Abstract
Secondary cardiac cancer most frequently originates from primary lung cancer and most commonly occurs in the pericardium. On electrocardiographic examination, patients with secondary cardiac cancer occasionally show ST segment elevation that mimics acute coronary syndrome, despite the absence of coronary artery occlusion. We herein describe a rare case of secondary cardiac cancer that presented with ST segment elevation and review the literature regarding ST segment elevation caused by secondary cardiac cancer. A 73-year-old Japanese woman was admitted to the hospital with chest pain. Electrocardiography showed abnormal ST segment elevation in the precordial and lateral leads, suggestive of ST-elevation myocardial infarction. Emergency coronary angiography showed occlusion of the distal left anterior descending coronary artery (LAD), and plain old balloon angioplasty of the LAD was performed. The ST segment elevation initially resolved after angioplasty, but recurred after 7 days. Contrast-enhanced chest computed tomography showed primary lung cancer in the left lower lobe, pericardial metastasis, and myocardial metastasis in the intraventricular septum and posterolateral wall of the left ventricle. Histopathological examination of the lung cancer was not performed. Patients with ST segment elevation due to secondary cardiac cancer may have symptoms and electrocardiographic changes mimicking anteroseptal or lateral infarction without the development of abnormal Q waves. These findings are frequently associated with posterolateral or anteroseptal invasion by primary lung cancer and may indicate a poor prognosis. In conclusion, physicians should be aware that secondary cardiac cancer may present with symptoms and ST segment elevation mimicking acute coronary syndrome, indicating a poor prognosis.
Keywords: Primary lung cancer, secondary cardiac cancer, ST segment elevation
Introduction
Secondary cardiac cancer most frequently originates from primary lung cancer [1,2]. Cardiac metastasis is detected in 25% to 30% of autopsy examinations of patients with primary lung cancer, but it is difficult to diagnose before death because it is often asymptomatic [2]. The most common site of cardiac metastasis is the pericardium, and metastasis to the myocardium or endocardium is rare [3].
Secondary cardiac cancer may result in various abnormalities on electrocardiography (ECG) including bradycardia, atrial fibrillation, ST-T changes, and premature atrial or ventricular contractions [3]. Patients with direct transmural invasion or myocardial metastasis from primary lung cancer may have ST-T changes on ECG that mimic ST elevation myocardial infarction (STEMI), even in the absence of coronary artery occlusion [3]. Tumor compression or invasion of a major coronary artery leading to occlusion has been reported, but is rare [4,5].
We herein present a rare case of myocardial and pericardial metastasis of primary lung cancer that presented with coronary artery occlusion probably caused by external tumor compression of the left anterior descending coronary artery (LAD). Interestingly, the ST segment was re-elevated after percutaneous coronary intervention (PCI), and the ST segment elevation persisted during hospitalization. We also herein review the literature describing STEMI-like ECG abnormalities in patients with secondary cardiac cancer.
Case report
A 73-year-old woman was admitted to our hospital with a 1-week history of intermittent chest pain, which was not clearly related to exercise and had gradually increased and become persistent half an hour before admission. She had a history of atrial fibrillation and had taken rivaroxaban (15 mg/day) for the past 2 years to prevent thromboembolism, but had no other remarkable medical or family history. She was a nonsmoker and did not consume alcohol. An ECG from 1 year before admission did not show significant ST-T changes (Figure 1A).
Figure 1.
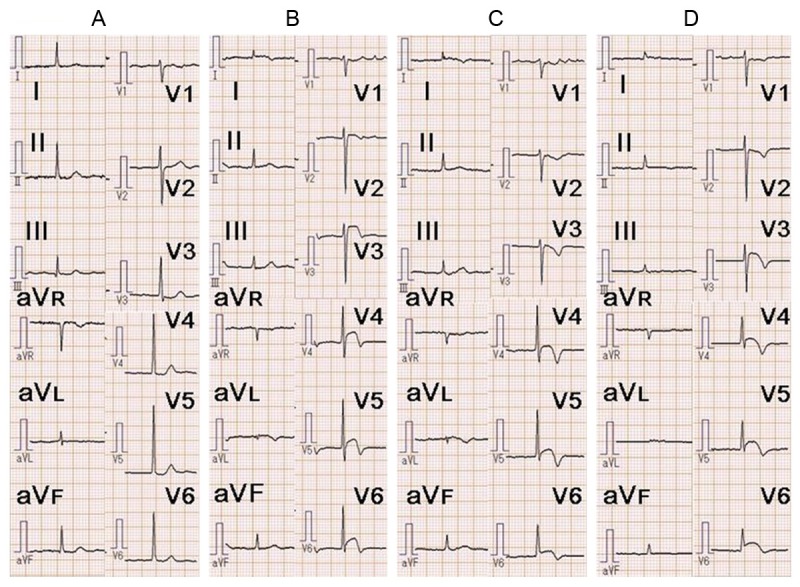
Electrocardiography (ECG) findings in our patient. A. ECG 1 year before admission showed no significant ST-T changes. B. ECG on admission showed ST segment elevation in leads I, aVL, and V3-V6. C. ECG on day 2 showed improvement of the ST segment elevation and terminal T-wave inversion in leads I, aVL, and V3-V6. D. ECG on day 5 showed recurrence of the ST segment elevation in leads I, aVL, and V3-V6. The ST segment elevation persisted throughout the remainder of the hospital admission.
On admission, her height was 147.3 cm, weight was 50.0 kg, body temperature was 36.8°C, and blood pressure was 110/58 mmHg. Her heart rate was 72 beats per minute and was irregular. Physical examination revealed no significant abnormalities. She presented with shortness of breath in room air, with a blood O2 saturation of 96%, partial O2 pressure of 82 mmHg, and partial CO2 pressure of 37 mmHg. Chest X-ray showed moderate cardiomegaly (cardiothoracic ratio of 63%), but no congestion or pleural effusion was observed. ECG showed atrial fibrillation with ST segment elevation in leads I, aVL, and V2-V6, suggestive of STEMI (Figure 1B). Laboratory tests showed a high white cell count (13,400/mm3; normal, <9000/mm3) and high levels of troponin (Tn)-I (2.05 ng/ml; normal, <0.04 ng/ml), brain natriuretic peptide (456.3 pg/ml; normal, <18.4 pg/ml), and C-reactive protein (6.05 mg/dl; normal, <0.30 mg/dl). Levels of cardiac enzymes, including aspartate aminotransferase (AST), creatine phosphokinase (CK), CK-MB, and lactate dehydrogenase (LDH), were normal. Levels of lipid parameters, including low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides, were also normal. Transthoracic echocardiography (TTE) had poor penetration, but did not show massive pericardial effusion or other obvious abnormalities except for apical hypokinesis. Sublingual nitroglycerin improved but did not completely resolve the patient’s chest pain. She was treated with oral aspirin (200 mg), oral clopidogrel (300 mg), and intravenous heparin (5000 IU). Emergency coronary angiography (CAG) was performed via the right femoral artery using 6-Fr sheath and showed occlusion of the distal LAD (Figure 2A). The occlusion did not change after intracoronary injection of isosorbide dinitrate. There were no other stenotic lesions or visible coronary calcifications in the left or right coronary arteries. Based on these findings, PCI was performed. A guiding catheter (6-Fr Launcher JL 3.5; Goodman, Nagoya, Japan) was advanced into the left coronary artery, and a guidewire (Asahi Sion Blue; Asahi Intecc, Nagoya, Japan) was passed through the lesion into the distal LAD. Thrombus aspiration was attempted using an Eliminate 3 catheter (Terumo, Tokyo, Japan) but was unsuccessful. The intravascular ultrasound catheter could not be advanced through the lesion. Therefore, plain old balloon angioplasty (POBA) was performed using a Tenku catheter (2.0-mm diameter, 15-mm length; St. Jude Medical, Saint Paul, MN, USA). No indentation of the balloon was observed during inflation. After POBA, TIMI grade 3 flow was observed, but the LAD lesion was still visible (Figure 2B). POBA was repeated twice with no further improvement. Considering the small diameter of the target lesion, stent implantation was not performed.
Figure 2.
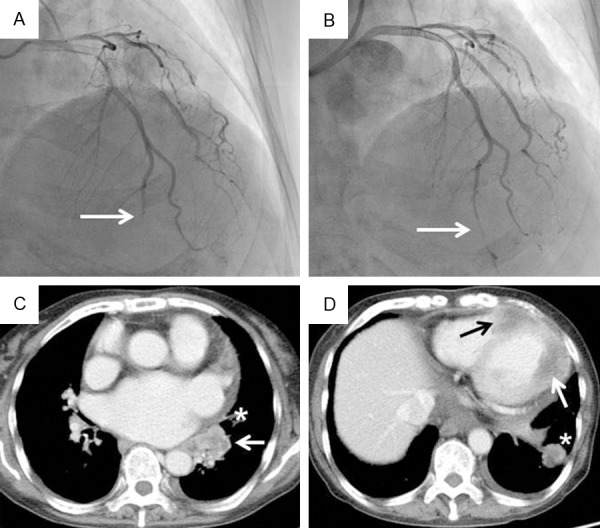
(A, B) Coronary angiography (CAG) and (C, D) contrast-enhanced chest computed tomography (CT) findings. (A) CAG on admission showed occlusion of the distal left anterior descending coronary artery (white arrow). (B) CAG after plain old balloon angioplasty showed revascularization with persistent residual stenosis (white arrow). (C) Contrast-enhanced CT on day 7 showed an irregularly shaped mass in the left lower lobe of the lung adjacent to the left atrium (white arrow). Part of the pericardium was thickened (asterisk). (D) Myocardial masses were present in the apex and intraventricular septum (black arrow) and in the left lateral wall (white arrow). An intrapulmonary metastasis is also visible (asterisk).
The patient’s chest pain resolved after the POBA procedure. On day 2 after the procedure, the ST segment elevation in leads I, aVL, and V2-V6 was obviously improved (Figure 1C). The cardiac enzyme levels (AST, CK, CK-MB, and LDH) were normal, but the Tn-I level remained high (1.58 ng/ml). On day 5, the Tn-I level had returned to normal. On day 6, the patient complained of frequent lower back pain, but there were no changes on ECG, laboratory data, or chest X-ray findings. On day 7, ECG showed ST segment re-elevation in the precordial leads, but the patient did not complain of chest pain (Figure 1D). Repeated TTE showed no additional changes. Contrast-enhanced computed tomography (CT) of the chest and abdomen showed an irregularly shaped mass in the lower lobe of the left lung adjacent to the left atrium (Figure 2C). No significant pericardial effusion was observed, but the left lateral part of the pericardium was thickened and slightly enhanced, suggesting pericardial metastasis of the lung cancer. There were also myocardial lesions with necrotic areas in the intraventricular septum and posterolateral wall of the left ventricle (Figure 2D). Bone metastases in the L1 vertebra and right sacrum and multiple metastatic pulmonary nodules were simultaneously observed. There were no abnormal masses in the neck or abdomen. These findings strongly suggested left ventricular myocardial metastasis of primary lung cancer. Tumor marker levels were elevated as follows: carbohydrate antigen 19-9 (71.6 U/ml; normal, <36.9 U/ml), cytokeratin 19 fragment (27.0 ng/ml; normal, <3.5 ng/ml), and neuron-specific enolase (28.4 ng/ml; normal, <16.3 ng/ml). ECG showed persistent ST segment elevation. Because sputum cytology findings were negative, we recommended that the patient undergo transbronchial lung biopsy to obtain a histopathological diagnosis. However, the patient refused further investigation and treatment for her lung cancer. She was prescribed oral oxycodone (80 mg/day) and was transferred to a hospice on day 28. Two months after discharge, she died of respiratory failure due to progression of the lung lesion. Autopsy could not be performed.
Discussion
Secondary cardiac cancer, especially metastatic cancer, most frequently originates from primary lung cancer [2]. However, it can also originate from bronchogenic carcinoma, breast cancer, esophageal cancer, and non-solid cancers such as leukemia, lymphoma, and Kaposi’s sarcoma [6]. Diagnosis of metastatic cardiac cancer while the patient is alive can be difficult because of the lack of specific signs or symptoms [6]. Some patients with secondary cardiac cancer, including metastatic cancer and direct invasion of cancer into the heart, may develop symptoms mimicking acute coronary syndrome (ACS) and exhibit ECG changes suggestive of STEMI. To the best of our knowledge, only 18 patients with secondary cardiac cancer who had STEMI-like ECG changes and evidence of myocardial involvement on imaging examinations such as CT or echocardiography have been previously reported (Table 1) [7-24]. Seventeen of these 18 patients were male.
Table 1.
Characteristics of patients with secondary cardiac cancer and electrocardiographic changes mimicking ST elevation myocardial infarction
| Author | Year | Age, sex | ECG leads showing ST elevation | Symptoms | Primary lesion | Histo-pathology | Metastasis or direct invasion | Massive pericardial effusion | Coronary angiogram | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Yao [7] | 1997 | 71 M | I, II, aVL aVF, V4-6 | Chest pain | Lt lung (lower lobe) | S | I (posterolateral) | (-) | Not done | Unknown |
| Astorri [8] | 2000 | 73 M | I, aVL | Chest pain | Lt lung | S | I (posterolateral) | (-) | Not done | Unknown |
| Vallot [9] | 2001 | 69 M | I, aVL V4-6 | Dyspnea | Lt lung (lower lobe) | S | M (apex) | (+) | Not done | Dead (15 days) |
| Konishi [10] | 2001 | 69 M | V1-4 | Chest pain | Rt lung (lower lobe), Stomach | A | M (anteroseptum) | (+) | Normal | Dead |
| Daher [11] | 2003 | 54 M | V2-5 | Chest pain, dyspnea | Lt lung (upper lobe) | S | M (apex) | (-) | Not done | Unknown |
| Rodrigues [12] | 2003 | 56 M | I, aVL V4-6 | Chest pain | Lt lung (upper lobe) | S | M (pericardium) | (-) | Normal | Dead |
| Kinjo [13] | 2005 | 65 M | I, aVL V2-5 | Dyspnea, cough | Lt lung (lingual lobe) | L | I (anterolateral) | (-) | Not done | Dead |
| Samaras [14] | 2007 | 69 M | V2-5 | Chest pain, dyspnea | Rt lung (upper lobe) | S | M (anteroseptum) | (-) | Not done | Unknown |
| Okwuosa [15] | 2008 | 61 M | V2-6 | Chest pain, diaphoresis | Rt lung (upper lobe) | AS | M (apex) | (-) | LAD occlusion | Unknown |
| Roubille [16] | 2008 | 51 M | I, aVL V4-6 | Chest pain, dyspnea | Lt lung (lower lobe) | A | I (apex) | (-) | Normal | Unknown |
| Aggarwala [17] | 2008 | 71 F | II, III, aVf | Chest pain | Hematologic malignancy | Hematolymphoid neoplasm | M (RA, TA, AV groove) | (-) | RCA stenosis | Dead (1 month) |
| Guha [18] | 2010 | 47 M | I, aVL V4-6 | Chest Pain | Lt lung (hilar mass) | SC | I (posterolateral) | (-) | Not done | Unknown |
| Vivas [19] | 2010 | 24 M | I, aVL V5-6 | Chest pain, dyspnea | Osteo-sarcoma (lt lung metastasis) | Osteo-sarcoma | I (posterolateral) | (+) | Normal | Dead (a few days) |
| Roy [20] | 2011 | 48 M | V4-6 | Chest pain | Lt lung | S | I (posterior) | (-) | Not done | Dead (two days) |
| Toledano [21] | 2012 | 59 M | V1-5 | Chest pain | Lung | L | M (anterior) | (-) | Normal | Unknown |
| Oliveira [22] | 2012 | 81 M | V2-4 | Chest discomfort | Esophageal cancer | S | M (anteroseptum) | (-) | Normal | Dead |
| Ciurus [23] | 2013 | 58 M | V2-6 | Chest pain | Lt lung | SC | I (anterior) | (-) | LCX stenosis | Dead (3 months) |
| Kim [24] | 2014 | 62 M | I, aVL | Chest pain | Lt lung | S | I (posterolateral) | (-) | Normal | Unknown |
Abbreviations: Lt, left; Rt, right; S, squamous cell carcinoma; A, adenocarcinoma; L, large cell carcinoma; AS, adenosquamous cell carcinoma; SC, small cell carcinoma; I, direct invasion; M, metastasis; RA, right atrium; TA, tricuspid annulus; AV, atrioventricular; LAD, left anterior descending coronary artery; RCA, right coronary artery; LCX, left circumflex coronary artery.
Symptoms, ECG changes, and laboratory findings
All the previously reported patients complained of chest pain, discomfort, or dyspnea mimicking ACS or congestive heart failure. Some patients had intermittent symptoms mimicking effort angina that persisted for several weeks before admission [7,10,20]. However, half of the previously reported patients had sudden-onset or progressive symptoms that started within a few days of admission, mimicking ACS [9,11,14,15,17,18,21,23,24]. In addition, most of the patients showing ACS-like symptoms had dyspnea at the time of admission [9,11-14,16,18,20]. Post-admission symptoms were clearly reported in only two patients; both had presented with persistent chest pain [15,17]. Notably, half of the patients did not have a diagnosis of malignancy at the time of admission with cardiac symptoms [10,12-14,16,18,20,23,24]. Why symptoms mimicking effort angina or ACS may occur in such patients is unclear; interestingly, however, most of the patients had a transmural myocardial tumorous lesion. Considering the frequency of dyspnea at the time of presentation, left ventricular dysfunction resulting from a cardiac tumorous lesion may contribute to the symptoms mimicking angina or ACS in such patients.
Most of the patients had ST segment elevation in the precordial or lateral leads. The most frequent abnormality was ST segment elevation in the lateral leads (I, aVL, and V5/V6), which occurred in 10 patients [7-9,12,13,16,18-20,24]. The next most frequent abnormality was ST segment elevation in the precordial leads (V1, V2, and V3/V4), which occurred in seven patients [10,11,14,15,21-23]. Only one patient had ST segment elevation in the inferior leads [17]. Metastatic or invasive myocardial cancer can usually be classified as apical, anteroseptal, anterolateral, or posterolateral. The six patients with a posterior or posterolateral tumor had ST segment elevation in the lateral leads [7,8,18-20,24]. The five patients with an anterior or anteroseptal tumor had ST segment elevation in the precordial leads [10,14,21-23]. The five patients with an apical or anterolateral tumor had ST segment elevation in both the lateral and precordial leads [9,11,13,15,16]. The patient with diffuse pericardial metastases affecting all cardiac chambers and symptoms mimicking constrictive-effusive pericarditis had ST segment elevation in the precordial leads [12]. These findings indicate that the leads with ST segment elevation may reflect the location of the myocardial tumor, similar to the way in which ST segment elevation reflects the location of STEMI. Interestingly, only two patients had ST segment elevation in the inferior leads [7,17], suggesting that metastatic myocardial cancer of the inferior wall is rare. Moreover, ST segment elevation due to metastatic cardiac cancer is characterized by the persistence of these changes and absence of evolution, such as the development of Q waves [8,10,13,14,18,20,23,24]. Additional ST segment elevation may develop a few months later in leads that did not have significant ST-T changes at the time of admission [14]. Abnormal Q waves were rare, being reported in only two patients [10,23]. In our patient, the ST segment elevation and chest pain initially improved after angioplasty. The ST segment elevation subsequently recurred, but without further chest pain. These findings suggest that initial ST segment elevation may reflect both myocardial ischemia and metastasis, and that recurrent and persistent ST segment elevation after PCI without the development of abnormal Q waves may reflect progression of the myocardial metastasis. To the best of our knowledge, STEMI-like ECG changes have not been reported in patients with primary cardiac tumors. In our review of the literature, we found no reports describing ST-T changes in adults with primary cardiac tumors, which are rare. Various tachyarrhythmias and bradyarrhythmias have been reported in pediatric patients with primary cardiac tumors, but no clear ST-T changes have been reported in this population [25].
Cardiac enzyme data on admission, including AST, CK, CK-MB, and LDH levels, were reported in 11 patients, and were all normal [7,8,10,13-16,18,20,23,24]. Tn-T or Tn-I levels were reported in six patients [11,14,15,18,23,24] and were high in four patients [11,15,23,24]. Tn levels may stay elevated or gradually decrease after admission [11,15,23]. Two patients had a high brain natriuretic peptide level [14,16]. Considering these findings, a normal cardiac enzyme level may be another distinctive feature in patients with ST segment elevation due to metastatic cardiac cancer.
Imaging modalities used for diagnosis
The most common imaging modality used for the initial diagnosis of metastatic cardiac cancer was TTE, which was used to detect myocardial metastasis or myocardial invasion from an adjacent tumor in 12 patients [7,9,12,15-19,21-24]. Five patients were initially diagnosed based on contrast-enhanced chest CT findings [8,10,13,14,20]. Contrast-enhanced magnetic resonance imaging of the heart may be an alternative to CT, but was used for the initial diagnosis in only one patient [11]. It may be useful for secondary investigation after CT or TTE for the definitive diagnosis of metastatic cardiac cancer [10,16,17,19,23]. Myocardial perfusion single-photon emission CT (SPECT) using technetium-99m-hexakis-methoxy-isobutil-isonitrile (MIBI) can show a myocardial cancer lesion as a photon-deficient area, but this is similar to the appearance of myocardial infarction [10] and is therefore not specific for myocardial cancer. In our case, TTE was not very useful for initial diagnosis of the myocardial lesion because of poor penetration. A combination of TTE and contrast-enhanced CT may therefore be useful for the initial diagnosis of secondary cardiac cancer. CAG was performed in 10 patients and showed normal findings in eight [10,12,16,19,21,22,24]. No feeding arteries to the cardiac tumor were found in any of the patients who underwent CAG. Two patients had significant stenosis on CAG. One of these patients had stenosis in the right coronary artery with ST segment elevation in the inferior leads [17]. The other patient had stenosis in the left circumflex artery; however, a myocardial tumor was present in the anterior wall with ST segment elevation in the precordial leads [23], suggesting that the ECG changes may have reflected tumor invasion rather than myocardial ischemia. Another patient had obstruction of the LAD and underwent a failed attempt to pass a guidewire through the occlusion [15]. This patient had occlusion of the distal LAD and an apical metastatic lesion, similar to our patient. Whether the metastatic lesion adjacent to the LAD caused direct vascular occlusion is unclear. However, it should be noted that coronary artery obstruction may be an infrequent complication of secondary cardiac cancer. Left ventriculography was performed in three patients [12,19,21] and showed a left ventricular mass in two of these patients. Left ventriculography may therefore be a useful investigation technique in patients with suspected secondary cardiac cancer.
Location of the primary cancer, histopathological findings, and outcome
Unfortunately, we were unable to determine the histopathological features of the primary lung cancer in our patient. The metastatic cancer originated from primary lung cancer in 14 of the 18 previously reported patients [7-9,11-16,18,20,21,23,24], and the remaining four patients had combined primary lung and stomach cancer, a hematolymphoid neoplasm, a primary osteosarcoma, and primary esophageal cancer, respectively [10,17,19,22]. The primary lesion was in the left lung in 11 of the 14 patients with primary lung cancer [7-9,11-13,16,18,20,23,24]. Direct invasion into the heart occurred in eight of these 14 patients [7,8,13,16,18,20,23,24], all of whom had a primary lesion in the left lung and ST segment elevation in the lateral leads. Actually, five of these eight patients showed direct invasion into the lateral wall, suggesting that the lateral wall is frequently affected by direct invasion of primary lung cancer [7,8,13,18,24]. The histopathological findings in these eight patients included squamous cell carcinoma (n = 4), small cell carcinoma (n = 2), adenocarcinoma (n = 1), and large cell carcinoma (n = 1). Cardiac metastasis occurred in the remaining six of the 14 patients, including two patients with right-sided primary lung cancer [9,11,12,14,15,21]. Interestingly, cardiac metastasis occurred mainly in the apical and anteroseptal areas [9,11,14,15,21], except in one patient who had diffuse pericardial metastasis [12]. These patients often had ST segment elevation in the precordial leads [11,14,15,21]. The histopathological findings in these six patients included squamous cell carcinoma (n = 4), adenosquamous cell carcinoma (n = 1), and large cell carcinoma (n = 1). These findings suggest that squamous cell carcinoma is the most frequent cause of secondary cardiac cancer, both in cases of direct invasion and in cases of cardiac metastasis from primary lung cancer. Squamous cell carcinoma accounts for 20% to 30% of non-small-cell lung cancers and commonly arises from the epithelial cells of the central airways [26]. Squamous cell carcinoma generally exhibits locally aggressive growth with invasion into adjacent organs and metastasis to the regional bronchial lymph nodes; it metastasizes to distant organs less frequently than does adenocarcinoma [26]. In general, the most common cardiac site for metastasis is the pericardium [3], and previous studies have reported that the most probable metastatic pathway from the lung to the heart is via retrograde lymphatic extension [27,28]. However, more recent studies have reported that pericardial metastasis is associated with massive pericardial effusion in more than half of affected patients and that the most common locations of metastatic nodules in the pericardium are the free wall of the right ventricle and the right atrioventricular groove [29]. Most of the previously reported patients with cardiac metastasis rather than direct invasion had an intramural mass rather than a pericardial lesion and little or no pericardial effusion; massive pericardial effusion was present in only two patients [9,10]. The previously reported patients had cardiac metastatic lesions in the anteroseptal or apical areas. The anterior wall of the left ventricle, anterior two-thirds of the interventricular septum, and most of the apex usually receive their blood supply from the LAD [30]. The LAD is considered to be the most important coronary artery because it supplies more than 50% of the left ventricular mass [31]. Hematogenous metastasis via the LAD may therefore play an important role in the cardiac metastasis of lung cancer.
Autopsy was performed in two of the previously reported patients [10,13], one of which had normal CAG findings [10]. Interestingly, autopsy showed infiltration of tumor cells into the myocardium in both cases, with no evidence of myocardial necrosis due to ischemia. These findings may help us to understand the mechanisms underlying ST segment elevation in patients with secondary cardiac cancer. A previous report described a patient with acute myocardial infarction due to intravascular tumor thromboembolism in the intramural branches of the coronary artery, but no ST segment elevation was present on ECG [32]. Moreover, most of the previously reported cases showed transmural secondary cardiac cancer on CT, MRI, or TTE. Together, these findings suggest that transmural myocardial damage by infiltration of tumor cells may be the cause of persistent ST segment elevation without the development of abnormal Q waves in these patients, rather than ischemic necrosis.
The clinical outcome was described in nine of the previously reported patients [9,10,12,13,17,19,20,22,23]. In these nine patients, the survival time after admission ranged from 2 days to 3 months, suggesting that STEMI-like ECG changes due to secondary cardiac cancer may indicate a poor prognosis. Physicians should be aware of the possibility that such patients may die within a few days.
Conclusions
Patients with secondary cardiac cancer may have symptoms mimicking ACS and STEMI-like ECG changes, even in the absence of coronary artery stenosis or occlusion. Patients may have persistent ST segment elevation in the precordial and/or lateral leads without the development of abnormal Q waves. ST segment elevation most frequently occurs in the posterolateral or anteroseptal leads, consistent with invasion of the myocardium in these areas. Patients with secondary cardiac cancer who present with symptoms and ECG changes mimicking anteroseptal or lateral infarction have a poor prognosis, and physicians should be aware of the possibility of early death.
Acknowledgements
We thank Miss Hisae Kuribara for providing secretarial assistance, which was funded by Gunma Chuo Hospital.
Disclosure of conflict of interest
None.
References
- 1.Prichard RW. Tumors of the heart: review of the subject and report of one hundred and fifty cases. AMA Arch Pathol. 1951;51:98–128. [PubMed] [Google Scholar]
- 2.Reynen K, Köckeritz U, Strasser RH. Metastases to the heart. Ann Oncol. 2004;15:375–81. doi: 10.1093/annonc/mdh086. [DOI] [PubMed] [Google Scholar]
- 3.Abe S, Watanabe N, Ogura S, Kunikane H, Isobe H, Yamaguchi E, Munakata M, Kawakami Y. Myocardial metastasis from primary lung cancer: myocardial infarction-like ECG changes and pathologic findings. Jpn J Med. 1991;30:213–8. doi: 10.2169/internalmedicine1962.30.213. [DOI] [PubMed] [Google Scholar]
- 4.Brazdzionyte J, Mickeviciene A, Gailys R, Unikas R, Andriulis M, Macas A. A rare clinical case: myocardial infarction caused by coronary occlusion by cancer cells. Medicina (Kaunas) 2002;38:631–6. [PubMed] [Google Scholar]
- 5.Bulava A, Skvarilová M, Marek D, Kociánová E, Lukl J. Acute myocardial infarct as a result of external compression caused by an expanding pulmonary adenocarcinoma. Vnitr Lek. 2004;50:321–4. [PubMed] [Google Scholar]
- 6.Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics. 2001;21:439–49. doi: 10.1148/radiographics.21.2.g01mr15439. [DOI] [PubMed] [Google Scholar]
- 7.Yao NS, Hsu YM, Liu JM, Chen LT, Liau CS. Lung cancer mimicking acute myocardial infarction on electrocardiogram. Am J Emerg Med. 1999;17:86–8. doi: 10.1016/s0735-6757(99)90026-8. [DOI] [PubMed] [Google Scholar]
- 8.Astorri E, Fiorina P, Pattoneri P, Paganelli C. Persistent ST segment elevation in a patient with metastatic involvement of the heart. Minerva Cardioangiol. 2001;49:81–5. [PubMed] [Google Scholar]
- 9.Vallot F, Berghmans T, Delhaye F, Dagnelie J, Sculier JP. Electrocardiographic manifestations of heart metastasis from a primary lung cancer. Support Care Cancer. 2001;9:275–7. doi: 10.1007/s005200000212. [DOI] [PubMed] [Google Scholar]
- 10.Konishi S, Kojima T, Ichiyanagi K, Kinuya S, Yokoyama K, Taki J, Nakajima K, Michigishi T, Tonami N. A case of double cancers with myocardial metastasis mimicking acute myocardial infarction both on an electrocardiogram and on Tc-99m-MIBI myocardial SPECT. Ann Nucl Med. 2001;15:381–5. doi: 10.1007/BF02988248. [DOI] [PubMed] [Google Scholar]
- 11.Daher IN, Luh JY, Duarte AG. Squamous cell lung cancer simulating an acute myocardial infarction. Chest. 2003;123:304–6. doi: 10.1378/chest.123.1.304. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues AC, Abreu E, Demarchi LM, Mathias W Jr, Leal SM, Andrade JL. Lung neoplasm mimicking an acute lateral myocardial infarction. J Am Soc Echocardiogr. 2003;16:1198–200. doi: 10.1067/S0894-7317(03)00642-4. [DOI] [PubMed] [Google Scholar]
- 13.Kinjo Y, Nagasaki A, Teruya I, Nakachi K, Higa M, Komiya I, Takasu N. Cardiac involvement of lung cancer presenting myocardial infarction-like electrocardiographic changes. Intern Med. 2006;45:113–4. doi: 10.2169/internalmedicine.45.1559. [DOI] [PubMed] [Google Scholar]
- 14.Samaras P, Stenner-Liewen F, Bauer S, Goerres GW, von Boehmer L, Kotrubczik N, Jenni R, Renner C, Knuth A. Images in cardiovascular medicine. Infarction-like electrocardiographic changes due to a myocardial metastasis from a primary lung cancer. Circulation. 2007;115:e320–1. doi: 10.1161/CIRCULATIONAHA.106.650762. [DOI] [PubMed] [Google Scholar]
- 15.Okwuosa TM, Williams KA. “Mass-ive” infarction: case report and review of myocardial metastatic malignancies. J Nucl Cardiol. 2008;15:719–26. doi: 10.1016/j.nuclcard.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Roubille F, Cayla G, Gahide G. Cardiac tumor mimicking acute myocardial infarction. Arch Cardiovasc Dis. 2008;101:677–8. doi: 10.1016/j.acvd.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwala G, Iyengar N, Horwitz P. Cardiac mass presenting as ST-elevation myocardial infarction: case report and review of the literature. J Invasive Cardiol. 2008;20:628–30. [PubMed] [Google Scholar]
- 18.Guha S, Mookerjee S, Karmakar RN, Mani S, Pande A, Bhattacharya R, Hema MB. Left atrial extension of lung malignancy with ECG changes resembling STEMI. Indian Heart J. 2010;62:81–3. [PubMed] [Google Scholar]
- 19.Vivas D, Ruiz-Mateos B, Franco E. Acute myocardial infarction secondary to direct myocardial infiltration by a malignant neoplasia. Eur Heart J. 2010;31:2261. doi: 10.1093/eurheartj/ehq177. [DOI] [PubMed] [Google Scholar]
- 20.Roy PP, Dwari AK, Dey SK, Sarkar A, Bhattacharya A, Saha AK. A rare case of lung cancer with invasion in the heart giving rise to electrocardiographic features simulating myocardial infarction. J Indian Med Assoc. 2011;109:498–9. [PubMed] [Google Scholar]
- 21.Toledano FJ, de Lezo JS, Segura J, de Lezo JS, Mesa D. An electrocardiographic sign of tumor invasion of the myocardium. Am J Emerg Med. 2012;30:2078, e1–5. doi: 10.1016/j.ajem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira SM, Gonçalves A, Cruz C, Almeida J, Madureira AJ, Amendoeira I, Maciel MJ. Cardiac metastasis from epidermoid esophageal cancer mimicking anterior myocardial infarction. Rev Port Cardiol. 2012;31:163–6. doi: 10.1016/j.repc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Ciurus T, Maciejewski M, Lelonek M. Cardiac involvement of lung cancer mimicking myocardial infarction. Kardiol Pol. 2013;71:994. doi: 10.5603/KP.2013.0245. [DOI] [PubMed] [Google Scholar]
- 24.Kim KH, Jeong MH, Yoon HJ, Ahn Y, Cho JG, Park JC, Kang JC. A case of myocardial involvement in lung cancer that mimics ST segment elevation in myocardial infarction. Korean J Intern Med. 2014;29:525–8. doi: 10.3904/kjim.2014.29.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake CY, Del Nido PJ, Alexander ME, Cecchin F, Berul CI, Triedman JK, Geva T, Walsh EP. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol. 2011;58:1903–9. doi: 10.1016/j.jacc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol. 2012;13:e418–26. doi: 10.1016/S1470-2045(12)70291-7. [DOI] [PubMed] [Google Scholar]
- 27.Kline IK. Cardiac lymphatic involvement by metastatic tumor. Cancer. 1972;29:799–808. doi: 10.1002/1097-0142(197203)29:3<799::aid-cncr2820290338>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Onuigbo WI. The spread of lung cancer to the heart, pericardium and great vessels. Jpn Heart J. 1974;15:234–8. doi: 10.1536/ihj.15.234. [DOI] [PubMed] [Google Scholar]
- 29.Prakash P, Kalra MK, Stone JR, Shepard JA, Digumarthy SR. Imaging findings of pericardial metastasis on chest computed tomography. J Comput Assist Tomogr. 2010;34:554–8. doi: 10.1097/RCT.0b013e3181d77d7e. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell RN. Heart. In: Kumar V, Abbas AK, Aster JC, editors. Robbins Basic Pathology. Philadelphia: Elsevier Saunders; 2013. pp. 365–406. [Google Scholar]
- 31.Gopaldas RR, Chu D, Bakaeen FG. Acquired Heart Disease: Coronary Insuffuciency. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL., editors. Sabiston Textbook of Surgery: the Biological Basis of Modern Surgical Practice. Philadelphia: Elsevier Saunders; 2012. pp. 1650–78. [Google Scholar]
- 32.Buckendahl AC, Martens F, Scholman HJ, Denkert C, Dietel M, Weichert W. Acute myocardial infarction caused by coconary tumor thromboembolism: a rare primary manifestation of malignant tumor disease. Hum Pathol. 2006;37:236–8. doi: 10.1016/j.humpath.2005.10.016. [DOI] [PubMed] [Google Scholar]


