Abstract
The present study was to investigate the effects of transcutaneous electrical acupoint stimulation (TEAS) in alleviating the hyperandrogenism of polycystic ovarian syndrome (PCOS) model rats induced by testosterone propionate and the possible underlying mechanism. Thirty-six female Sprague-Dawley rats were randomly divided into normal control, PCOS model and TEAS groups with twelve rats in each group. The PCOS model rats were established by single injection of testosterone propionate at 9th day after birth, and the status of estrous cyclicity for each rat was observed. When the 8-week TEAS treatment completed, the weight of body, uterus and ovaries of the rats were respectively measured. The serum levels of total testosterone (TT), sex hormone binding globulin (SHBG), androstenedione, luteinizing hormone (LH), follicle stimulating hormone (FSH) and estradiol (E2) were detected. The mRNA and protein expression levels of aromatase cytochrome P450 (P450arom) and connective tissue growth factor (CTGF) in the ovaries of the rats were respectively measured with real-time quantitative PCR and immunohistochemistry. The TEAS treatment significantly improved the estrous cycles of the PCOS rats and the TEAS group displayed significantly lower average body and ovaries weights than the PCOS model group (P < 0.05). TEAS significantly decreased the serum TT, free androgen index (FAI), androstenedione and LH/FSH levels, and increased the serum FSH levels of the PCOS rats (P < 0.05). The TEAS treatment significantly increased the P450arom mRNA as well as protein expression levels and significantly decreased the CTGF mRNA as well as protein expression levels in the ovaries of the PCOS rats (P < 0.05). We concluded that it is through regulating the P450arom and CTGF expression levels in the ovaries that TEAS significantly alleviates the hyperandrogenism of PCOS rats induced by testosterone propionate.
Keywords: Transcutaneous electrical acupoint stimulation, polycystic ovarian syndrome, hyperandrogenism, aromatase cytochrome P450, connective tissue growth factor
Introduction
As one of the heterogeneous endocrine disorders, polycystic ovary syndrome (PCOS) affects about one in 15 women worldwide and the excessive androgen secretion or activity is the major endocrine disruption [1]. The adrenal androgen excess or the abnormal paracrine/endocrine control of androgen synthesis may lead to the hyperandrogenism of PCOS [2-4]. The ovarian hyperandrogenism have been found to induce the dysfunctions of granulosa cells (GCs) and to hamper the follicular development in PCOS women and the PCOS model rats [5,6]. As we know, the oral contraceptives are the most common therapy for the PCOS patients, which are effective in alleviating hirsutism and acne, however, they have exhibited negative effects on the glucose tolerance, coagulability and fertility [7].
As an important non-pharmaceutical treatment, acupuncture is often used by PCOS patients as an alternative therapy in clinical practices, and some PCOS women chosen acupuncture as an adjunct while undergoing infertility treatment [8]. More than 50 studies have been conducted to explore the effects of acupuncture on PCOS, however, few of them has explored the effects of acupuncture in alleviating the hyperandrogenism of PCOS [8,9]. Transcutaneous electrical acupoint stimulation (TEAS) is a non-invasive acupuncture, which is a new development of traditional Chinese acupuncture under the advancement of modern science [10,11]. For the PCOS patients who are unwilling to receive the traditional invasive or painful acupuncture treatment, TEAS is a good choice [10,11]. The present study was designed to investigate the effects of TEAS in alleviating the hyperandrogenism of PCOS based on the PCOS model rats induced by testosterone propionate, and to explore the possible underlying mechanism.
Materials and methods
Animals, groups and administration
Thirty-six neonatal female Sprague-Dawley rats were provided by Experimental Animal Centre, School of Medicine, Zhejiang University (Hangzhou, China). They were housed in a temperature-controlled room with a 12-hour light/dark cycle and maintained at a constant temperature of 25°C and humidity of 55% and fed with standard pelleted food and plain tap water ad libitum. The study was conducted based on the National Research Council Guide for the care and use of laboratory animals, and was approved by the Ethics Committee.
In the study, a randomization chart constructed in Microsoft Excel was used to divide the rats into normal control, PCOS model and TEAS groups (n = 12 in each group). The model rats with PCOS were established with the subcutaneous injection of testosterone propionate (0.1 mg in 0.004 ml olive oil/weight (g)) at the 9th day after birth [6,12]. The testosterone propionate was provided by Jinyao Amino Acid Ltd. (Tianjin, China). The rats in the control group only received olive oil. All pups were weaned from their mothers at the age of 21 days. From the 22nd day, the rats in TEAS group were acupunctured at the following acupoints: Jizhong (Du6), Dazhui (Du14), Zhongwan (Ren12) and Guanyuan (Ren 4). The rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) to minimize any restraint stress before TEAS treatment. TEAS was applied to rats through self-adhesive surface electrodes using a TEAS device (HANS-200A, Nanjing Jisheng Medical Technology Co., Ltd, Nanjing, China). The electrodes were cut into 5-mm squares and applied to the denuded skin. The TEAS treatment lasted for 30 min. The stimulation parameters were set as a frequency of 2/100 Hz and an intensity of 5-25 mA, strong enough to elicit visible muscle contraction. The parameters of TEAS treatment regarding selection of the acupoints, frequency, pulse duration, application time and intensity were in accordance to those used in the clinical practices. The TEAS treatment was taken once every day for consecutive 8 weeks.
Collection and measurement of the samples
During the study period, one rat in the PCOS model group passed away. From the age of 70 days, the vaginal smears were examined daily for 10 consecutive days. On the day following the end of the 8week treatment, after fasting for 12 h, the body weight of each rat was measured. The rats were then intraperitoneally anesthetized with urethane (1.2 g/kg). The blood samples were drawn from the abdominal aorta into the heparinized injectors, and then centrifuged at 3,000 rpm at 4°C for 10 min. The supernatant serum was then transferred to clean Eppendorf tubes and stored at -80°C. The rats were sacrificed following collection of the samples. The uterus and ovaries were immediately removed, washed with physiological saline and then weighed. The left ovary was sliced and the tissue slices were fixed in 10% neutralbuffered formalin for 24 h. The right ovary was frozen and stored at -80°C. Laser capture micro-dissection system (ASLMD, Leica, Germany) was used for ovary dissection. The serum levels of total testosterone (TT), sex hormone binding globulin (SHBG), androstenedione, luteinizing hormone (LH), follicle stimulating hormone (FSH) and E2 (estradiol) were measured with commercial enzyme-linked immunosorbent assay kits (R&D Systems, MN, USA). Free androgen index (FAI) was calculated as TT (nmol/l) divided by SHBG (nmol/l) × 100 [13]. All measurements were performed in duplicate and were conducted according to the manufacturer’s instructions. Intra and interassay coefficients of variation were < 10%. The mRNA and protein expression levels of aromatase cytochrome P450 (P450arom) and connective tissue growth factor (CTGF) in the ovaries of the rats were receptively measured with real-time quantitative PCR and immunohistochemistry.
Detection of P450arom and CTGF mRNA expression in the ovary tissues with quantitative polymerase chain reaction (qPCR)
Total RNA was isolated with RNAiso™ reagent (Takara Biotechnology, Dalian, China) according to the instructions of the manufacturer. The purity and concentration of the RNAs were detected with a NanoDrop® ND100 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The cDNA was prepared from 500 ng total RNA by reverse transcription (RT) with the PrimeScript™ RT Reagent kit (Perfect Real Time; Takara Biotechnology). The cDNA samples were then diluted in DNase and RNasefree water at a proportion of 1:3 prior to further analysis. PCR was performed using the iCycler iQ RealTime PCR Detection system (BioRad, Hercules, CA, USA). The rat P450arom and CTGF genespecific primers were provided by Sangon Biological Engineering Technology (Shanghai, China). The sequences of the primers were as follows: P450arom forward, 5’-CTGGCTACTGTCTGGGAATC-3’ and reverse, 5’-TTGCTGCCGAATCTGGAG3’; CTGF forward, 5’-CAAGCTGCCCGGGAAAT-3’ and reverse, 5’-CGGTCCTTGGGCTCATCA-3’; GAPDH forward, 5’-GCAAGTTCAACGGCACAG-3’ and reverse, 5’-CGCCAGTAGACTCCACGAC-3’. PCR reactions were performed using 2 μl cDNA, 10 μM each primer, and 2X SYBR® Premix Ex Taq™ (Takara Biotechnology) in 20-μl reactions. Thermal cycling conditions were as follows: 95°C for 10 sec, followed by 30 cycles of 95°C for 10 sec and 60°C for 40 sec. A final melting curve was used to verify singleproduct formation. Gene starting quantity was based on the cycle threshold (Ct) method. Each value was normalized to GAPDH to control the amount of input cDNA. The Ct value for GAPDH mRNA was subtracted from that of the target gene, and the mRNA levels of P450arom and CTGF were expressed as 2-ΔCt.
Detection of P450arom and CTGF protein expression in the ovary tissues with immunohistochemistry
Once the fixed ovary tissue slices were embedded in paraffin, sectioned, deparaffinized and rehydrated, they were cut into sections and mounted on slides. For immunohistochemical staining of P450arom and CTGF, the sections were incubated with monoclonal P450arom antibody (1:100 dilution; Bioss Inc., Massachusetts, U.S.A), and monoclonal CTGF antibody (1:500 dilution; Abcam, Cambridge, UK) at 4°C overnight. The biotinylated secondary antibody and the horseradish peroxidaseconjugated streptavidin were applied to the ovary sections after washing the slides with Trisbuffered saline (TBS) twice. The expression was visualized by adding 3,3’diaminobenzidine substrate and the positive staining was colored in deep brown. The stained sections were evaluated using an Olympus BX50 optical microscope equipped with an Image-Pro Plus software (version 6.0, Media Cybernetics, Inc, MD, U. S. A.) by a pathologist who was blinded to the identity of the groups. The integrated optical density (IOD) was calculated by measuring 10 consecutive visual fields for each sample using a 200 × objective.
Statistical analysis
All data were analyzed with Statistical Package for Social Sciences (SPSS, version 15.0 for Windows; SPSS, Inc., Chicago, IL, U. S. A.). Analysis of variance was employed to analyze all the data. A 5% significance level (P < 0.05) and two-tailed tests were applied for all hypothesis tests.
Results
The estrous cycles
As shown in Table 1, PCOS model rats displayed persistently keratinized vaginal cells, based on vaginal smears. The TEAS treatment was found to significantly improve the estrous cycles of the PCOS rats (P < 0.05).
Table 1.
The estrous cycles and the weights of body, uterus and ovaries
| Items | Normal control group (n = 12) | PCOS model group (n = 11) | TEAS group (n = 12) |
|---|---|---|---|
| Estrous cycles (n) | 1.83 ± 0.39 | 0.00 ± 0.00* | 1.33 ± 0.65*,# |
| Body weight (g) | 189.26 ± 17.24 | 260.36 ± 33.45* | 219.22 ± 25.07*,# |
| Uterus weight (mg) | 298.62 ± 26.90 | 292.78 ± 51.61 | 332.60 ± 45.03* |
| Ovaries weight (mg) | 91.15 ± 13.79 | 170.83 ± 34.34* | 130.26 ± 39.82*,# |
Note: Data are presented as the mean ± standard deviation. P < 0.05 was considered to indicate a statistically significant difference.
P < 0.05, compared with the normal control group (analysis of variance);
P < 0.05, compared with the PCOS model group (analysis of variance).
PCOS, polycystic ovarian syndrome; TEAS, transcutaneous electrical acupoint stimulation.
Weights of body, uterus and ovaries
As shown in Table 1, following the 8week treatment, the average body and ovaries weights of the normal control rats were significantly lower than the other two groups (P < 0.05). Compared with the model group, the TEAS group displayed significantly lower average body and ovaries weights (P < 0.05). No significant differences existed between normal control and PCOS model groups on the uterus weight (P > 0.05), however, the TEAS group exhibited a significantly higher uterus weight than the other two groups (P < 0.05).
Serum levels of hormones
As shown in Table 2, the serum levels of TT, FAI and androstenedione in the normal control group were significantly lower than those of the other groups (P < 0.05). The TEAS treatment significantly decreased the serum TT, FAI and androstenedione levels of the PCOS rats (P < 0.05). TEAS group exhibited a significantly lower serum SHBG level than the other two groups (P < 0.05) and no differences were noted between the normal control and PCOS model groups (P > 0.05). In the normal control group, the serum levels of LH and LH/FSH were significantly lower, and the serum FSH and E2 levels were significantly higher than the other two groups (P < 0.05) (Table 2). The TEAS treatment was found to significantly increase the serum FSH levels and to significantly decrease the LH/FSH levels of the PCOS rats (P < 0.05), however, there were no significant differences between the PCOS model and TEAS groups on the serum LH and E2 levels (P > 0.05).
Table 2.
The serum levels of hormones
| Items | Normal control group (n = 12) | PCOS model group (n = 11) | TEAS group (n = 12) |
|---|---|---|---|
| TT (nmol/l) | 7.66 ± 1.08 | 26.33 ± 4.36* | 13.93 ± 2.59*,# |
| SHBG (nmol/l) | 22.15 ± 3.39 | 20.45 ± 3.20 | 15.89 ± 2.71*,# |
| FAI | 35.08 ± 5.93 | 132.07 ± 32.72* | 89.25 ± 18.34*,# |
| Androstenedione (ng/mL) | 0.67 ± 0.15 | 1.70 ± 0.31* | 0.79 ± 0.09*,# |
| LH (IU/L) | 4.28 ± 0.38 | 6.13 ± 1.73* | 6.38 ± 1.09* |
| FSH (IU/L) | 7.46 ± 1.76 | 3.91 ± 0.91* | 6.22 ± 0.80*,# |
| LH/FSH | 0.60 ± 0.14 | 1.67 ± 0.68* | 1.03 ± 0.18*,# |
| E2 (pmol/L) | 124.47 ± 8.13 | 107.34 ± 9.53* | 112.35 ± 9.01* |
Note: Data are presented as the mean ± standard deviation. P < 0.05 was considered to indicate a statistically significant difference.
P < 0.05, compared with the normal control group (analysis of variance);
P < 0.05, compared with the PCOS model group (analysis of variance).
PCOS, polycystic ovarian syndrome; TEAS, transcutaneous electrical acupoint stimulation; TT, total testosterone; SHBG, sex hormone binding globulin; FAI, free androgen index; LH, luteinizing hormone; FSH, follicle stimulating hormone; E2: estradiol.
P450arom and CTGF mRNA expression levels in the ovaries of rats
In the normal control group, the P450arom mRNA expression levels of the ovaries were significantly higher, and the CTGF mRNA expression levels of the ovaries were significantly lower than the other two groups (P < 0.05) (Figure 1). The PCOS model group displayed the lowest P450arom mRNA expression levels and the highest CTGF mRNA expression levels in the ovaries among all the groups (P < 0.05). The TEAS treatment significantly increased the P450arom mRNA expression levels and significantly decreased the CTGF mRNA expression levels in the ovaries of the PCOS rats (P < 0.05).
Figure 1.
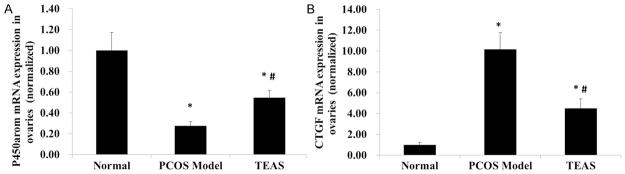
(A) Aromatase cytochrome P450 (P450arom) and (B) connective tissue growth factor (CTGF) mRNA expression levels in the rat ovaries. Normal, normal control group; PCOS Model, polycystic ovarian syndrome (PCOS) model group; TEAS, transcutaneous electrical acupoint stimulation (TEAS) group. Data are presented as the mean ± standard deviation (n = 12, 11 and 12 in Normal, PCOS Model and TEAS groups respectively). P < 0.05 was considered to indicate a statistically significant difference. *P < 0.05, compared with the normal control group (analysis of variance); #P < 0.05, compared with the PCOS model group (analysis of variance).
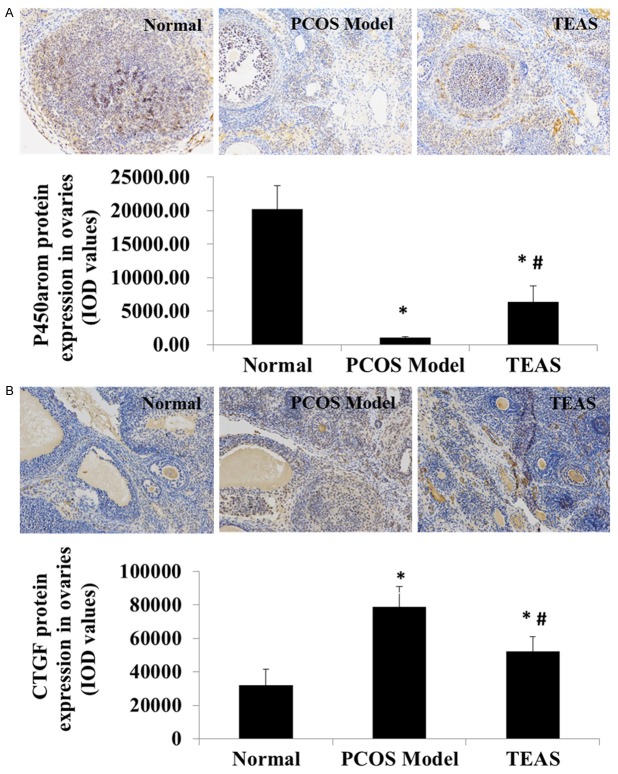
P450arom and CTGF protein expression in the ovaries of rats
As shown in Figure 2, the normal control rats exhibited the highest P450arom protein expression levels and the lowest CTGF protein expression levels in the ovaries among all the groups (P < 0.05). In the PCOS model group, the P450arom protein expression levels in the ovaries of the rats were significantly lower and the CTGF protein expression levels were significantly higher than the other two groups (P < 0.05) (Figure 2). The TEAS treatment significantly increased the protein expression of P450arom and significantly decreased the protein expression levels of CTGF in the ovary tissues of the PCOS rats (P < 0.05).
Figure 2.
(A) Aromatase cytochrome P450 (P450arom) and (B) connective tissue growth factor (CTGF) proteinexpression levels in the rat ovaries. Normal, normal control group; PCOS Model, polycystic ovarian syndrome (PCOS) model group; TEAS, transcutaneous electrical acupoint stimulation (TEAS) group. The integrated optical density (IOD) was calculated by measuring 10 consecutive visual fields for each sample using a 200× objective. Data are presented as the mean ± standard deviation (n = 12, 11 and 12 in Normal, PCOS Model and TEAS groups respectively). P < 0.05 was considered to indicate a statistically significant difference. *P < 0.05, compared with the normal control group (analysis of variance); #P < 0.05, compared with the PCOS model group (analysis of variance).
Discussion
We found in the present study that the TEAS treatment significantly alleviated the hyperandrogenism of PCOS rats induced by testosterone propionate through regulating the P450arom and CTGF expression levels in the ovaries. In the study, FAI and androstendione were selected to represent the hyperandrogenism of the rats, as they have the highest clinical utility to evaluate the PCOS-associated hyperandrogenism [14]. We found that the serum levels of TT, FAI and androstenedione in the normal control group were significantly lower than those of the other groups, and the TEAS treatment significantly decreased the serum TT, FAI and androstenedione levels of the PCOS rats. An “androgen-sensitive period” existed in the first ten days of life in the rats and mice, especially on the 9th day following birth [12,15]. In the present study, the PCOS model rats were successfully established by a single subcutaneous injection of testosterone propionate on the 9th day after birth, which was characterized by polycystic ovary histology, hyperandrogenemia and anovulation.
To explore the possible mechanism underlying TEAS alleviating the hyperandrogenism of PCOS rats, we detected the mRNA and protein expression levels of P450arom and CTGF in the ovaries of the rats. P450arom, encoding by CYP19 gene, is a rate-limiting enzyme in the final step of the estrogen biosynthesis, which was expressed both in the follicles before ovulation and in the ovarian luteal cells of the reproductive-aged women [16,17]. The increased P450arom mRNA expression was found to be consistent with the development of associated follicles in the immature ovaries [18]. CTGF plays a key role not only during the early stages of follicular development but also in the formation of the corpus luteum after ovulation [19]. CTGF mRNA was found to be abundantly expressed in the GCs of the preantral and early antral follicles [19] and the CTGF expression levels increased in the GCs of the porcine ovaries during early antral follicle development [20]. We found that the TEAS treatment significantly increased the P450arom mRNA as well as protein expression levels and significantly decreased the CTGF mRNA as well as protein expression levels in the ovaries of the PCOS rats.
It has been demonstrated that acupuncture possesses effective curative effects in treating PCOS through increasing the blood flow to the ovaries, reducing the ovarian volume as well as the number of ovarian cysts, controlling the hyperglycaemia, decreasing the cortisol levels and improving the weight loss and anorexia [21,22]. The low frequency electro-acupuncture displayed positive effects in modulating the ovarian blood flow and improving the functions of the hypothalamus-pituitary-ovary system [23-26]. We found that TEAS significantly alleviated the hyperandrogenism of PCOS rats by regulating the P450arom and CTGF expression levels in the ovaries. The present study demonstrated that TEAS has promises in alleviating the hyperandrogenism of PCOS, however, more studies are expected to be conducted on the epigenetic and transcriptional regulation mechanism underlying TEAS alleviating the hyperandrogenism of PCOS in the near future.
Acknowledgements
This work was supported by Zhejiang Province Top Key Discipline of Chinese Medicine-Acupuncture & Tuina (No. ZTK2014A02), Zhejiang Traditional Chinese Medicine Foundation (No. 2008YB010) and China Scholarship Council (No. 201308330139).
Disclosure of conflict of interest
None.
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Loughlin T, Cunningham S, Moore A, Culliton M, Smyth PP, McKenna TJ. Adrenal abnormalities in polycystic ovary syndrome. J Clin Endocrinol Metab. 1986;62:142–147. doi: 10.1210/jcem-62-1-142. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Woods KS, Bartolucci AA, Azziz R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2005;62:644–649. doi: 10.1111/j.1365-2265.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- 4.Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF 3rd, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 5.Qu F, Wang FF, Lu XE, Dong MY, Sheng JZ, Lv PP, Ding GL, Shi BW, Zhang D, Huang HF. Altered aquaporin expression in women with polycystic ovary syndrome: hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphatidylinositol 3-kinase pathway. Hum Reprod. 2010;25:1441–1450. doi: 10.1093/humrep/deq078. [DOI] [PubMed] [Google Scholar]
- 6.Qu F, Wang FF, Yin R, Ding GL, El-Prince M, Gao Q, Shi BW, Pan HH, Huang YT, Jin M, Leung PC, Sheng JZ, Huang HF. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J Mol Med (Berl) 2012;90:911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- 7.Lanham MS, Lebovic DI, Domino SE. Contemporary medical therapy for polycystic ovary syndrome. Int J Gynaecol Obstet. 2006;95:236–241. doi: 10.1016/j.ijgo.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Stener-Victorin E, Jedel E, Manneras L. Acupuncture in polycystic ovary syndrome: current experimental and clinical evidence. J Neuroendocrinol. 2008;20:290–298. doi: 10.1111/j.1365-2826.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- 9.Zheng YH, Su NJ, Ding T. [Treatment of PCOS by acupuncture: present experimental and clinical reports] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1436–1439. [PubMed] [Google Scholar]
- 10.Zhang Q, Gao Z, Wang H, Ma L, Guo F, Zhong H, Xiong L, Wang Q. The effect of pre-treatment with transcutaneous electrical acupoint stimulation on the quality of recovery after ambulatory breast surgery: a prospective, randomised controlled trial. Anaesthesia. 2014;69:832–839. doi: 10.1111/anae.12639. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Feng XJ, Guan Q, Cui W, Zheng Y, Sun W, Han JS. Increase of success rate for women undergoing embryo transfer by transcutaneous electrical acupoint stimulation: a prospective randomized placebo-controlled study. Fertil Steril. 2011;96:912–916. doi: 10.1016/j.fertnstert.2011.07.1093. [DOI] [PubMed] [Google Scholar]
- 12.Tamura N, Kurabayashi T, Nagata H, Matsushita H, Yahata T, Tanaka K. Effects of testosterone on cancellous bone, marrow adipocytes, and ovarian phenotype in a young female rat model of polycystic ovary syndrome. Fertil Steril. 2005;84(Suppl 2):1277–1284. doi: 10.1016/j.fertnstert.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Doi SA, Al-Zaid M, Towers PA, Scott CJ, Al-Shoumer KA. Steroidogenic alterations and adrenal androgen excess in PCOS. Steroids. 2006;71:751–759. doi: 10.1016/j.steroids.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Morreale HF, Asuncion M, Calvo RM, Sancho J, San Millan JL. Receiver operating characteristic analysis of the performance of basal serum hormone profiles for the diagnosis of polycystic ovary syndrome in epidemiological studies. Eur J Endocrinol. 2001;145:619–624. doi: 10.1530/eje.0.1450619. [DOI] [PubMed] [Google Scholar]
- 15.Sun F, Yu J. The effect of a special herbal tea on obesity and anovulation in androgen-sterilized rats. Proc Soc Exp Biol Med. 2000;223:295–301. doi: 10.1177/153537020022300312. [DOI] [PubMed] [Google Scholar]
- 16.Sen K, Hackett JC. Coupled electron transfer and proton hopping in the final step of CYP19-catalyzed androgen aromatization. Biochemistry. 2012;51:3039–3049. doi: 10.1021/bi300017p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okubo T, Mok SC, Chen S. Regulation of aromatase expression in human ovarian surface epithelial cells. J Clin Endocrinol Metab. 2000;85:4889–4899. doi: 10.1210/jcem.85.12.7067. [DOI] [PubMed] [Google Scholar]
- 18.Sakurada Y, Shirota M, Inoue K, Uchida N, Shirota K. New approach to in situ quantification of ovarian gene expression in rat using a laser microdissection technique: relationship between follicle types and regulation of inhibin-alpha and cytochrome P450aromatase genes in the rat ovary. Histochem Cell Biol. 2006;126:735–741. doi: 10.1007/s00418-006-0205-2. [DOI] [PubMed] [Google Scholar]
- 19.Harlow CR, Hillier SG. Connective tissue growth factor in the ovarian paracrine system. Mol Cell Endocrinol. 2002;187:23–27. doi: 10.1016/s0303-7207(01)00702-x. [DOI] [PubMed] [Google Scholar]
- 20.Wandji SA, Gadsby JE, Barber JA, Hammond JM. Messenger ribonucleic acids for MAC25 and connective tissue growth factor (CTGF) are inversely regulated during folliculogenesis and early luteogenesis. Endocrinology. 2000;141:2648–2657. doi: 10.1210/endo.141.7.7576. [DOI] [PubMed] [Google Scholar]
- 21.Lim CE, Wong WS. Current evidence of acupuncture on polycystic ovarian syndrome. Gynecol Endocrinol. 2010;26:473–478. doi: 10.3109/09513591003686304. [DOI] [PubMed] [Google Scholar]
- 22.Lim DC, Chen W, Cheng LN, Xue CC, Wong FW, O’Sullivan AJ, Liu JP. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev. 2011:CD007689. doi: 10.1002/14651858.CD007689.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Stener-Victorin E, Kobayashi R, Kurosawa M. Ovarian blood flow responses to electro-acupuncture stimulation at different frequencies and intensities in anaesthetized rats. Auton Neurosci. 2003;108:50–56. doi: 10.1016/j.autneu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Stener-Victorin E, Kobayashi R, Watanabe O, Lundeberg T, Kurosawa M. Effect of electro-acupuncture stimulation of different frequencies and intensities on ovarian blood flow in anaesthetized rats with steroid-induced polycystic ovaries. Reprod Biol Endocrinol. 2004;2:16. doi: 10.1186/1477-7827-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stener-Victorin E, Waldenstrom U, Tagnfors U, Lundeberg T, Lindstedt G, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2000;79:180–188. [PubMed] [Google Scholar]
- 26.Sun J, Jin C, Wu H, Zhao J, Cui Y, Liu H, Wu L, Shi Y, Zhu B. Effects of electro-acupuncture on ovarian P450arom, P450c17alpha and mRNA expression induced by letrozole in PCOS rats. PLoS One. 2013;8:e79382. doi: 10.1371/journal.pone.0079382. [DOI] [PMC free article] [PubMed] [Google Scholar]