Abstract
The alcohol dehydrogenase 2 (ADH2) gene has been implicated in the development of alcoholic liver cirrhosis (ALC). However, the results are inconsistent. In this study, a meta-analysis was performed to assess the associations between the ADH2 polymorphism and the risk of ALC. Relevant studies were retrieved by searching PubMed, Web of Science, CNKI, Wanfang and VIP databases up to January 10, 2015. The pooled odds ratio (OR) with a 95% confidence interval (CI) was calculated using the fixed- or random effects model. A total of 21 case-control studies included 1812 cases and 3468 controls were included. Overall, the ADH2 polymorphism was associated with a decreased risk of ALC in all four genetic models (dominant model: OR=0.56, 95% CI: 0.38-0.83; recessive model: OR=0.59, 95% CI: 0.39-0.91; *1/*2 vs. *1/*1: OR=0.58, 95% CI: 0.40-0.85; *2/*2 vs *1/*1: OR=0.35, 95% CI: 0.16-0.75). Besides, in stratification analysis by ethnicity, similar results were observed in Asian populations, however, we detected no association in Caucasian populations under recessive and homozygote comparison model. The pooled evidence suggests that ADH2 polymorphism may be an important protective factor for alcoholic liver cirrhosis, especially for Asians.
Keywords: ADH2, polymorphism, alcoholic liver cirrhosis, meta-analysis
Introduction
Alcoholic liver disease (ALD) refers to a wide spectrum of liver abnormalities, ranging from fatty liver to acute alcoholic hepatitis, and alcoholic liver cirrhosis (ALC). The most severe of these, ALC, causes an estimated 373, 000 deaths per year [1]. The burden of ALD is highest in the developed world, where it may account for as much as 9.2% of all disability-adjusted life years [2]. It has been demonstrated that a clear correlation exists between cumulative alcohol intake and ALD; however, only a small portion of the alcohol abusers develop signs of liver disease, which suggests some of the genetic variations are involved in the etiology of ALD [3,4].
The ADH2 (also named ADH1B) gene is located on chromosome 4q21-q23. There are several polymorphism sites in the ADH2 gene, and the Arg47His polymorphism (rs1229984, with the Arg corresponding to *1 allele, and His corresponding to *2 allele) has been the most frequently studied. The β2β2 enzyme encoded by ADH2 2*2 is approximately 20-fold more active in ethanol oxidation than the β1β1 enzyme [5]. Individuals who inherit the ADH2*2 allele have homodimeric and heterodimeric β2-containing isozymes and could be expected to have faster rates of alcohol metabolism and possibly higher concentrations of acetaldehyde production after alcohol consumption [6]. Furthermore, the variant ADH2*2 allele is prevalent in East Asian individuals, but is rare in non-Asians [7]. To date, many studies have investigated the association between the ADH2 polymorphism and the risk of alcoholic liver cirrhosis [8-30]. However, the results remain controversial. In this study, we conduct a meta-analysis to evaluate the association between the polymorphism and alcoholic liver cirrhosis risk.
Materials and methods
Search strategy
Relevant articles published before January 10, 2015 were identified through a search of PubMed, Web of Science, CNKI, Wanfang and VIP databases using the following terms: “alcohol dehydrogenase 2 or ADH2 or alcohol dehydrogenase 1B or ADH1B” and “genetic polymorphism or polymorphisms or variant” and “alcoholic liver disease or ALD or alcoholic liver cirrhosis or ALC or cirrhosis”. The search was restricted to humans without language restrictions. Additional studies were identified by a hand search of references of original or review articles on this topic.
Inclusion criteria and exclusion criteria
Studies included in this meta-analysis have to meet the following criteria: (1) studies that evaluated the association between the ADH2 polymorphism and alcoholic liver cirrhosis, (2) in a case-control study design, (3) had detailed genotype frequency of cases and controls or could be calculated from the article text. Studies were excluded when they were: (1) case-only study, case reports, and review articles, (2) based on incomplete data, (3) duplicate of previous publication.
Data extraction
For each study, the following data were extracted independently by two investigators: the first author’s name, year of publication, country of origin, ethnicity, genotyping methods, number of cases and controls, and Hardy-Weinberg equilibrium (HWE) in controls (P value). The results were compared, and disagreements were discussed among all authors and resolved with consensus.
Statistical analysis
HWE was evaluated for each study using an internet-based HWE calculator (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). The risk of alcoholic liver cirrhosis associated with the ADH2 polymorphism was estimated for each study by odds ratio (OR) and 95% confidence interval (95%CI). Four different ORs were calculated: the dominant model (*1/*2+*2/*2 vs. *1/*1), the recessive model (*2/*2 vs. *1/*2+*1/*1), heterozygote comparison (*1/*2 vs. *1/*1), and homozygote comparison (*2/*2 vs. *1/*1). A χ 2-test-based Q statistic test was performed to assess the between-study heterogeneity [31]. We also quantified the effect of heterogeneity by I 2 test. When a significant Q test (P>0.1) or I 2<50% indicated homogeneity across studies, the fixed effects model was used [32], or else the random effects model was used [33]. Then, we performed stratification analyses on ethnicity. Analysis of sensitivity was performed to evaluate the stability of the results. Finally, potential publication bias was investigated using Begg’s funnel plot and Egger’s regression test [34,35]. P<0.05 was considered statistically significant.
All analyses were performed using the Cochrane Collaboration RevMan 5.2 and STATA package version 12.0 (Stata Corporation, College Station, Texas).
Results
Study characteristics
The search strategy retrieved 73 potentially relevant studies. According to the inclusion criteria, 23 studies [8-30] with full-text were included in this meta-analysis and 50 studies were excluded. Because two studies [29,30] did not present detailed genotyping information, we excluded them. Therefore, as shown in Table 1, there were 21 case-control studies with 1812 cases and 3468 controls concerning ADH2 polymorphism. Of the 21 eligible studies, two ethnicities were addressed: eleven studies [9-12,18-21,26-28] were conducted on Asian populations and ten studies [8,13-17,22-25] on Caucasian populations. The distribution of genotypes in the controls was consistent with the HWE for all selected studies, except for two studies [20,28].
Table 1.
Characteristics of studies included in the meta-analysis
| Author | Year | Country | Ethnicity | Genotyping methods | Genotype (case/control) | HWE | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total | 1/1 | 1/2 | 2/2 | ||||||
| Borras | 2000 | Mixed | Caucasian | PCR-RFLP | 180/224 | 175/214 | 5/10 | 0/0 | 0.733 |
| Chao | 1994 | China | Asian | PCR-RFLP | 27/47 | 4/3 | 15/19 | 8/25 | 0.808 |
| Chao | 1997 | China | Asian | PCR-RFLP | 75/100 | 17/6 | 39/41 | 19/53 | 0.600 |
| Chao | 2000 | China | Asian | PCR-RFLP | 116/105 | 20/7 | 62/43 | 34/55 | 0.717 |
| Chao | 2003 | China | Asian | PCR-RFLP | 159/100 | 33/7 | 74/38 | 52/55 | 0.901 |
| Cichoz-Lach | 2007 | Poland | Caucasian | PCR-RFLP | 57/54 | 56/48 | 1/6 | 0/0 | 0.666 |
| Couzigou | 1990 | France | Caucasian | PCR | 46/39 | 44/38 | 2/1 | 0/0 | 0.935 |
| Day | 1991 | England | Caucasian | PCR | 59/79 | 59/78 | 0/1 | 0/0 | 0.955 |
| Frenzer | 2002 | Australia | Caucasian | PCR-RFLP | 57/200 | 57/184 | 0/16 | 0/0 | 0.556 |
| Garcia-Banuelos | 2012 | Mexico | Caucasian | PCR-RFLP | 32/66 | 31/58 | 1/8 | 0/0 | 0.600 |
| Kee | 2003 | Korea | Asian | PCR-RFLP | 27/39 | 11/18 | 14/18 | 2/3 | 0.603 |
| Khan | 2010 | India | Asian | PCR-RFLP | 175/255 | 141/222 | 34/33& | NA | |
| Kim | 2004 | Korea | Asian | PCR-RFLP | 20/77 | 13/36 | 5/23 | 2/18 | 0.001 |
| Lee | 2001 | Korea | Asian | PCR-RFLP | 56/64 | 7/6 | 11/18 | 38/40 | 0.084 |
| Ogurtsov | 2001 | Russia | Caucasian | PCR | 37/50 | 13/15 | 23/29 | 1/6 | 0.160 |
| Poupon | 1992 | France | Caucasian | Starch-Gel Electrophoresis | 23/42 | 23/41 | 0/1 | 0/0 | 0.938 |
| Rodrigo | 1999 | Spain | Caucasian | PCR-RFLP | 120/200 | 108/173 | 12/27 | 0/0 | 0.306 |
| Vidal | 2004 | Spain | Caucasian | PCR-RFLP | 99/64 | 85/54 | 13/10 | 1/0 | 0.498 |
| Yamauchi | 1995a | Japan | Asian | PCR-RFLP | 46/60 | 8/2 | 12/22 | 26/36 | 0.534 |
| Yamauchi | 1995b | Japan | Asian | PCR-RFLP | 42/60 | 8/2 | 12/22 | 22/36 | 0.534 |
| Yokoyama | 2013 | Japan | Asian | PCR-RFLP | 359/1543 | 75/441 | 128/497 | 156/605 | 0.000 |
HWE: Hardy-Weinberg equilibrium; &Numbers of *1/*2+*2/*2; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; NA: not available.
Quantitative data synthesis
Overall, the ADH2 polymorphism was associated with a decreased risk of ALC in all four genetic models (dominant model: OR=0.56, 95% CI: 0.38-0.83; recessive model: OR=0.59, 95% CI: 0.39-0.91; *1/*2 vs. *1/*1: OR=0.58, 95% CI: 0.40-0.85; *2/*2 vs *1/*1: OR=0.35, 95% CI: 0.16-0.75) (Table 2; Figure 1).
Table 2.
Summary of OR of the ADH2 polymorphism and alcoholic cirrhosis risk
| Variables | Na | dominant model | recessive model | *1/*2 vs. *1/*1 | *2/*2 vs. *1/*1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| OR (95% CI) | Pb | I2 | OR (95% CI) | Pb | I2 | OR (95% CI) | Pb | I2 | OR (95% CI) | Pb | I2 | ||
| Total | 21 | 0.56 (0.38, 0.83) | <0.0001 | 66 | 0.59 (0.39, 0.91) | <0.0001 | 73 | 0.58 (0.40, 0.85) | 0.006 | 50 | 0.35 (0.16, 0.75) | <0.00001 | 81 |
| Ethnicity | |||||||||||||
| Asian | 11 | 0.53 (0.30, 0.93) | <0.00001 | 80 | 0.60 (0.39, 0.93) | <0.00001 | 77 | 0.54 (0.30, 0.95) | 0.0005 | 70 | 0.34 (0.15, 0.77) | <0.00001 | 84 |
| Caucasian | 10 | 0.60 (0.41, 0.88) | 0.72 | 0 | 0.39 (0.08, 1.85) | 0.25 | 24 | 0.60 (0.41, 0.89) | 0.71 | 0 | 0.40 (0.08, 1.94) | 0.25 | 24 |
Number of comparisons.
Test for heterogeneity.
Figure 1.
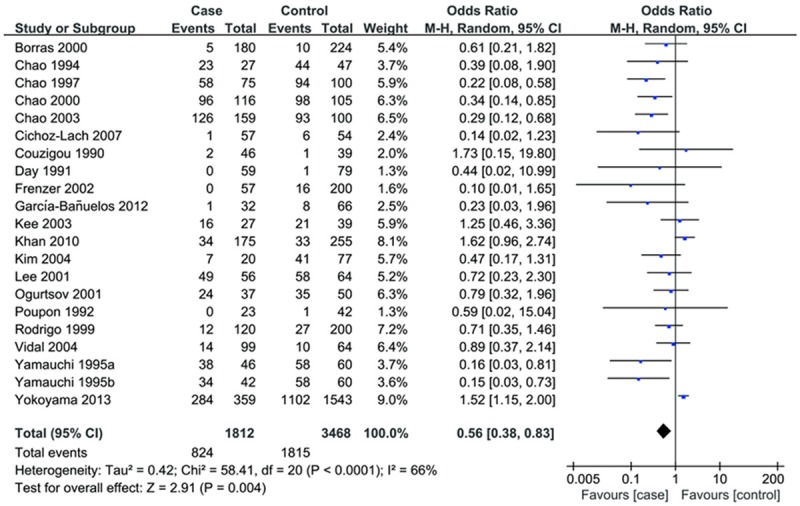
Forest plots for the association of ADH2 polymorphism and ALC risk. (dominant model).
In stratification analysis by ethnicity, similar results were observed in Asian population, however, we detected no association in Caucasian populations under recessive and homozygote comparison model (Table 2; Figure 2).
Figure 2.
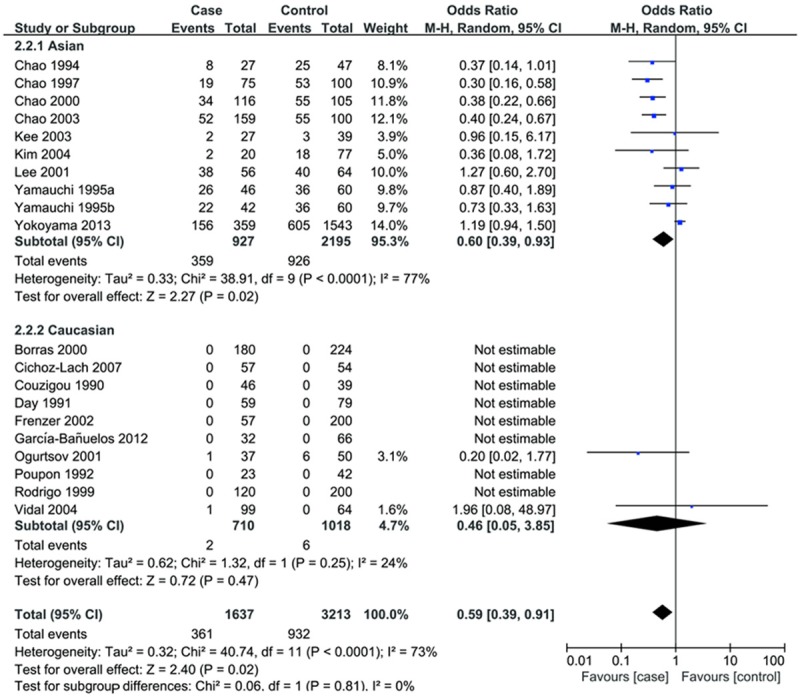
Forest plots for subgroup analysis by ethnicity for the association of ADH2 polymorphism and ALC risk. (recessive model).
Sensitivity analyses were conducted to determine whether modification of the inclusion criteria of the meta-analysis affected the final results. We examined the influence of these studies on the pooled OR by repeating the meta-analysis while excluding one study at a time. The estimated pooled ORs change quite little, indicating that our results were statistically robust.
Test of heterogeneity
There was significant heterogeneity for overall comparisons (dominant model: P<0.0001, I2=66%; recessive model: P<0.0001, I2=73%; *1/*2 vs. *1/*1: P=0.006, I2=50%; *2/*2 vs. *1/*1: P<0.00001, I2=81%). In the subgroup analysis by ethnicity, results were similar in the Asian population. However, there was no significant heterogeneity in the Caucasian population.
Publication bias
The Begg’s funnel plot and Egger’s test was used to address potential publication bias in the available literature. The shape of funnel plots did not reveal any evidence of funnel plot asymmetry (Figure not shown). Egger’s test also showed that there was no statistical significance for the evaluation of publication bias (dominant model: P=0.432; recessive model: P=0.117; *1/*2 vs. *1/*1: P=0.381; *2/*2 vs. *1/*1: P=0.217).
Discussion
Alcohol dependence (AD), which is multifactorial and chronic relapsing disorders, is a major public health problem. Among the patients with AD, ALC occurs in around 10% [36]. The enzyme encoded by ADH2 is a member of the alcohol dehydrogenase family, which metabolizes alcohol into acetaldehyde. ADH2 was hypothesized to be an important ethanol oxidizing enzyme that may alter genetic susceptibility to ALD [37].
Recently, a previous meta-analysis conducted by Li et al [38] has evaluated the association between ADH2 polymorphism and the risk of alcohol dependence and alcohol-induced medical diseases and the results of that provide confirmation of the involvement of the human ADH2 gene in the pathogenesis of alcohol dependence and abuse as well as alcohol-induced medical illnesses in the multiple ethnic populations-in particular, certain Asian populations. However, only 12 studies focusing on ALC were included in the above meta-analysis, due to the limited studies, further analyses was not conducted. Compared with it, we conducted a comprehensive literature search in different databases (i.e. Web of science, CNKI, Wanfang and VIP) and added 9 studies, which allowed for a larger number of subjects and more precise risk estimation. In this study, 21 case-control studies included 1812 cases and 3468 controls were included. We found that the ADH2*2 allele is associated with ALC. Individuals with the ADH2*2/*2 and/or *1/*2 genotype had a lower risk of developing ALC when compared to individuals with the ADH2*/1*1 genotype. They might be explained that ALDH2*2 allele encodes a superactive allozyme, which oxidize ethanol into acetaldehyde fast. The accumulation of acetaldehyde could develop intense facial flushing responses with nausea, headache, drowsiness and other unpleasant symptoms resulting from high blood acetaldehyde levels after alcohol consumption [39]. This unpleasant discomfort may prevent people from consuming alcohol and may keep them from developing alcoholism thus they have much lower chance to expose to the acetaldehyde [40], which may decrease the risk of developing ALC. In addition, in stratification analysis by ethnicity, significant associations were observed in Asian populations. However, we detected no association in Caucasian populations under recessive and homozygote comparison models. There are some possible explanations for the discrepant results. Due to Linkage disequilibrium (LD), the ADH2*2 allele could be coinherited with other ADH (such ADH3) variants that might affect the risk of alcoholism and that could differ between Caucasians and Asians [41]. Another reason may be found in the population genetics of alcohol metabolizing enzyme variants.
Two significant issues should be addressed in this study, that is, heterogeneity and publication bias, which may influence the results of meta-analysis. We don’t detect a significant publication bias in this meta-analysis, suggesting the reliability of our results. With regard to heterogeneity, in this meta-analysis, heterogeneity was found in overall comparison under all four genetic models, when stratified by ethnicity, the heterogeneity was partly decreased or removed in Caucasian populations. However, heterogeneity still existed in Asian population. The results above suggest that the ethnic background might be the source of heterogeneity. Then sensitivity analyses were conducted by successively excluding one study, the estimated pooled odd ratio changed quite little, strengthening the results from this metaanalysis.
This meta-analysis has limitations that must be acknowledged. First, because of incomplete raw data or publication limitations, some relevant studies could not be included in our analysis. Second, moderate to higher heterogeneity existed for the analyses especially for the subgroup of Asian. Third, our results were based on unadjusted estimates, which may cause serious confounding bias. In addition, all of the studies were conducted in Asian and Caucasians, which may generate selective bias. More studies focused on Africans are needed.
In summary, this meta-analysis suggests that ADH2 polymorphism may be an important protective factor for alcoholic liver cirrhosis, especially for Asians. Since potential confounders could not be ruled out completely, further studies are needed to confirm these results.
Disclosure of conflict of interest
None.
References
- 1.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 3.Monzoni A, Masutti F, Saccoccio G, Bellentani S, Tiribelli C, Giacca M. Genetic determinants of ethanol-induced liver damage. Mol Med. 2001;7:255–262. [PMC free article] [PubMed] [Google Scholar]
- 4.Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012;61:150–159. doi: 10.1136/gutjnl-2011-301239. [DOI] [PubMed] [Google Scholar]
- 5.Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, Beckmann G, Bhatia K, Chen LZ, Fang B, Lisker R, Paik YK, Rothhammer F, Saha N, Segal B, Srivastava LM, Czeizel A. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. 1992;88:344–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- 6.Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T, Harada S. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol Alcohol. 1994;29:707–710. [PubMed] [Google Scholar]
- 7.Osier M, Pakstis AJ, Kidd JR, Lee JF, Yin SJ, Ko HC, Edenberg HJ, Lu RB, Kidd KK. Linkage disequilibrium at the ADH2 and ADH3 loci and risk of alcoholism. Am J Hum Genet. 1999;64:1147–1157. doi: 10.1086/302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borràs E, Coutelle C, Rosell A, Fernández-Muixi F, Broch M, Crosas B, Hjelmqvist L, Lorenzo A, Gutiérrez C, Santos M, Szczepanek M, Heilig M, Quattrocchi P, Farrés J, Vidal F, Richart C, Mach T, Bogdal J, Jörnvall H, Seitz HK, Couzigou P, Parés X. Genetic polymorphism of alcohol dehydrogenase in europeans: the ADH2*2 allele decreases the risk for alcoholism and is associated with ADH3*1. Hepatology. 2000;31:984–989. doi: 10.1053/he.2000.5978. [DOI] [PubMed] [Google Scholar]
- 9.Chao YC, Liou SR, Chung YY, Tang HS, Hsu CT, Li TK, Yin SJ. Polymorphism of alcohol and aldehyde dehydrogenase genes and alcoholic cirrhosis in Chinese patients. Hepatology. 1994;19:360–366. [PubMed] [Google Scholar]
- 10.Chao YC, Young TH, Tang HS, Hsu CT. Alcoholism and alcoholic organ damage and genetic polymorphisms of alcohol metabolizing enzymes in Chinese patients. Hepatology. 1997;25:112–117. doi: 10.1002/hep.510250121. [DOI] [PubMed] [Google Scholar]
- 11.Chao YC, Wang LS, Hsieh TY, Chu CW, Chang FY, Chu HC. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol. 2000;95:2958–2964. doi: 10.1111/j.1572-0241.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 12.Chao YC, Wang SJ, Chu HC, Chang WK, Hsieh TY. Investigation of alcohol metabolizing enzyme genes in Chinese alcoholics with avascular necrosis of hip joint, pancreatitis and cirrhosis of the liver. Alcohol Alcohol. 2003;38:431–436. doi: 10.1093/alcalc/agg106. [DOI] [PubMed] [Google Scholar]
- 13.Cichoz-Lach H, Partycka J, Nesina I, Celinski K, Slomka M, Wojcierowski J. Alcohol dehydrogenase and aldehyde dehydrogenase gene polymorphism in alcohol liver cirrhosis and alcohol chronic pancreatitis among Polish individuals. Scand J Gastroenterol. 2007;42:493–498. doi: 10.1080/00365520600965723. [DOI] [PubMed] [Google Scholar]
- 14.Couzigou P, Fleury B, Groppi A, Cassaigne A, Begueret J, Iron A. Genotyping study of alcohol dehydrogenase class I polymorphism in French patients with alcoholic cirrhosis. The French Group for Research on Alcohol and Liver. Alcohol Alcohol. 1990;25:623–626. doi: 10.1093/oxfordjournals.alcalc.a045058. [DOI] [PubMed] [Google Scholar]
- 15.Day CP, Bashir R, James OF, Bassendine MF, Crabb DW, Thomasson HR, Li TK, Edenberg HJ. Investigation of the role of polymorphisms at the alcohol and aldehyde dehydrogenase loci in genetic predisposition to alcohol-related end-organ damage. Hepatology. 1991;14:798–801. doi: 10.1002/hep.1840140509. [DOI] [PubMed] [Google Scholar]
- 16.Frenzer A, Butler WJ, Norton ID, Wilson JS, Apte MV, Pirola RC, Ryan P, Roberts-Thomson IC. Polymorphism in alcohol-metabolizing enzymes, glutathione S-transferases and apolipoprotein E and susceptibility to alcohol-induced cirrhosis and chronic pancreatitis. J Gastroenterol Hepatol. 2002;17:177–182. doi: 10.1046/j.1440-1746.2002.02670.x. [DOI] [PubMed] [Google Scholar]
- 17.García-Bañuelos J, Panduro A, Gordillo-Bastidas D, Gordillo-Bastidas E, Muñoz-Valle JF, Gurrola-Díaz CM, Sánchez-Enríquez S, Ruiz-Madrigal B, Bastidas-Ramírez BE. Genetic polymorphisms of genes coding to alcohol-metabolizing enzymes in western Mexicans: association of CYP2E1*c2/CYP2E1*5B allele with cirrhosis and liver function. Alcohol Clin Exp Res. 2012;36:425–431. doi: 10.1111/j.1530-0277.2011.01617.x. [DOI] [PubMed] [Google Scholar]
- 18.Kee JY, Kim MO, You IY, Chai JY, Hong ES, An SC, Kim H, Park SM, Youn SJ, Chae HB. Effects of genetic polymorphisms of ethanol-metabolizing enzymes on alcohol drinking behaviors. Taehan Kan Hakhoe Chi. 2003;9:89–97. [PubMed] [Google Scholar]
- 19.Khan AJ, Husain Q, Choudhuri G, Parmar D. Association of polymorphism in alcohol dehydrogenase and interaction with other genetic risk factors with alcoholic liver cirrhosis. Drug Alcohol Depend. 2010;109:190–197. doi: 10.1016/j.drugalcdep.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Kim MS, Lee DH, Kang HS, Park HS, Jung S, Lee JW, Kwon KS, Kim PS, Kim HG, Shin YW, Kim YS, Baek I, Lee MS. Genetic polymorphisms of alcohol-metabolizing enzymes and cytokines in patients with alcohol induced pancreatitis and alcoholic liver cirrhosis. Korean J Gastroenterol. 2004;43:355–363. [PubMed] [Google Scholar]
- 21.Lee HC, Lee HS, Jung SH, Yi SY, Jung HK, Yoon JH, Kim CY. Association between polymorphisms of ethanol-metabolizing enzymes and susceptibility to alcoholic cirrhosis in a Korean male population. J Korean Med Sci. 2001;16:745–750. doi: 10.3346/jkms.2001.16.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogurtsov PP, Garmash IV, Miandina GI, Guschin AE, Itkes AV, Moiseev VS. Alcohol dehydrogenase ADH2-1 and ADH2-2 allelic isoforms in the Russian population correlate with type of alcoholic disease. Addict Biol. 2001;6:377–383. doi: 10.1080/13556210020077109. [DOI] [PubMed] [Google Scholar]
- 23.Poupon RE, Nalpas B, Coutelle C, Fleury B, Couzigou P, Higueret D. Polymorphism of alcohol dehydrogenase, alcohol and aldehyde dehydrogenase activities: implication in alcoholic cirrhosis in white patients. The French Group for Research on Alcohol and Liver. Hepatology. 1992;15:1017–1022. doi: 10.1002/hep.1840150608. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo L, Alvarez V, Rodriguez M, Pérez R, Alvarez R, Coto E. N-acetyltransferase-2, glutathione S-transferase M1, alcohol dehydrogenase, and cytochrome P450IIE1 genotypes in alcoholic liver cirrhosis: a case-control study. Scand J Gastroenterol. 1999;34:303–307. doi: 10.1080/00365529950173735. [DOI] [PubMed] [Google Scholar]
- 25.Vidal F, Lorenzo A, Auguet T, Olona M, Broch M, Gutiérrez C, Aguilar C, Estupiñà P, Santos M, Richart C. Genetic polymorphisms of ADH2, ADH3, CYP4502E1Dra-I and Pst-I, and ALDH2 in Spanish men: lack of association with alcoholism and alcoholic liver disease. J Hepatol. 2004;41:744–750. doi: 10.1016/j.jhep.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi M, Maezawa Y, Mizuhara Y, Ohata M, Hirakawa J, Nakajima H, Toda G. Polymorphisms in alcohol metabolizing enzyme genes and alcoholic cirrhosis in Japanese patients: a multivariate analysis. Hepatology. 1995;22:1136–1142. doi: 10.1016/0270-9139(95)90621-5. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi M, Maezawa Y, Toda G, Suzuki H, Sakurai S. Association of a restriction fragment length polymorphism in the alcohol dehydrogenase 2 gene with Japanese alcoholic liver cirrhosis. J Hepatol. 1995;23:519–523. doi: 10.1016/0168-8278(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama A, Mizukami T, Matsui T, Yokoyama T, Kimura M, Matsushita S, Higuchi S, Maruyama K. Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol Clin Exp Res. 2013;37:1391–1401. doi: 10.1111/acer.12108. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzo A, Auguet T, Vidal F, Broch M, Olona M, Gutiérrez C, López-Dupla M, Sirvent JJ, Quer JC, Santos M, Richart C. Polymorphisms of alcohol-metabolizing enzymes and the risk for alcoholism and alcoholic liver disease in Caucasian Spanish women. Drug Alcohol Depend. 2006;84:195–200. doi: 10.1016/j.drugalcdep.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka F, Shiratori Y, Yokosuka O, Imazeki F, Tsukada Y, Omata M. High incidence of ADH2*1/ALDH2*1 genes among Japanese alcohol dependents and patients with alcoholic liver disease. Hepatology. 1996;23:234–239. doi: 10.1053/jhep.1996.v23.pm0008591846. [DOI] [PubMed] [Google Scholar]
- 31.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis. 1988;8:12–25. doi: 10.1055/s-2008-1040525. [DOI] [PubMed] [Google Scholar]
- 37.Zintzaras E, Stefanidis I, Santos M, Vidal F. Do alcohol-metabolizing enzyme gene polymorphisms increase the risk of alcoholism and alcoholic liver disease? Hepatology. 2006;43:352–361. doi: 10.1002/hep.21023. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical disorders. Biol Psychiatry. 2011;70:504–512. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enomoto N, Takase S, Yasuhara M, Takada A. Acetaldehyde metabolism in different aldehyde dehydrogenase-2 genotypes. Alcohol Clin Exp Res. 1991;15:141–144. doi: 10.1111/j.1530-0277.1991.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 40.Thomasson HR, Edenberg HJ, Crabb DW, Mai XL, Jerome RE, Li TK, Wang SP, Lin YT, Lu RB, Yin SJ. Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet. 1991;48:677–681. [PMC free article] [PubMed] [Google Scholar]
- 41.Edenberg HJ. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]


