Abstract
Background and aims: It remains a challenge to prevent the local recurrence or distant metastasis of gastric carcinoma after D2 gastrectomy. Cytokine-induced killer (CIK) cells have shown promising activity against solid tumors in vitro and in vivo. We investigated the effect of adjuvant chemotherapy combined with autologous CIK therapy after D2 gastrectomy compared with adjuvant chemotherapy alone after D2 gastrectomy in patients with stage II-III gastric cancer. Methods: From January 2009 to December 2011, 226 patients with stage II-III gastric cancer who had had curative D2 gastrectomy were enrolled. Eighty-nine patients (CIK group) received adjuvant chemotherapy combining with autologous CIK therapy and 137 patients (control group) received adjuvant chemotherapy alone. Disease-free survival (DFS) and overall survival (OS) were evaluated. Results: Patients in the CIK group had longer DFS and OS than patients in the control group (DFS 41.0 months vs. 32.0 months, OS 45.0 months vs. 44.0 months, by log-rank test P = 0.006 and P = 0.028, respectively). In subgroup analysis, no significant differences in DFS and OS were observed between the two groups for the patients with stages II disease. Patients with stage III disease in the CIK group had longer median DFS and OS than patients in the control group (P = 0.031 and P = 0.038, respectively). In multivariate analysis, the stage and the interaction of stage and CIK therapy were independent prognostic factors for DFS and OS. Conclusions: The data suggest that adjuvant chemotherapy combined with autologous CIK therapy can improve prognosis for gastric carcinoma patients after D2 gastrectomy, especially for the patients with stage III disease.
Keywords: Gastric cancer, immunotherapy, cytokine-induced killer cells, adjuvant chemotherapy, D2 gastrectomy
Introduction
Gastric cancer is the fourth most common malignant disease worldwide and the second most common cause of death from cancer, with 988,000 new cases (7.8% of all cancers) and 736,000 deaths per year [1-3]. Surgery is the main treatment for operable gastric cancer; however, recurrence rates are as high as 40-80% in advanced cases [3,4]. In East Asia, D2 gastrectomy is the standard surgical treatment for localized gastric cancer, and chemotherapy alone as adjuvant treatment is now considered the standard of care after adequate D2 gastrectomy [5-7]. However, adjuvant treatment results in only a modest reduction of the risk of cancer-related death by 25-30%, translating into an absolute 5-year survival benefit of only 10-15% [8]. This poor outcome has prompted major efforts to explore different effective adjuvant therapies to prolong the survival of patients with gastric cancer.
In recent years, immune therapy has become the fourth important treatment modality for malignant tumors following surgery, radiotherapy and chemotherapy [9-11]. Adoptive immunotherapy, as an adjuvant or alternative treatment, is anticipated to be an innovative approach to treat most malignancies. The general concept of immunotherapy is to stimulate the patient’s immune system ex vivo or in vivo in order to induce an antitumor immune response and to restore the patient’s immune status. Cytokine-induced killer (CIK) cells meet all requirements for application in adoptive immunotherapy [12].
The CIK cells are heterogeneous ex vivo-expanded T lymphocytes, with a mixed T-NK phenotype, able to exert a wide MHC-unrestricted antitumor activity against both solid and hematologic malignancies [13,14]. Among CIK cells it is possible to distinguish two main subsets, positive (CD3+CD56+) and negative (CD3+CD56-) for the membrane expression of CD56. The antitumor activity of CIK cells is mainly due to the CD3+CD56+ fraction, while the CD3+CD56- cells are more similar to conventional T lymphocytes [13,15,16]. The high proliferation of CD3+CD56+ cells and cytotoxic activity of T-cell receptor alpha/beta (TCR-α/β) cells in CIK cell cultures leads to the primary effective and substantial lytic activity of CIK cells toward tumor cells by releasing of cytoplasmic cytotoxic granule contents to the extracellular space on stimulation by susceptible target cells [17,18]. The cytotoxicity of these CD3+CD56+ cells is non-MHC-restricted, perforin-mediated and induced via the natural killer group 2 member D (NKG2D) cell-surface receptors [19,20]. The NKG2D ligands, for example, MHC class I-related chain (MIC) A/B and UL-16 binding protein 1-4, are over-expressed on both solid and hematologic tumor cells, turning these cells into a favored targets for CIK cells [21,22].
Our previous studies showed that the high expression of MHC class I chain-related gene A (MICA) is one of the indicators of a poor prognosis for advanced non-small cell lung cancer patients [23], but there is limited data about the CIK in gastric cancer [24-26]. In the present study, we evaluate the clinical outcome of CIK cell immunotherapy as an adjuvant therapy for postoperative patients with gastric cancer.
Methods
Ethics statement
All procedures were conducted in accordance with the Helsinki Declaration, and with approval from the Ethics Committee of Fujian Provincial Cancer Hospital. Written informed consent was obtained from all participants.
Patients
We retrospectively studied all the patients with stage II-III gastric carcinoma diagnosed after surgery who were admitted into the Department of Medical Oncology of Fujian Provincial Cancer Hospital from January 2009 to December 2011. Two hundred and twenty-six consecutive patients were recruited into the study according to the following criteria: 1) All the patients had R0 gastrectomy with D2 lymphadenectomy, 2) All patients were diagnosed and histologically confirmed with stages IIA, IIB, IIIA, IIIB, or IIIC disease according to the American Joint Committee on Cancer (AJCC) TNM Staging Classification for Carcinoma of the Stomach (7th ed., 2010), 3) Eastern Cooperative Oncology Group performance status between 0 and 2 before adjuvant chemotherapy, 4) Complete medical records were available, 5) All patients had received at least four cycles of adjuvant chemotherapy based on 5-fluorouracil (5-FU) or capecitabine doublet regimens, 6) Patients non-randomly received adjuvant immmunotherapy with CIK cells, and patients who received CIK cell treatment were given at least two cycles, 7) Adequate bone marrow function with leukocyte counts 3,000-12,000/mm3, hemoglobin ≥ 8.0 g/dl, and platelet counts ≥ 100,000/mm3, 8) Adequate liver function with total serum bilirubin ≤ 2.0 mg/dl and serum transaminases ≤ 100/UI, 9) Adequate renal function with serum creatinine within the upper limit of normal, 10) An expected survival period of > 3 months.
CIK cells preparation
The CIK cells were isolated and cultured according to a standard protocol as described in our previous studies [23]. Briefly, peripheral blood (50 ml) was drawn from patients using heparin as an anticoagulant. Mononuclear cells were isolated by Ficoll-Conray density gradient centrifugation and their viability assessed by trypan blue exclusion. About 2.0 × 106/ml mononuclear cells were plated onto six-well dishes and cultured with Medium I containing RPMI 1640 plus 1.0 × 106 U/L human interferon gamma (IFN-γ), 5.0 × 105 U/L recombinant human interleukin-2 (IL-2), 10% heat inactivated human serum, 25 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. The cells were incubated in a humidified atmosphere with 5% CO2 at 37°C. After 24 h, 100 µg/L of monoclonal antibody (MAb) against CD3 and 1.0 × 105 U/L IL-1α were added. After another 48 h, the supernatant was aspirated and the cells were cultured in Medium II (Medium I in the absence of INF-γ). The medium was changed every three days. Cell viability was determined using trypan blue staining. The CIK cells were transfused back into the donors following eight days of culture. All CIK cell cultures were tested for contamination (bacteria, fungi, and mycoplasma) throughout the study to assure culture quality and transfusion safety.
Adjuvant chemotherapy
All patients had received at least four cycles of adjuvant chemotherapy based on 5-fluorouracil (5-FU) or capecitabine doublet regimens, including Xelox (Capecitabine, 1,000 mg/m² twice daily on days 1 to 14 of each cycle. Oxaliplatin, 130 mg/m² on day 1 of each cycle), Folfox4 (Oxaliplatin, 85 mg/m² on day 1, 5-florouracil, 2,400 mg/m2 46-hour infusion Leucovorin (400 mg/m2 on day 1 of each cycle), PF (Paclitaxel, 135 mg/m² on day 1, 5-florouracil, 2,400 mg/m2 46-hour infusion, and Leucovorin, 400 mg/m2 on day 1 of each cycle).
CIK cells treatment
As shown in Table 1, 137 patients (control group) received adjuvant chemotherapy alone, and 89 patients (CIK group) received adjuvant chemotherapy combined with autologous CIK cell therapy. The patients in the CIK group received at least three cycles of CIK cell therapy, the first one of which was given within two weeks after surgery, and the others were given once per month starting within six weeks after adjuvant chemotherapy. For each cycle, patients were given an infusion of at least 1.0 × 1010 CIK cells. The patients were eligible for CIK cells therapy until they no longer agreed to continue treatment or until disease recurrence.
Table 1.
Patient characteristics
| Characteristics | Total 226 | Control group 137 | CIK group 89 | P-value | |
|---|---|---|---|---|---|
| Sex | Male | 178 | 108 | 70 | 0.974 |
| Female | 48 | 29 | 19 | ||
| Age | < 65 | 155 | 92 | 63 | 0.565 |
| ≥ 65 | 71 | 45 | 26 | ||
| Histological grade | G1-G2 | 105 | 57 | 48 | 0.069 |
| G3-G4 | 121 | 80 | 41 | ||
| Stage | II | 72 | 38 | 34 | 0.099 |
| III | 154 | 99 | 55 | ||
| Adjuvant Chemotherapy | Xelox Folfox4 | 142 | 89 | 53 | 0.168 |
| PF | 84 | 48 | 36 | ||
With the eventual failure of this adjuvant therapy, the first-line, second-line chemotherapy according to 2010 NCCN Clinical Practice Guidelines in Oncology were recommended to all patients if their performance status was preserved.
Evaluation of toxicity and efficacy
All patients were followed up at the outpatient clinic from the date of surgery to December 31, 2014, or to the time of death. During treatment, patients were evaluated with abdominal computed tomography (CT) scans every 1-2 months according to the Response Evaluation Criteria In Solid Tumors (RECIST) criteria [27]. A complete blood cell count and measurements of liver and renal function were assessed at least once a week during the treatment. Non-hematological toxicities were also verified at least once a week by patient interview and physical examination. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0 [28]. Disease-Free-Survival (DFS) was measured from the day of surgery to the first evidence of recurrence or death. Overall Survival (OS) was defined from the date of surgery to death from any cause.
Statistical methods
Statistical analysis was performed with the SPSS software (Version 18.0, SPSS). For all analyses, the significance level was specified as P< 0.05. Comparisons between the immunotherapy and control groups were analyzed using the χ2 test and the Fisher exact probability test. The OS and DFS variables were estimated by the Kaplan-Meier method and survival curves were plotted. Two-sided log-rank tests were used to compare survival rates between groups. Multivariate analyses using the Cox proportional hazards regression model were performed to assess the impact of the variables on DFS and OS.
Results
From January 2009 to December 2011, 226 patients with stage II-III gastric carcinoma were enrolled in this study and were fully evaluated for DFS and OS. One hundred and thirty-seven patients received adjuvant chemotherapy alone, and 89 patients received adjuvant chemotherapy combining with autologous CIK cell therapy. Patient characteristics are listed in Table 1, showing that there were no statistically significant differences in patient characteristics between the two groups.
Phenotypic analysis of CIK cells
The phenotype of the cultured cells was determined by flow cytometry (BD FACSCalibur). The cells were labeled with monoclonal antibodies (mAbs) that recognize human CD3, CD4, CD8, CD56, CD19, CD25, and CD127. Phenotypic analysis of cells in the 89 patients after 14 days of culture demonstrated that the percentages of CD8+, CD4+, CD3+, CD3+CD56+, CD19+, and CD4+CD25+CD127- cells were 72.8 ± 4.5%, 8.3 ± 3.2%, 88.4 ± 7.5%, 23.2 ± 6.5%, 0.4 ± 0.2%, and 0.3 ± 0.2% respectively.
DFS and OS
For the 226 patients, the median follow-up period was 44.1 months, 95% CI = 41.9-46.3 months. By the end of follow-up 93 patients (41.2%) had died and 96 patients (42.5%) had been diagnosed with recurrence. The median DFS was 39.0 months, 95% CI = 35.7-42.3 months, and median OS was 45.0 months, 95% CI = 40.2-49.8 months, and the 3-year DFS rate and 3-year OS rate were 59.3% and 69.9%, respectively (Figure 1A).
Figure 1.
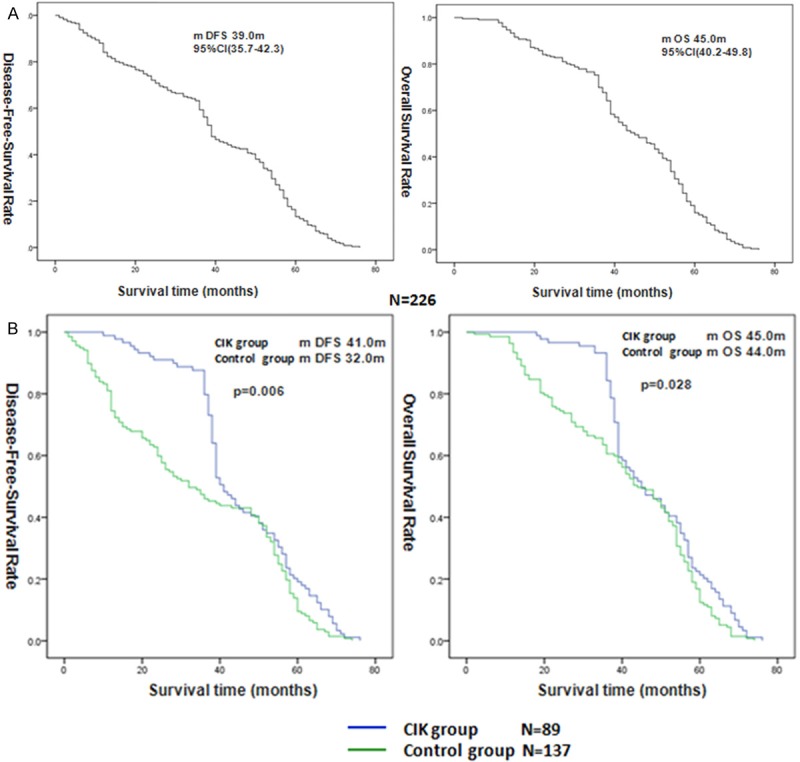
Kaplan-Meier curves for Disease-Free-Survival (DFS) and Overall Survival (OS) in 226 patients (A). DFS and OS in the patients received adjuvant chemotherapy combining with or not CIK therapy (B).
Univariate survival analysis
In the univariate analysis, stage, and CIK therapy were associated with DFS and OS, and histological grade was associated with DFS but not with OS. While the sex, age, and the regimens of adjuvant chemotherapy were not correlated with DFS or OS (Table 2). The median DFS and OS of patients in the CIK group were significantly longer than for those in the control group, DFS 41.0 months vs. 32.0 months, OS 45.0 months vs. 44.0 months, by log-rank test P = 0.006 and P = 0.028, respectively (Figure 1B).
Table 2.
Univariate analysis of factors associated with survival of 226 patients
| Variable | n | m DFS | P-value | m OS | P-value | |
|---|---|---|---|---|---|---|
| Sex | Male | 178 | 39 | 0.794 | 45 | 0.942 |
| Female | 48 | 39 | 45 | |||
| Age | < 65 | 155 | 39 | 0.427 | 46 | 0.611 |
| ≥ 65 | 71 | 38 | 43 | |||
| Histological grade | G1-G2 | 105 | 44 | 0.048 | 49 | 0.090 |
| G3-G4 | 121 | 37 | 42 | |||
| Stage | II | 72 | 48 | 0.007 | 50 | 0.013 |
| III | 154 | 36 | 42 | |||
| CIK therapy Yes | 89 | 41 | 0.006 | 45 | 0.028 | |
| No | 137 | 32 | 44 | |||
| Adjuvant chemotherapy | Xelox Folfox4 | 142 | 39 | 0.883 | 46 | 0.721 |
| PF | 84 | 39 | 43 | |||
In the subgroup analysis, it was found that the median DFS and OS of patients with stage III disease in the CIK group were both longer than the median DFS and OS for patients in the control group, P = 0.031 and P = 0.038, respectively (Figure 2B). For the patients with stage II disease, the median DFS and OS were not significantly different between the CIK group and control group, P = 0.799 and P = 0.727, respectively (Figure 2A).
Figure 2.
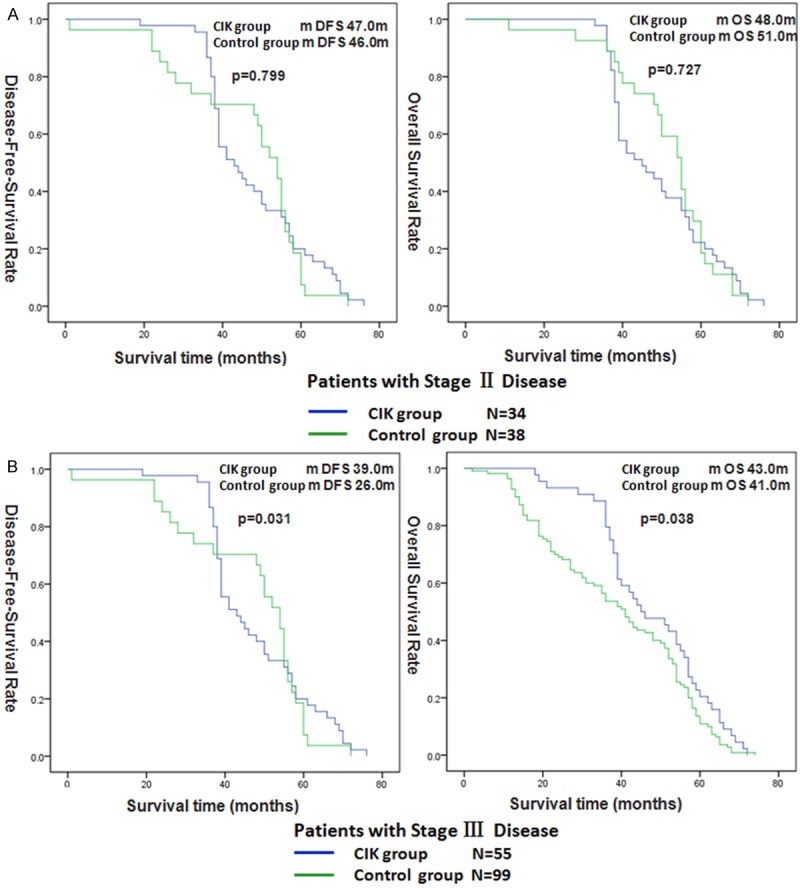
Disease-Free-Survival (DFS) and Overall Survival (OS) in the patients with stage II by CIK therapy (A). DFS and OS in the patients with stage III by CIK therapy (B).
Multivariate survival analysis
In the multivariate analysis, variables that included sex, age, histological grade, stage, CIK therapy, adjuvant chemotherapy, and the interaction of stage and CIK therapy, were tested to determine their independent effect on DFS and OS. The CIK therapy was an independent prognostic factor for DFS (Table 3). The stage and the interaction of stage and CIK therapy were independent prognostic factors for DFS and OS (Table 3). This shows that the CIK therapy has a good effect in patients with stage III disease, which was consistent with the results of the univariate subgroup analysis.
Table 3.
Multivariate analysis (Cox model) of factors associated with disease-free-survival (DFS) and overall survival (OS) of 226 patients
| Variable | DFS | OS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| P value | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | |
| Stage | 0.047 | 1.347 | 1.004-1.894 | 0.018 | 1.408 | 1.061-1.869 |
| CIK therapy | 0.043 | 1.336 | 1.009-1.769 | - | - | - |
| Stage\CIK Interaction | 0.001 | 1.146 | 1.058-1.241 | 0.003 | 1.129 | 1.041-1.224 |
Safety of CIK cell treatment
In the CIK group, autologous CIK cell treatment was generally well tolerated. CIK-treatment-related adverse events (Grade 1/2) were observed in 23.6% of patients, with the most common events being flu-like symptoms such as fever and fatigue (13 patients, 14.6%), rash (five patients, 5.6%), and diarrhea (three patients, 3.4%). No patient experienced adverse events consistent with grade 3/4 toxicity or an autoimmune reaction.
Discussion
In China, gastric cancers are usually diagnosed at a relatively advanced stage. Surgery is the main treatment for operable gastric cancer, and adjuvant chemotherapy is a standard component of resectable gastric cancer therapy and improves patient outcomes, however, recurrence rates are as high as 40-80% in advanced cases [5,7,29,30]. CIK cells, or NK T-lymphocytes, hold great promise in the quest for new therapeutic approaches in the setting with solid tumors refractory to standard treatments. In the last 10 years efforts have been made to evaluate the clinical value of CIK cell therapy and to improve cellular effectivity. CIK cell therapy has been combined with different therapies, resulting in promising clinical responses [12,31].
In this study, we evaluated the potential effects of adjuvant chemotherapy combined with CIK cells in patients with locally advanced gastric carcinoma after gastrectomy. To our knowledge, the present report is the largest prognostic study in gastric cancer treated with adjuvant CIK cells immunotherapy.
Liu et al. combined CIK cell therapy with Folfox4 chemotherapy for the postoperative treatment of 98 patients with gastric carcinoma [32]. Compared with the control group, the 1-, 2- and 3-year recurrence rates were significantly (P < 0.05) reduced in the immunotherapy group while the survival rates were significantly (P < 0.05) improved. In a different study, 151 postoperative gastric carcinoma patients who had received six cycles of adjuvant chemotherapy based on 5-FU were divided into a control group receiving no additional treatment and an immunotherapy group treated with adjuvant CIK cell therapy [33]. Immunotherapy significantly (P = 0.044) prolonged 5-year DFS (28.3% vs. 10.4%) but not 5-year OS (32.4% in the immunotherapy group vs. 23.4% in the control group, P = 0.071).
In order to balance the baseline of our patients, we had enrolled the patients with stage II and III gastric carcinoma who had accepted a D2 radical operation and at least four cycles of postoperative chemotherapy. Our result shows that adjuvant chemotherapy combined with CIK cells could prolong the survival time of patients with stage II-III gastric carcinoma after D2 gastrectomy. However, the single-factor subgroup analysis revealed that the population of beneficiaries was the patients with gastric carcinoma in stage III, which has been substantiated in multi-factor analysis. In the multivariate analysis, the stage and the interaction of stage and CIK therapy were independent prognostic factors for DFS and OS. This means that CIK therapy has a better effect in patients with stage III disease, which is consistent with the results of the univariate subgroup analysis.
We considered that this could be explained by two possibilities. First, the 5-year survival rate of the patients with gastric carcinoma in phase II exceeds 60%. There were almost half of the patients who were not expected to relapse in this time frame among the group under investigation from 2009 to 2011, and the patients in stage II account for a large proportion of the total patient population. So within the period of follow-up visits, it remains impossible to observe a statistically significant effect; perhaps a positive statistical result can be observed over a longer period of follow-up visits. Second, according to other findings, the later the state of malignant cancer, typically the more significantly the patients’ immunologic function is inhibited, and relapse tends to occur within three years in patients with stage III gastric carcinoma. CIK can restore the immune function in patients after an operation for a solid tumor. Thus the survival benefit CIK brings to patients with stage III disease is particularly apparent in the survival curve because of restored immune function. But our findings need a larger sample and longer follow-up visit periods in future studies in order to verify this.
We found patients have good tolerance of CIK therapy with only slight untoward side-effects. This result is consistent with other centers’ research. With measurable benefits and limited side-effects patients are advised to accept the CIK therapy after the postoperative chemotherapy for gastric carcinoma provided that their condition permits.
Our results are limited by the heterogeneity in the study design and the retrospective nature of the analysis. Therefore, a treatment model for systemic chemotherapy combined with CIK therapy needs further investigation with an expanded sample size in further prospective clinical studies.
In conclusion, these results indicate that CIK cell therapy combined with adjuvant chemotherapy is associated with an improved prognosis for gastric carcinoma patients after D2 gastrectomy, especially for the patients with stage III disease. The side effects of this treatment are mild. A prospective randomized trial in our hospital is being carried out to further validate these findings.
Acknowledgements
We thank everyone at our institution who helped with this study. The project was supported by the Foundation of the Key Science and Technology Projects in Fujian Social Development Program (Grant No. 2013YZ0002) and the Foundation of the Natural Science Foundation of Fujian Province (Grant No. 2015J01435).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237–3242. doi: 10.3748/wjg.v12.i20.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzen F, Noh SH. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 6.Park JM, Kim YH. Current approaches to gastric cancer in Korea. Gastrointest Cancer Res. 2008;2:137–144. [PMC free article] [PubMed] [Google Scholar]
- 7.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO) Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584–91. doi: 10.1016/j.ejso.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Ilson DH. Adjuvant treatment for gastric cancer: too much is not enough. Lancet Oncol. 2014;15:788–789. doi: 10.1016/S1470-2045(14)70293-1. [DOI] [PubMed] [Google Scholar]
- 9.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137:305–310. doi: 10.1007/s00432-010-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 12.Jakel CE, Schmidt-Wolf IG. An update on new adoptive immunotherapy strategies for solid tumors with cytokine-induced killer cells. Expert Opin Biol Ther. 2014;14:905–916. doi: 10.1517/14712598.2014.900537. [DOI] [PubMed] [Google Scholar]
- 13.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 14.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, Introna M. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol. 2009;37:616–628. doi: 10.1016/j.exphem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Sangiolo D, Martinuzzi E, Todorovic M, Vitaggio K, Vallario A, Jordaney N, Carnevale-Schianca F, Capaldi A, Geuna M, Casorzo L, Nash RA, Aglietta M, Cignetti A. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. Int Immunol. 2008;20:841–848. doi: 10.1093/intimm/dxn042. [DOI] [PubMed] [Google Scholar]
- 17.Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood. 1995;86:3493–3499. [PubMed] [Google Scholar]
- 18.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–1679. [PubMed] [Google Scholar]
- 19.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301–3310. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 20.Verneris MR, Ito M, Baker J, Arshi A, Negrin RS, Shizuru JA. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol Blood Marrow Transplant. 2001;7:532–542. doi: 10.1016/s1083-8791(01)70014-6. [DOI] [PubMed] [Google Scholar]
- 21.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 22.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Lin G, Guo ZQ, Zhou ZF, He ZY, Ye YB. Effects of MICA expression on the prognosis of advanced non-small cell lung cancer and the efficacy of CIK therapy. PLoS One. 2013;8:e69044. doi: 10.1371/journal.pone.0069044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Fan Y, Li H, Yu J, Liu L, Cao S, Ren B, Yan F, Ren X. Immunotherapy with Cytokine-Induced Killer Cells as an Adjuvant Treatment for Advanced Gastric Carcinoma: A Retrospective Study of 165 Patients. Cancer Biother Radiopharm. 2013;28:303–309. doi: 10.1089/cbr.2012.1306. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61:2251–2259. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang JT, Shen YP, Wu CP, Zhu YB, Wei WX, Chen LJ, Zheng X, Sun J, Lu BF, Zhang XG. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16:6155–6162. doi: 10.3748/wjg.v16.i48.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 29.Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237–3242. doi: 10.3748/wjg.v12.i20.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Semin Radiat Oncol. 2002;12:150–161. doi: 10.1053/srao.2002.30817. [DOI] [PubMed] [Google Scholar]
- 31.Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo DL, Carnevale-Schianca F, Fagioli F, Piacibello W, Aglietta M, Sangiolo D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–684. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Song J, Yang Z, Zhang X. Effects of cytokine-induced killer cell treatment combined with FOLFOX4 on the recurrence and survival rates for gastric cancer following surgery. Exp Ther Med. 2013;6:953–956. doi: 10.3892/etm.2013.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61:2251–2259. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


