Abstract
The present study demonstrates the effect of (R)-3-oxobutyl 3-hydroxybutanoate (OBHB) on hyperketonemia in 2 patients with Alzheimer’s disease dementia who were performed a mini-mental state examination score of above 11 and 10. The patients were treated with OBHB for 24 months and received usual diet. The patients were administered 15 g of OBHB three times per day for two days. The dosage of OBHB was increased to 30 g three times daily from the day 4. OBHB was always taken after adding with soda-flavoured syrups in order to mask the bitter taste. The measurement of plasma β-hydroxybutyrate (βHB) levels after every week was performed to determine OBHB plasma βHB dose-response relationships. Precision Xtra Glucose and Ketone Monitoring System (Abbott Diabetes Care, Inc., Alameda, CA, USA) was used to measure βHB levels in the blood samples. We did not observe any adverse effects of OBHB in any of the patients and it was well tolerated throughout the 24 months treatment period. Both of the patients showed marked improvement in mood, behaviour, self-care, cognitive and daily activity performance. The results revealed a marked improvement in conversation and interaction after administration of OBHB doses. The biochemical investigation of the blood samples before, during OBHB treatment and after 24 months of the treatment revealed only minor changes in the plasma lipids. There was a decrease in cholesterol level from 251 to 158 and 247 to 152 mg/dL in the two patients respectively after 24 months of the treatment. Similarly the level of high-density lipoprotein cholesterol was found to decrease from 157 to 79 and 149 to 76 mg/dL, respectively in two patients. Thus OBHB can be a promising agent in the treatment of hyperketonemia and can be taken as an oral supplement without changing the habitual diet.
Keywords: Neurons, ketone bodies, Alzheimer’s disease, β-hydroxybutyrate, cognitive
Introduction
Alzheimer’s disease (AD) in the initial stage is characterised by the failure of brain to utilize glucose which leads to the progression of the disease [1,2]. The use of fluorodeoxyglucose positron emission tomography has clearly shown a decrease in cerebral metabolic rate of glucose (CMRglu) in patients with preclinical AD [3]. It is reported that clinical symptoms of AD follow CMRglu decreases which may be due to the brain insulin resistance [3,4]. Since KB-derived acetyl CoA enters Krebs cycle pathway distal to the rate-limiting mitochondrial enzyme, pyruvate dehydrogenase (PDH), thus bypassing blocks in glucose utilization caused by the inhibiting effect of insulin resistance on PDH activity [5,6]. Thus during glucose utilization impairment the alternate source of energy in brain are ketone bodies (KBs) [7,8]. Therefore supplying brain with sufficient KB can help the AD brain to overcome the energy crisis. For KB to be utilized as energy source in glucose-deprived parts of the AD brain the concentration of KB needs to be increased above 0.2 mM which is present in the individuals with normal metabolism [8,9].
During hyperketonemia, the rate-limiting step for KB utilization is their transport into brain, with the utilization rate increasing nearly proportionally with plasma KB concentration [10]. It is reported that medium-chain triglyceride (MCTG) administration at a dose 20 g per day for 1 week, then 30 g for 3 weeks could contribute up to 9% of brain’s energy metabolism [7]. The methods have been established to induce therapeutic hyperketonemia through ketogenic diet (KD) [11] and/or repetitive ingestion of MCTG [12]. Use of very low-carbohydrate diet (5%-10% of calories) for 8 weeks was shown to improve verbal memory performance in patients with mild cognitive impairment (MCI) [13]. In patients with MCI and AD 20 g of MCTG per day along with usual diet increased β-hydroxybutyrate (βHB) concentration from ~0.09 mM to 0.3-0.4 mM after 2 hours. The present study demonstrates the effect ofa potent ketogenic agent, OBHB (Figure 1) on hyperketonemiain two patients with younger onset sporadic AD [14]. Ketone monoester (KME), the reduced derivative of OBHB has undergone extensive animal [15] and human toxicity tests [14] and was declared to be safe by Food and Drug Administration. It is reported that KME improves cognitive performance and reduces amyloid-b and tau deposition in cognition-relevant areas in a mouse AD model [16]. Taking into account the biological importance of KME, we devised a study to investigate the effect of OBHB (Figure 1), analogue of KME on hyperketonemia in two patients with younger onset sporadic AD [14].
Figure 1.

Structure of (R)-3-oxobutyl 3-hydroxybutanoate (OBHB).
Materials and methods
Drugs
(R)-3-oxobutyl 3-hydroxybutanoate and medium-chain triglyceride (MCTG) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Patient
Two male patients, 67 and 58years old with younger onset, sporadic AD, were selected for the study. The patients were displaying characteristic features of AD dementia. Both the patients showed marked memory loss, poor concentration and organization, misplacing important items and inability to carry out activities of daily living. The mini-mental state examination (MMSE) score for both the patients declined from 24 to 11 and 25 to 10, respectively. Brain magnetic resonance imaging (MRI) showed diffuse involutional changes of frontal and parietal lobes and moderate left-sided and severe right-sided atrophy of amygdala and hippocampus, consistent with AD. The patients were observed to be APOE ε4 positive.
Treatment
After the patients became increasingly depressed and began to experience confusion with images in the mirror and his whereabouts in the home. He began wandering, lost interest in yard and housework, and required step-by-step instruction and considerable assistance to dress and complete hygiene related tasks.
Results
A written consent was taken from the caregivers of both of the patients confirming that they are aware of the nature and type of the study performed. The patients were treated with OBHB at home for 24 months. The patients received usual diet and were also given the doses of MCTG/CO mixture during the whole treatment period. OBHB was always taken after adding with soda-flavoured syrups [25% syrup, 25% OBHB, and 50% water] in order to mask the bitter taste. Treatment of the patients with 15 g three times per day doses of OBHB for two days resulted in a marked improvement in mood and the ability to recite and write out the complete alphabet. A more prominent improvement was observed on the day 3, when the patients spontaneously selected clothes and dressed themselves. The dosage of OBHB was increased to 30 g three times daily from the day 4. With the increase in dosage the patients began to initiate and complete many other activities without the assistance from caregivers. The commonly performed activities of both the patients included showering, shaving, brushing teeth, finding his way around the house, and choosing and ordering food from a menu. At the end of the one month of OBHB treatment abstract thinking, insight, and a subtle sense of humour was noticed back in the conversation of both the patients. The patients themselves stated that they are feeling good, happier and more energetic. They were able to do the things easily after one month of the OBHB treatment. After 2 months of OBHB treatment using 30 g three times per day, both the patients showed improvement in memory retrieval, spontaneously discussing events that occurred up to a week earlier. The patients were able to perform more complex tasks such as vacuuming, washing dishes by hand and yard work.
The measurement of plasma βHB levels after every week was performed to determine OBHB plasma βHB dose-response relationships (Figure 2). The results revealed a marked improvement in conversation and interaction after administration of OBHB doses.
Figure 2.
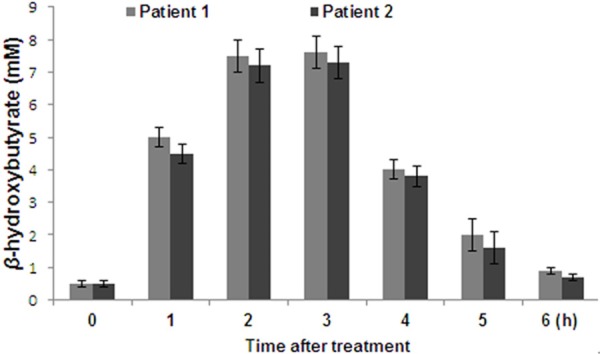
Effect of OBHB treatment on β-Hydroxybutyrate (βHB) concentrations in patients with Alzheimer’s disease dementia.
The biochemical investigation of the blood samples before, during OBHB treatment and after 24 months of the treatment revealed only minor changes in the plasma lipids. There was a decrease in cholesterol level from 251 to 158 and 247 to 152 mg/dL in the two patients respectively after 24 months of the treatment (Figure 3A). Similarly the level of high-density lipoprotein cholesterol was found to decrease from 157 to 79 and 149 to 76 mg/dL, respectively in two patients (Figure 3B).
Figure 3.

Effect of OBHB treatment on total cholesterol and high-density lipoprotein cholesterol levels.
Discussion
Earlier people used prolonged fasting or a very high-fat and very low-carbohydrate containing diet to raise the plasma KB levels [5,11]. However, the side effects like dehydration, urate nephrolithiasis, substantial weight changes, and amenorrhea associated with these methods hindered their applicability. The fact that relatively high plasma KB levels (6-8 mM) can be maintained indefinitely when KME is ingested at 3- to 4-hour intervals raises questions about the long-term safety of this degree of hyperketonemia. In the present study OBHB was administered regularly as a food supplement to observe its effect onhyperketonemia in patients with long-standing AD dementia.
Treatment of the patients initially with 15 g three times per day doses of OBHB for 2 days resulted in a marked improvement in mood and the ability to recite and write out the complete alphabet. The results from our study demonstrate that after 2 months of OBHB treatment using 30 g three times per day, the patients showed improvement in memory retrieval, spontaneously discussing events. The patients were able to perform more complex tasks such as vacuuming, washing dishes by hand, and yard work. The measurement of plasma βHB levels after every week was performed to determine OBHB plasma βHB dose-response relationships. The results revealed a marked improvement in conversation and interaction after administration of OBHB doses. The biochemical investigation of the blood samples revealed only minor changes in the plasma lipids. However, the cholesterol level was decreased in both the patients after 24 months of the treatment. Similarly the level of high-density lipoprotein cholesterol was also found to decrease.
Conclusion
KME can be a safe and promising agent to induced hyperketonemia and can be administered regularly as a food supplement without changing the habitual diet. KME induced elevations of circulating βHB levels improved behaviour, cognitive and daily activity performance in patients with long-standing AD dementia.
Disclosure of conflict of interest
None.
References
- 1.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging and Alzheimer’s disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosconi L, Berti V, McHugh P, Pupi A, de Leon MJ. A Tale of Two Tracers: Glucose Metabolism and Amyloid Positron Emission Tomography Imaging in Alzheimer’s Disease. In: Ashford JW, editor. Handbook of Imaging the Alzheimer Brain. Amsterdam: IOS Press; 2011. pp. 219–34. [Google Scholar]
- 4.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denton RM, Randle PJ, Bridges BJ, Cooper RH, Kerbey AL, Pask HT, Severson DL, Stansbie D, Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975;9:27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- 6.Kim VI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin resistant states. Diabetes. 2006;55:2311–7. doi: 10.2337/db05-1606. [DOI] [PubMed] [Google Scholar]
- 7.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–95. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanltallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Nutr Rev. 2003;61:327–41. doi: 10.1301/nr.2003.oct.327-341. [DOI] [PubMed] [Google Scholar]
- 9.Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, Castellano CA, Cunnane SC. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition. 2013;29:635–40. doi: 10.1016/j.nut.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Blomqvist G, Alvarsson M, Grill V, Von Heijne G, Ingvar M, Thorell JO, Stone-Elander S, Widén L, Ekberg K. Effect of acute hyperketonemia on the cerebral uptake of ketone bodies in nondiabetic subjects and IDDM patients. Am J Physiol Endocrinol Metab. 2002;283:E20–8. doi: 10.1152/ajpendo.00294.2001. [DOI] [PubMed] [Google Scholar]
- 11.Kossoff EH, Freeman J, Turner Z, Rubenstein J. Ketogenic Diets: Treatments for Epilepsy and Other Disorders. 5th edition. New York: Demos; 2011. pp. 1–341. [Google Scholar]
- 12.Henderson ST, Vogel JL, Barr L, Garvin F, Jones JJ, Costantini LG. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33:425, e19–2. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke K, Tchabanenko K, Pawlosky R, Carter E, King MT, Musa-Veloso K, Ho M, Roberts A, Robertson J, Vanitallie TB, Veech RL. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–8. doi: 10.1016/j.yrtph.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight NS, Murray AJ, Cochlin LE, King MT, Wong AW, Roberts A, Robertson J, Veech RL. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol. 2012;63:196–208. doi: 10.1016/j.yrtph.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognitive-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:1530–9. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerndt PR, Naughton JL. Fasting: the history, pathophysiology and complications. West J Med. 1982;137:379–99. [PMC free article] [PubMed] [Google Scholar]


