Abstract
Vascular cognitive impairment (VCI) refers to different degrees of cognitive dysfunction syndrome caused by all kinds of cerebral vascular disease and vascular factors. Before in the development of vascular dementia (VaD), early diagnosis and intervention can prevent and delay the progress of VCI, even reverse cognitive impairment. In this review, we summarized the research progress of vascular cognitive impairment in pathophysiology, biomarkers and treatments, etc.
Keywords: Vascular dementia, cognitive impairment, biomarkers, treatments, molecular mechanism
Introduction
Vascular cognitive impairment (VCI) refers to different degrees of cognitive dysfunction syndrome caused by all kinds of cerebral vascular disease (CVD) and vascular factors. Historically, the clinical manifestation of VaD (vascular dementia) was firstly described since the 15th century by Thomas Willis. From the concept of VaD emerging since 19th century to VCI recognized, the content of VCI improved and encompassed not only VaD but also Alzheimer’s disease (AD) with cerebrovascular disorder (AD with CVD or mixed dementia) and VCI with no dementia (VCIND) [1]. Thus, VCI is a more broad term that encompasses all the states of cognitive impairment associated with CVD.
Around one-third of patients with AD demonstrate evidences of vascular pathology, while up to two-thirds of patients with CVD develop at least some degree of AD pathology in the brain. Cerebral small vessel diseases (cSVD) are thought to be the most frequent pathological neurological processes and have a crucial role in at least three fields: stroke, dementia and aging. Cerebral small vessel disease has an important role in cerebrovascular disease and is a leading cause of VCI in the elderly [2]. Unlike AD, VCI is thought to be preventive and even reversible in early-stage, thus SVD should be a main target for preventive and treatment strategies. In this article, the authors aimed to review pathophysiologic mechanisms, biomarkers, treatments, of VaD/VCI based on the classification of SVD.
Pathophysiology of SVD with vascular cognitive impairment
Recently, there has been a growing interest in the clinical significance of SVD as cause of VCI. So far, the main pathology and neuroimaging features of SVD including lacunar infarcts, white matter lesions (WMLs), cerebral microbleeds (CMBs), enlarged perivascular spaces (Virchow-Robin Spaces, VRS), are known to be strongly implicated for the presence of VCI (Figure 1).
Figure 1.
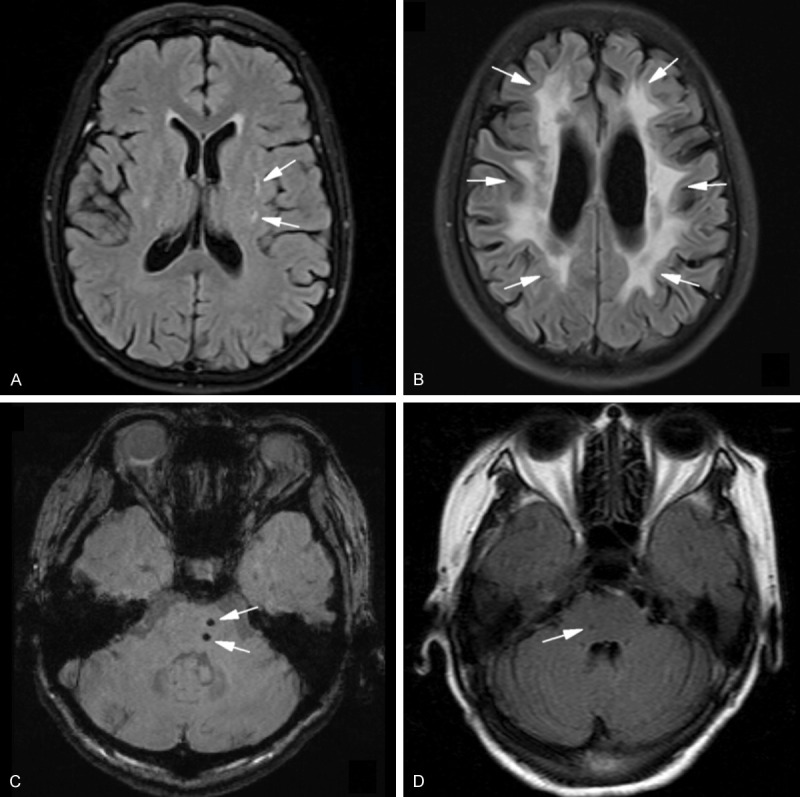
Key imaging characteristics of features of cerebral small vessel disease. A. Lacunar infarcts in the left basal ganglia on a FLAIR MRI. B. Multiple white matter lesions or hyperintensities on a MRI (FLAIR imaging) around the ventriculus of a patient with possible CADASIL. C. Multiple microbleeds (small foci of hypointensity) in the brain stem as shown on a SWI MRI sequence. D. Virchow-Robin Spaces (VRS) in the brain stem. basal ganglia and centrum semiovale. (White arrows = representative features of cerebral small vessel disease). FLAIR = fluid-attenuated inversion recovery; SWI = susceptibility weighted imaging; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencepalopathy.
Deep brain infarction
It is important to note, that the classic lacunar syndromes did not include cognitive impairment as a feature or dedicated syndrome. However, small deep brain infarcts are known to cause so-called “strategic infarct dementia” and the lesion burdens have also been associated with dementia risk. In acute period, deep brain infarcts are better detected by MRI than by CT, and hyperintense appears on diffusion-weighted imaging (DWI), and within hours to days on T2-weighted imaging or fluid attenuated inversion recovery (FLAIR) sequences [3]. On the other hand, chronically, deep brain infarcts present hypointense on T1 and FLAIR, and often have a hyperintense rim around them on the latter sequence [3]. Once macrophages have removed to infarcted area, irregular cavities are left with surrounding gliosis, and lipid- rich and hemosiderin-rich macrophages are left in surrounding gliotic tissue along with extravasated plasma proteins, fibrinoid necrosis, and vascular fragments [4]. Many risk factors are associated with deep brain infarcts, such as older age, and particularly hypertension, as well as diabetes mellitus, smoking, excess alcohol consumption, and dyslipidemia, are shared with those of superficial infarcts [5]. Deep brain infarcts have overall better outcomes, but have a greater risk of developing cognitive impairment [6,7]. In a recent cohort study, the presence of thalamic lacunes was associated with poor global cognitive performance, low motor activity and executive function performance; and the presence of lacunes in the pallidum or putamen was associated with memory dysfunction [8]. Above all, deep brain infarcts have been associated with a number of outcomes in relation to VCI, including cognitive decline, dementia, but also gait disturbance, urinary incontinence, and disability.
White matter lesions (WMLs)
This commonly phenotype of SVD represents a different group than deep brain infarcts, but they often co-exist [1]. On MRI, WMLs are recognized as white matter hyperintensities (WMH) on T2 and FLAIR sequences. Such WMLs are seen in white matter tracts surrounding the ventricular system, though they are also seen in other areas and in the immediate subcortical white matter [9]. Magnetic resonance-based diffusion tensor imaging (DTI) provides a promising measure the diffusion of water in white matter tracts, allowing researchers to examine the integrity of axonal pathways in patients with SVD [10]. Recent epidemiological studies have demonstrated that WMLs are associated with cognitive decline. Moreover, the combination of WMLs in patients with deep brain infarcts is also associated with more cognitive decline [11]. WMLs are seen in at least 30% of patients with AD and in 60% of patients with dementia. It has been found that a greater burden of WMLs is also associated with incontinence, gait dyspraxia, and incident falls. An appraisal of 16 studies confirmed the association between WMLs and cognitive decline in different patient groups, from hospital-based to population-based [12]. Finally, a large meta-analysis of 46 observational studies, demonstrated that WMLs are associated with greater risk of future stroke, dementia and mortality [13].
Cerebral microbleeds (CMBs)
This phenotype of SVD refers to small deep or superficial hemorrhages of 2-10 mm in diameter seen by MRI. The T2* gradient-echo sequence and the newer susceptibility-weighted imaging (SWI), both provide sensitive methods for detecting microbleeds [1]. These lesions correspond to small collections of hemosiderin-laden macrophages around small perforating vessels. In recent years, there have been more evidences supported the cerebral microbleeds in relation to cognitive decline [14]. A greater burden of cerebral microbleeds has been found in relation to worse cognitive impairment [14]; it has been found in subjects with CADASIL that a greater burden of cerebral microbleeds associated with worse functional ability [15]. In the most recent study, CMBs were assessed in Chinese subjects from the population-based Singapore Chinese Eye Study, and independent of cardiovascular risk factors and other markers of cerebral small vessel disease, significantly associated with poorer cognitive function, including executive function, attention, and visuoconstruction [16]. In the other recent study, Karolinska Imaging Dementia Study, CMBs are present in 1504 patients with dementia of 22% and related to lower cognitive scores differently depending on dementia diagnosis, which reflects the inherent pathology of different dementia diagnoses [17]. Additional previous research of CMBs were comprehensively discussed in the remarkable review by Werring [18], strongly indicating that the presence of CMBs played an independent role in the path-physiology process of cognitive impairment.
VCI and AD have generally been considered as independent, non-overlapping diseases. It is oversimplified for both VCI and AD are diseases of older people and commonly co-occurrence. A key hypothesis supposed that AD and SVD either co-exist in individuals or do interact in pathological process [19]. Interestingly, CMB are commonly found in patients with AD of between about 20 and 30% [20]. It is indicated that the distribution of CMB in AD is similar to that in sporadic cerebral amyloid angiopathy (CAA), suggesting that CMB in AD are more likely to be related to CAA [20]. CAA is a small vascular damage characterized by vascular deposition of β-amyloid protein, which is almost invariable and found at autopsy in more than 90% of AD [21]. One possibility is that the intriguing process that may link SVD with AD is CAA, for which CMB is an increasingly important neuroimaging marker. Although the mechanisms by CMB might influence cognitive decline remain unclear, it is speculated that CMB may cause direct structural damage to surrounding brain tissue, leading to disrupting frontal-subcortical circuits and nearby neurons; or play an indirect effect including arteriolar narrowing causing hypoperfusion and micro-ischaemic damage [22]. Finally, it has been unknown whether CMBs may be a general neuroimaging marker of small vessel disease or have little influence on cognitive function. In a word, there is clearly a need to explore the CMB thoroughly, which may be a promising bridge linked between VCI and AD in pathogenesis.
SVD biomarkers
Combinatorial markers of general MRI features
It has been a long time that the SVD is strongly correlated with VCI. However, it is difficult to establish a stable animal model used for mechanism, so neuroimaging provides a surrogate marker for investigating it. The above mentioned four general MRI features of cSVD (lacunar infarct, VRS, WMLs, CMB) have all been individually linked to cognitive decline. Further, there have been a growing number of evidences that these general MRI features of brain damage are more likely to express co-occurrence rather than occur separately. Thus, researchers have paid much interest in the combination of general features for predicting cSVD. Baune et al found a combined occurrence of lacunar infarcts and WMLs in 10% of the population and a significant difference between those patients with only one lesion type and patients affected by both on information processing speed and memory [23]. In the LADIS study, Jokinen et al found that the relationship between progression of WMLs and acute lacunes with cognitive decline was additive, but not synergistic [24]. Huijts et al recently combined these four types of general features and found the significant associations between accumulating SVD burden and decreased performance on all cognitive domains [25]. As Wardlaw recently suggested, additional methods needed to assess SVD on neuroimaging rather than over-reliance on single general feature on MRI [2]. Therefore the combination of variety general neuroimaging features could be a feasible way and taken into clinical practice widely.
Connectivity features
A number of studies have correlated T2 lesion volume with clinical and cognitive parameters, but results have been conflicting. The conflicts are probably caused by that T2WI represents increased water content and may not differentiate between areas of mildly and severely damaged tissue [26]. DTI is more sensitive to white matter ultrastructural damage and DTI parameters have been shown to correlate more strongly with cognition than T2 lesion volume (Figure 2). In a longitudinal study, Nitkunan recruited 35 subjects with lacunar stroke and leukoaraiosis, and underwent multimodal MRI (brain volume, fluid-attenuated inversion recovery lesion load, lacunar infarct number, fractional anisotropy, and mean diffusivity from diffusion tensor imaging) and neuropsychological testing. Repeating MRI and neuropsychology at 1 year, diffusion tensor imaging particularly correlates best with executive function and is the most sensitive to change [27]. Similarly, Lawrence recruited 115 subjects in the ongoing SCANS study of cognitive impairment with SVD, and underwent neuropsychological assessment and multimodal MRI. The results showed that independent predictors of executive function in SVD included diffusivity of normal appearing white matter on DTI, that radial diffusivity was a stronger DTI predictor than axial diffusivity, suggesting ischaemic demyelination, may be an important predictor of cognitive impairment in SVD [28]. Severe asymptomatic stenosis of the internal carotid artery (ICA) leads to increased incidence of mild cognitive impairment (MCI) likely through silent embolic infarcts and/or chronic hypoperfusion, but the brain dysfunction is poorly understood and difficult to diagnose. In a recent study, thirty cognitively intact subjects with asymptomatic, severe (> 70%), unilateral stenosis of the ICA were compared with 30 healthy controls, on a battery of neuropsychiatric tests and diffusion tensor imaging. In the subjects group, their whole-brain mean fractional anisotropy (FA) was significantly reduced and regional functional connectivity (Fc) was significantly impaired in the dorsal attention network (DAN), frontoparietal network, sensorimotor network and default mode network. In particular, the Fc strength at the insula of the DAN was linearly related with attention performance. The multivariate pattern classification gave over 90% predictive accuracy of individual with MCI. Cognitive decline in stroke-free individuals with severe carotid stenosis may arise from nonselective widespread disconnections of long-range, predominantly interhemispheric non-hippocampal pathways [29].
Figure 2.
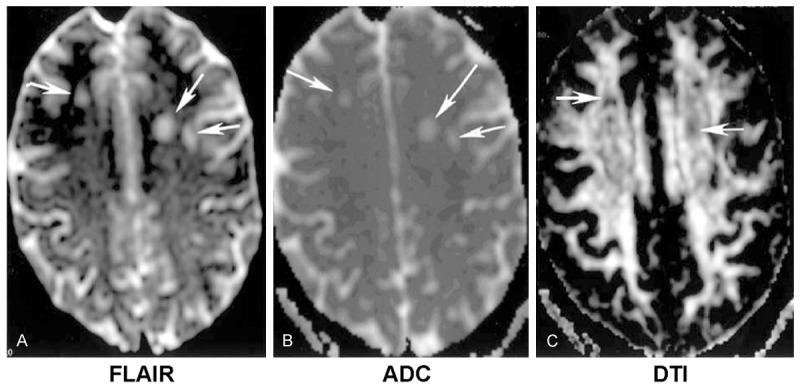
An old female patient with multiple leukoencepalopathy in centrum semiovale (white arrows). A. Hyperintensities on T2-weighted imaging. B. ADC imaging. C. Lower FA (fractional anisotropy) on diffusion-tensor imaging (DTI).
All these studies mentioned above indicated that, DTI, measuring connectivity, can be more sensitive to detect white matter impairment earlier and be strongly correlated with cognitive impairment. As the result, compared with general MRI, DTI has two additional advantages including being a surrogate marker for detecting VCI and an important method for investigating mechanism underlying VCI.
BBB permeability and neuroinflammation
A compromised BBB can be a source of albumin leakage, which can increase protein concentration in the CSF as it crosses the BBB with less resistance. Accumulating evidences, from clinical, neuroimaging to pathological studies, indicated the durable link between BBB and cognitive impairment. Yang and Rosenberg concluded in 2011 that the presence of hypoxia induced by breakdown of BBB leads to death of oligodendrocytes and extensive gliosis in the WMLs, which are highly related to the presence of VCI. They also demonstrated that, in addition to hypertension and diabetes, disrupted BBB was possibly caused by the inflammatory process contributing to glial activation, inflammatory mediators release, secondary microvascular injury, neurovascular unit dysfunction [30].
In a recent study, 60 patients suspected VCI underwent permeability measurements with DCEMRI and lumbar puncture to measure albumin index (Qalb). The result indicated that there was abnormal permeability in white matter in patients with subcortical ischemic vascular disease as shown by DCEMRI and Qalb. On the other hand, another neuroimaging study in patients with non-disabling lacunar or cortical stroke and BBB permeability MR imaging following their original stroke revealed that long term poor functional outcome was associated with increased basal ganglia BBB permeability about 3 years later [31]. Although the inconsistent damage locations were possibly due to the duration followed up and functional assessment, these two studies indicated disrupted BBB contributed to poor cognition and long function.
Metalloproteinases are markers of neuroinflammation as they attack the basal lamina and tight junctions in blood vessels, in addition to the disruption of myelin. In comparison with AD, the levels of metalloproteinases-9 (MMP-9) in the CSF were increased in patients with VCI [32]. However samples, like inflammatory markers, in the CSF by lumbar puncture are difficult to obtained, therefore circulating ones have been gotten more interested in. In a recent systematic review, the result indicated that markers of inflammation (C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6)) were higher in lacunar stroke versus non-stroke, while there were no studies measuring TNF-α chronically and a single study measuring IL-6 chronically showed no difference between lacunar stroke and non-stroke [33]. Subjects in the Lothian Birth Cohort 1936, all born in 1936, were measured plasma fibrinogen, C-reactive protein (CRP) and interleukin-6 (IL-6) and rated PVS in three brain regions, as well as WMH volumetrically and visually using the Fazekas scale. Circulating inflammatory markers are associated with MR-visible PVS but not directly with WMH [34]. In 163 first-ever lacunar stroke patients and 183 essential hypertensive patients, Rouhl assessed SVD manifestations on brain magnetic resonance imaging (MRI) and levels of C-reactive protein (CRP), neopterin, as well as circulating soluble adhesion molecules (sICAM-1, sVCAM-1, sE-selectin, sP-selectin). Neopterin, sICAM-1 and sVCAM-1 levels were higher in patients with extensive SVD manifestations than in those without that. Neopterin levels independently related to higher numbers of enlarged VRS [35]. It implies an inflammatory process with activated monocytes/macrophages may play an essential role in the increased permeability of the blood brain barrier in patients with SVD.
microRNAs (miRNAs) for brain ischemia and cognitive impairment
The discovery of posttranscriptional gene silencing by miRNAs has led to an explosion of new hypotheses in human disease. miRNAs are now implicated in most biological processes including embryonic development, cellular differentiation, metabolism, and many pathological processes [36]. In some research areas including cancer, liver and heart disease, miRNA researches have quickly developed from diagnosis into therapeutic programs. In the nervous system, the complexity and plasticity of the neuronal network and the functional specialization of neurons and glia depend on highly organized and coordinated gene expression. It has been estimated that at least 30% of protein-coding genes are regulated by miRNAs, so it is not surprising that initial studies characterizing miRNAs in the brain revealed a role in neuronal cell differentiation and embryonic development of the nervous system [37]. Moreover, changes in the miRNA expression profile have been observed in diseases of the brain, including cerebral ischemia and Alzheimer’s disease. Recent studies have demonstrated the relationships between miR146a and Alzheimer disease (AD) and implicated that miR146a may be involved in neuroinflammation and the metabolism of amyloid-β (Aβ), which are critical markers in AD pathology. The further research provided evidence that the rs57095329 polymorphism, risk AA genotype, in the miR146a promoter was related to the genetic susceptibility to AD, and may promoted proinflammatory cytokines, thus influencing the pathogenesis of AD. On the other hand, our colleagues recently concluded comprehensively the role of miRNAs in stroke in a remarkable review [38], therefore there is no need of repetition in this part.
All these evidences above implicate that miRNAs may have remarkable potential to be excellent biomarkers for brain ischemia and cognitive impairment. Indeed, miRNA-based diagnostic assays have already been developed and approved for certain diseases, like neoplasm. On the other hand, the other advantage for miRNAs served as potentially useful diagnostic biomarkers is less invasive sample procurement. Endogenous circulating miRNAs have been found to be stable and consistently detected in serum, plasma and other bodily fluids because of their packaging and secretion into the blood within exosomes [39]. Although most of recent researches are involved in the cerebral large vessel diseases and AD, thus more information of miRNAs in the SVD and VCI studies is rare, the potential diagnostic value of miRNAs is unlikely to be limited to certain diseases and likely to be developed in the SVD and VCI in the future.
Future perspectives-multivariate measures
Beside biomarkers discussed above, other emerging technologies (PET, proton magnetic resonance spectroscopy, Aβ and tau in CSF), have also been promising in future study, but beyond the content in this review. With technical developments for the integration of different modalities, future researches should aim to investigate all available various biomarkers of SVD (morphology, metabolism, inflammation, and molecular activities) and can be performed simultaneously. For that, even though there is no single measurement that can be used to diagnose patients with the small vessel progressive form of VCI, taking information together from the clinical examination, neuropsychological testing, white matter lesion pattern on traditional MRI, connectivity features on diffusion tensor imaging, BBB permeability, and inflammatory intermediates in circulation or CSF will provide a comprehensive picture that improves accuracy of diagnosis early in the course. If inflammation can be proven to be an important component of the progressive white matter damage, treatment trials with novel approaches may be possible.
Treatment strategies
As small vessel disease itself is one of the most important causes of VCI, clinical trials targeting classic vascular risk factors for VCI are ongoing. The ongoing SPRINT Memory and cognition IN Decreased hypertension (SPRINT-MIND)1 study will focus to determine whether lower systolic blood pressure (SBP) goals are associated with the rate of incident dementia and MCI, global and domain-specific cognitive function, and small vessel ischemic disease. The Prevention of Decline in Cognition after Stroke Trial (PODCAST) is testing the hypothesis that intensive blood pressure (SBP < 125 mmHg) and/or lipid-lowering [low density lipoprotein (LDL) < 2.0 mmol/L] versus moderate blood pressure (SBP < 140 mmHg) and LDL (< 3.0 mmol/L) is associated with less cognitive decline and VaD. The Aspirin in Reducing Events in the Elderly (ASPREE) trial is assessing the effect of aspirin on incident dementia and physical disability. The sub-study of the Secondary Prevention of Small Subcortical Strokes (SPS3) trial is looking at the rate of cognitive decline in patients treated with aspirin and/or clopidogrel [40]. The Efficacy and Safety Study of Nimodipine to Prevent Mild Cognitive Impairment after Acute Ischemic Strokes (NICE) is testing the hypothesis that the calcium channel blocker nimodipine may be associated with less cognitive decline and VaD.
On the other hand, it should be note that VCI management do not only confine medication, but non-medication like lifestyle improvement, regular monitor of blood pressure and glutamine, smoke free and so on, is also crucial for VCI. Therefore, additional ongoing trials are also interested in multi-target interventions. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial is testing if using lifestyle counseling including cognitive training, increased physical and social activity, nutritional guidance, and intensive monitoring of vascular and metabolic risk factors worked to prevent VCI. The Austrian Poly- intervention Study to Prevent Cognitive Decline after Ischemic Stroke (ASPIS) trial is using intensive control and motivation for better compliance with medication, regular blood pressure measurements, diet changes, and physical activity versus standard stroke care to prevent cognitive decline. The Prevention of Dementia by Intensive Vascular Care (PREDIVA) trial is using intensive vascular care with visiting a practice nurse every 4 months to monitor vascular risk factors like hypertension, hypercholesterolemia, diabetes, overweight, smoking, and level of physical exercise. Novel molecular interventions are using specific genetic approaches to affect different targeting of proteins, including NOTCH3, HTRA1, and APOE ε4, related to specific pathways of acute and chronic ischemia [41].
Conclusion ns
Small vessel disease is one of the most important causes of stroke and cognitive decline. More attention and targeted efforts should be needed to better explore the underlying mechanism of VCI caused by small vessel disease and to thoroughly define the clinical consequences of these diseases. Given the frequent coexistence of different manifestations of small vessel disease, such as white matter lesions and lacunar infarcts, and microbleeds, further research aim to VCI should take all available neuroimaging markers of SVD together rather than in isolation. Furthermore, specific preventive and therapeutic measures to reduce the burden of functional loss caused by small vessel disease needs to be designed. Neuroimaging could be a major tool to assess efficacy of these measures. More work remains to be done before the concept of VCI is widely accepted and put actively into clinical practice.
Acknowledgements
This study was supported by the Guide Project of Western Medicine from Shanghai Committee of Science (NO. 131119a6900).
Disclosure of conflict of interest
None.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke. 2011;6:47–59. doi: 10.1111/j.1747-4949.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1969;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 5.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, Anderson CS, Hankey GJ, Jamrozik K, Appelros P. Differing risk factor profiles of ischemic stroke subtypes evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- 6.Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 8.Benisty S, Gouw AA, Porcher R, Madureira S, Hernandez K, Poggesi A, van der Flier WM, Van Straaten EC, Verdelho A, Ferro J. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80:478–483. doi: 10.1136/jnnp.2008.160440. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, DeCarli C. White matter hyperintensity penumbra. Stroke. 2011;42:1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Swieten JC, Staal S, Kappelle LJ, Derix MM, Van Gijn J. Are white matter lesions directly associated with cognitive impairment in patients with lacunar infarcts? J Neurol. 1996;243:196–200. doi: 10.1007/BF02444014. [DOI] [PubMed] [Google Scholar]
- 12.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 13.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler M. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan A, Godin O, Jouvent E, O’Sullivan M, Gschwendtner A, Peters N, Duering M, Guichard JP, Holtmannspötter M, Dufouil C. Impact of MRI markers in subcortical vascular dementia: a multi-modal analysis in CADASIL. Neurobiol Aging. 2010;31:1629–1636. doi: 10.1016/j.neurobiolaging.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Hilal S, Saini M, Tan CS, Catindig JA, Koay WI, Niessen WJ, Vrooman HA, Wong TY, Chen C, Ikram MK. Cerebral microbleeds and cognition: the epidemiology of dementia in singapore study. Alzheimer Dis Assoc Disord. 2014;28:106–112. doi: 10.1097/WAD.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 17.Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, Cavallin L, Aspelin P, Kristoffersen-Wiberg M, Wahlund LO. Cerebral Microbleeds: Different Prevalence, Topography, and Risk Factors Depending on Dementia Diagnosis-The Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol. 2015;36:661–6. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Hennerici MG. What are the mechanisms for post-stroke dementia? Lancet Neurol. 2009;8:973–975. doi: 10.1016/S1474-4422(09)70261-3. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, Rogaeva E, Black SE. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 21.Iadecola C, Park L, Capone C. Threats to the mind aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charidimou A, Werring DJ. Cerebral microbleeds and cognition in cerebrovascular disease: an update. J Neurol Sci. 2012;322:50–55. doi: 10.1016/j.jns.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 23.Baune BT, Roesler A, Knecht S, Berger K. Single and combined effects of cerebral white matter lesions and lacunar infarctions on cognitive function in an elderly population. J Gerontol A Biol Sci Med Sci. 2009;64:118–24. doi: 10.1093/gerona/gln004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, Gouw A, Scheltens P, Barkhof F, Visser MC, Fazekas F. MRI-defined subcortical ischemic vascular disease: baseline clinical and neuropsychological findings. Cerebrovasc Dis. 2009;27:336–344. doi: 10.1159/000202010. [DOI] [PubMed] [Google Scholar]
- 25.Huijts M, Duits A, Van Oostenbrugge RJ, Kroon AA, De Leeuw PW, Staals J. Accumulation of MRI markers of cerebral small vessel disease is associated with decreased cognitive function. A study in first-ever lacunar stroke and hypertensive patients. Front Aging Neurosci. 2013;5:72. doi: 10.3389/fnagi.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. J Neurol Neurosurg Psychiatry. 2010;81:13–19. doi: 10.1136/jnnp.2008.167288. [DOI] [PubMed] [Google Scholar]
- 27.Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS. Multimodal MRI in cerebral small vessel disease its relationship with cognition and sensitivity to change over time. Stroke. 2008;39:1999–2005. doi: 10.1161/STROKEAHA.107.507475. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence AJ, Patel B, Morris RG, MacKinnon AD, Rich PM, Barrick TR, Markus HS. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s Cognition and Neuroimaging in Stroke (SCANS) study. PLoS One. 2013;8:e61014. doi: 10.1371/journal.pone.0061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin CJ, Tu PC, Chern CM, Hsiao FJ, Chang FC, Cheng HL, Tang CW, Lee YC, Chen WT, Lee IH. Connectivity features for identifying cognitive impairment in presymptomatic carotid stenosis. PLoS One. 2014;9:e85441. doi: 10.1371/journal.pone.0085441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2013;44:525–527. doi: 10.1161/STROKEAHA.112.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: systematic review and meta-analysis. Cerebrovasc Dis. 2013;37:64–75. doi: 10.1159/000356789. [DOI] [PubMed] [Google Scholar]
- 34.Aribisala BS, Wiseman S, Morris Z, Valdés-Hernández MC, Royle NA, Maniega SM, Gow AJ, Corley J, Bastin ME, Starr J. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke. 2014;45:605–607. doi: 10.1161/STROKEAHA.113.004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouhl RP, Damoiseaux JG, Lodder J, Theunissen RO, Knottnerus IL, Staals J, Henskens LH, Kroon AA, de Leeuw PW, Tervaert JW. Vascular inflammation in cerebral small vessel disease. Neurobiol Aging. 2012;33:1800–1806. doi: 10.1016/j.neurobiolaging.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Yan H, Fang M, Liu XY. Role of microRNAs in stroke and poststroke depression. ScientificWorldJournal. 2013;2013:459692. doi: 10.1155/2013/459692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R. The secondary prevention of small subcortical strokes (SPS3) study. Int J Stroke. 2011;6:164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43:3137–3146. doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]


