructions. The results of Western Blots were relatively quantified by ImageJ program inspired by NIH Image at Bethesda, Maryland.
ELISA assay for protein contents in the supernatant
At each time point after loading cyclic mechanical stress, the conditioned medium from each individual culture was collected and used for protein content quantification, using commercial ELISA kits for TGF β1 or type I collagen (R&D Systems, Minneapolis, MN) according to manufacturer’s recommendations.
Statistical analysis
All data were analyzed with statistical software of SPSS 11.5 and all the experiments were performed in triplicate. A two-tailed Student’s t-test was used to compare upper back to medial arm fibroblasts, which were both challenged with mechanical loads. Data were expressed as means ± standard deviation (SD). The results were taken to be statistically significant at a probability level of P<0.05.
Results
Cell proliferation response to mechanical strains
Under light microscopy, vigorous proliferation of skin fibroblasts after cyclic mechanical stimuli was observed (Figure 1A and 1B). In contrast, cell counting and CCK-8 assay showed no significant difference between non-stressed upper back and medial arm fibroblasts regardless of each time-point that were studied (P>0.05, Figure 1C). Although scapular upper back and medial arm fibroblasts proliferation index were not comparable when mechanical stress applied only for 24 hours (P>0.05), scapular upper back fibroblasts reached confluence more rapidly compared with medial arm fibroblasts when subjected to cyclic mechanical stimulation for 36 and 48 hours, respectively, with a significant difference of P<0.05 (Figure 1D).
Figure 1.
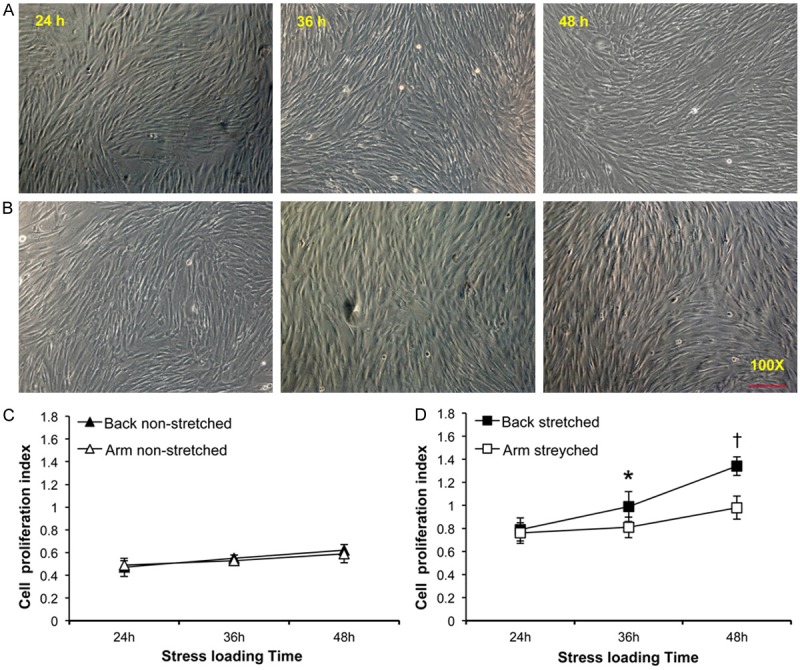
Effect of mechanical stress on the proliferation of cultured upper back and medial arm skin fibroblasts. Representative images showing the difference in cell proliferations of in vitro cultured skin fibroblasts from scapular upper back (A) and medial side of upper arm (B), respectively, at individual time-points under microscopic examination (scale bar=200 um). Panel (C) and (D) show the cell proliferation index of skin fibroblasts using CCK-8 cell count assays (C, non-stretched fibroblasts; D, stretched fibroblasts). Data are presented as mean values ± SD; * and † denote a significant difference (P<0.05) relative to medial arm fibroblasts stressed for 36 and 48 hours, respectively.
Target gene expression after mechanical strains
There were no significant differences for mRNA expression levels of integrin β1, p130Cas, TGF β1 and type I collagen between upper back and medial arm fibroblasts in the absence of mechanical loads (data not shown). When exposed to mechanical stress for only 24 hours, no significant change was observed for messenger RNA expressions of the above mentioned genes between the two groups (P>0.05, Figure 2A-C), except that collagen type I gene expression presented no differences at all given time-points studied in the experiments (Figure 2D). Interestingly, there was significantly higher messenger RNA expression levels of integrin β1, p130Cas and TGF β1 in upper back fibroblasts subjected to mechanical stimuli for 36 and 48 hours, respectively, when compared to medial arm fibroblasts (P<0.05, Figure 2A-C).
Figure 2.
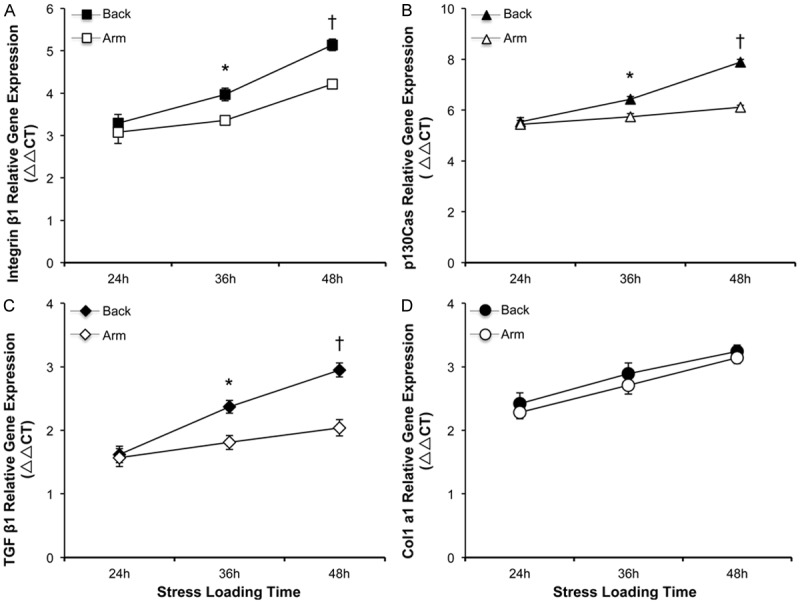
Effects of mechanical stress on target gene expressions in stretched upper back and medial arm skin fibroblasts. At each time points of mechanical loads, individual cultures of fibroblasts were collected and assayed for relative levels of gene expression of integrin β1 (Panel A), p130Cas (Panel B), TGF β1 (Panel C) and type I collagen (Panel D). Data are shown as the mean ± SD based on triplicate experiments. * and †P<0.05 versus medial arm fibroblasts stressed for 36 and 48 hours, respectively.
Measurement of protein productions in fibroblasts
Assays for protein productions in the stretched fibroblasts confirmed the above gene expression data. When exposed to mechanical stress for 36 and 48 hours, respectively, much higher expression levels of integrin β1, p130Cas (Figure 3A-C) and TGF β1 (Figure 3D) protein production were found in upper back fibroblasts as compared to those in medial arm fibroblasts (P<0.05). Moreover, the protein levels of type I collagen in the cell culture supernatant did not demonstrate significant differences between upper back and medical arm fibroblasts at any time throughout the study (P>0.05, Figure 3E).
Figure 3.
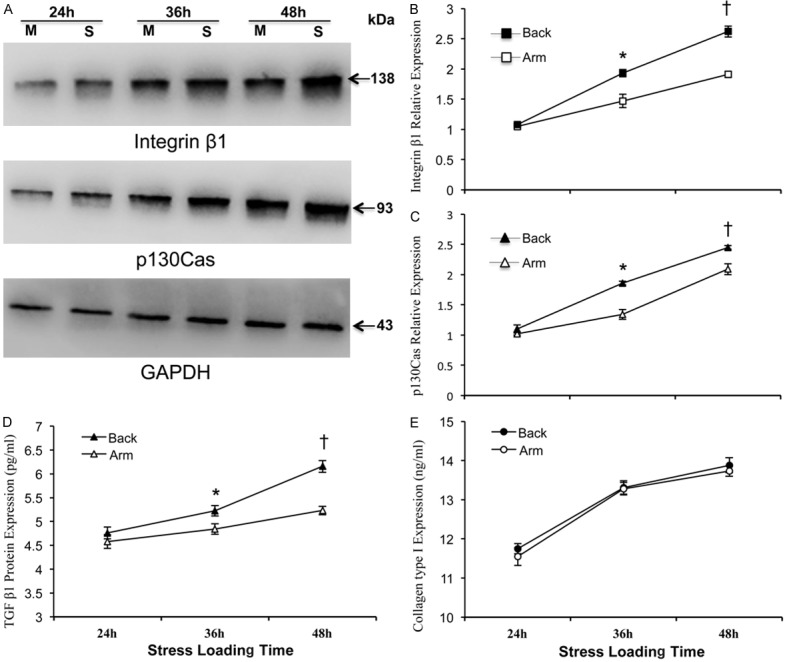
Effects of mechanical stress on target protein productions in stretched upper back and medial arm fibroblasts. At each time point following mechanical loads, each culture of fibroblasts was collected and used for Western-blot detection of integrin β1 and p130Cas (Panel A) and afterward semi-quantified and normalized to that of internal reference of GAPDH respectively (Panel B, C); or the conditioned supernatant from each group for ELISA assay to detect protein contents of TGF β1 and type I collagen (Panel D and Panel E, respectively). Data are shown as the mean ± SD based on triplicate experiments. * and †P<0.05 versus medial arm fibroblasts stressed for 36 and 48 hours, respectively. M: Medial side of upper arm; S: Scapular part of upper back skin.
Discussion
Mechanical stress is believed to be key players in the pathogenesis of human hypertrophic scar (HTS) [1]. Dermal fibroblasts represent a highly mechanoresponsive cell type known to contribute to this dermal form of fibroproliferative disorder, and the heterogeneity of these cell populations might play a major role in the HTS formation [2]. Therefore, a study on the mechanotransduction mechanisms by which human skin fibroblasts convert mechanical signals into cellular biological events is warranted. Static or dynamic mechanical stress have been reported to accelerate cell proliferation, collagen synthesis and growth factor production like TGF β1 by cultured skin fibroblasts [5,6]. Furthermore, mechanical stimulation could not only activate the paracrine TGF β1 signaling in fibroblasts, but also promote myofibroblasts to liberate and activate latent TGF β1 from preexisting and self-generated deposits in the ECM [7]. However, controversial results have also been reported that mechanical stress could induce the down-regulation of connective tissue growth factor (CTGF) expression, resulting in reduced cell proliferation rates and ECM expressions by cultured skin fibroblasts [8]. Thus it can be seen that skin fibroblasts are less well characterized in their responses to the mechanical stress.
As one of the largest organs of human body, skin tissues regarding their anatomical locations of the body demonstrate variant mechanical properties, resulting in the increased susceptibility to hypertrophic scar formation with diverse incidence rates and subsequent processes. Specific anatomic sites, such as anterior chest, shoulders, scapular part of upper back and suprapubic regions that are characterized by higher skin and muscle tension, are typical regions to produce scarring. Scalp, media side of upper arm, anterior lower leg and eyelids, in contrast, are usually less affected. Previous studies have suggested that fibroblasts, regarding their originated tissues and organs, respond differently to the applied mechanical stimulation [9-11]. Thus, we hypothesize that a difference exists in the response of dermal fibroblasts from different body sites to the mechanical forces, which is a necessary prerequisite to elucidate the underlying mechanisms for hypertrophic scar occurrence.
Integrin is a transmembrane receptor, and plays an important role in the initiation of signaling in response to mechanical and biochemical stimuli by providing two-way communication between the cells and its ECM [13]. When binding to specific extracellular proteins, the integrin elicits a cascade of events within the cell to assemble an integrin-adapter protein-cytoskeleton and signaling protein complex, which forms the basis of a focal adhesion (FA). Individual focal adhesions function as the miniature mechanosensors. In response to the applied mechanical forces, FAs re-organize their compositions that in turn serve as mechanotransducers to mediate specific cellular signals [14]. As a member of a growing family of structurally distinct protein tyrosine kinases, Focal adhesion kinase (FAK) is phosphorylated in response to integrin engagement, leading to the activation of cytoskeleton p130Cas which subsequently mediate transmission of mechanical signals across cell membrane to specific organelles inside the cells resulting in a series of biochemical signaling pathway [15-17].
Previous studies have demonstrated that mechanical loads efficiently accelerate gene expression of integrin β1 and cytoskeleton p130Cas in skin fibroblasts [18,19]. We examined the influence of cyclic mechanical stress on human skin fibroblasts derived from two different body sites, namely, scapular part of upper back and medial side of upper arm. The results demonstrate that applied mechanical stress increases the cell proliferation rates, and up-regulates the expression levels of integrin β1, cytoskeleton p130Cas, TGF β1 and type I collagen productions in the stretched dermal fibroblast. Most interestingly, there exists a difference in the levels of responsiveness to applied mechanical forces between the two sources of fibroblasts. When subjected to mechanical challenges for 36 and 48 hours, respectively, much higher expression of integrin β1 and p130Cas was found in fibroblasts from scapular upper back, which demonstrates an increased susceptibility to the mechanical stimulation in scapular upper back rather than medial upper arm fibroblasts. Meanwhile, higher cell proliferation and TGF β1 secretion were presented in stretched scapular upper back fibroblasts, suggesting that the mechanical stimuli may trigger a stronger biochemical response in the scapular upper back fibroblasts. In addition, no obvious differences exist in the synthesis and secretion of type I collagen between scapular upper back and medial upper arm fibroblasts, despite being subjected to the same described experimental conditions.
Taken together, these data document that dermal fibroblasts display a site-dependent diversity of mechanotransduction properties and biochemical reactions in response to applied mechanical loads, which might contribute to the increased susceptibility of hypertrophic scarring at different body sites. Although further studies with fibroblasts from multiple body sites are needed, our results provide new insight into the underlying biomechanical mechanisms for hypertrophic scar formation.
Acknowledgements
This work was supported by grants from Provincial Natural Science Foundation of Shandong, China, to Dr. Z. Wang [Grant number ZR2013HQ006], and by grants from Qingdao applied fundamental research program to Dr. R. Kuang [Grant number 13-1-4-161-jch].
Disclosure of conflict of interest
None.
References
- 1.Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, Stella M, Téot L, Wood FM, Ziegler UE. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Derderian CA, Bastidas N, Lerman OZ, Bhatt KA, Lin SE, Voss J, Holmes JW, Levine JP, Gurtner GC. Mechanical strain alters gene expression in an in vitro model of hypertrophic scarring. Ann Plast Surg. 2005;55:69–75. doi: 10.1097/01.sap.0000168160.86221.e9. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493–500. doi: 10.1016/j.mehy.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Ohmori Y, Akaishi S, Ogawa R, Hyakusoku H. The 4th Japan Scar Workshop. Tokyo, Japan: 2009. The analysis of keloid favorite site. [Google Scholar]
- 5.Shu MG, Yi CG, Han Y, Yang L, Zhang LX, Xia WS, Liu D, Guo SZ. The effect of mechanical stress on the expression of growth factor in human skin fibroblasts. Chinese Journal of Aesthetic Medicine. 2008;17:689–91. [Google Scholar]
- 6.Syedain ZH, Tranquillo RT. TGF-β1 diminishes collagen production during long-term cyclic stretching of engineered connective tissue: implication of decreased ERK signaling. J Biomech. 2011;44:848–55. doi: 10.1016/j.jbiomech.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanazawa Y, Nomura J, Yoshimoto S, Suzuki T, Kita K, Suzuki N, Ichinose M. Cyclical cell stretching of skin-derived fibroblasts downregulates connective tissue growth factor (CTGF) production. Connect Tissue Res. 2009;50:323–29. [PubMed] [Google Scholar]
- 9.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–26. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 10.Kjær M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 11.Jones LC, Tucci M, Frondoza C. Macrophages and fibroblasts respond differently to PMMA particles and mechanical strain. Biomed Sci Instrum. 2006;42:223–30. [PubMed] [Google Scholar]
- 12.Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011;19(Suppl 1):s2–9. doi: 10.1111/j.1524-475X.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- 13.Baker EL, Zaman MH. The biomechanical integrin. J Biomech. 2010;43:38–44. doi: 10.1016/j.jbiomech.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldmann WH. Mechanotransduction and focal adhesions. Cell Biol Int. 2012;36:649–52. doi: 10.1042/CBI20120184. [DOI] [PubMed] [Google Scholar]
- 15.Cheng M, Guan XM, Li H, Cui XD, Zhang XY, Li X, Jing X, Wu XY, Avsar E. Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement. PLoS One. 2013;8:e67675. doi: 10.1371/journal.pone.0067675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2013;196:375–85. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janoštiak R, Brábek J, Auernheimer V, Tatárová Z, Lautscham LA, Dey T, Gemperle J, Merkel R, Goldmann WH, Fabry B, Rösel D. CAS directly interacts with vinculin to control mechanosensing and focal adhesion dynamics. Cell Mol Life Sci. 2014;71:727–44. doi: 10.1007/s00018-013-1450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannafin JA, Attia EA, Henshaw R, Warren RF, Bhargava MM. Effect of cyclic strain and plating matrix on cell proliferation and integrin expression by ligament fibroblasts. J Orthop Res. 2006;24:149–58. doi: 10.1002/jor.20018. [DOI] [PubMed] [Google Scholar]
- 19.Demou ZN. Gene expression profiles in 3D tumor analogs indicate compressive strain differentially enhances metastatic potential. Ann Biomed Eng. 2010;38:3509–20. doi: 10.1007/s10439-010-0097-0. [DOI] [PubMed] [Google Scholar]


