Abstract
Objective: To investigate, whether interleukin (IL)-34 can be used as marker for treatment effectiveness in rheumatoid arthritis (RA). Methods: Serum samples were collected from 35 healthy participants and 83 patients with RA before as well as 4 weeks and 12 weeks after treatment initiation with the tumor necrosis factor α (TNF-α) inhibitor Etanercept. Related clinical data and hand radiograms of the patients were evaluated and serum IL-34, IL-6, IL-8, TNF-α, matrix metalloproteinase-3 (MMP-3) in addition to anti-cyclic citrullinated peptide (CCP) antibody concentrations were measured by ELISA. Results: Serum concentrations of IL-34, IL-6, IL-8, TNF-α, MMP-3 and anti-CCP antibodies were markedly elevated in RA patients compared with controls (P<0.001), significantly decreased during treatment and correlated with erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and RA disease activity (P<0.05). IL-34 correlated withIL-6, IL-8, TNF-α, MMP-3 and anti-CCP antibodies in RA patients at baseline (P<0.01) and also with IL-8, MMP-3, IL-6, and DAS28 changes during therapy. Patients in stage III of hand X-ray RA scores had higher IL-34 serum concentrations than in stage II (P<0.05). IL-34 level decreased significantly (P<0.01) starting from 4 weeks after therapy initiation. Conclusions: IL-34 serum concentrations correlated with inflammatory cytokines before and during therapy and were significantly higher in stage III of hand X-ray score patients than in stage II participants. IL-34 might be used both as a biomarker for RA diagnosis and therapy efficiency.
Keywords: IL-34, MMP-3, IL-8, Rheumatoid arthritis, IL-6, IL-8, TNF-α
Introduction
Rheumatoid Arthritis (RA) is a chronic autoimmune disease characterized by persistent synovitis, systemic inflammation, and autoantibodies (rheumatoid factors and citrullinated peptide), resulting in bone erosion, deformity and dysfunctional joints [1]. Interleukin (IL)-34 is a cytokine, which shares the same receptor with the macrophage colony-stimulating factor (M-CSF) [2,3]. Recent studies have shown that IL-34 might be involved in the pathogenesis of RA:1) IL-34 was increased in serum and synovial fluid of RA patients and decreased after treatments [4,5]; 2) Levels of IL-34 in synovial fluid of RA patients were increased by TNF-α stimulated synovial fibroblasts and might be related to the severity of synovitis [6]; 3) IL-34 might be involved in bone destruction in RA [7,8]. Evidence from previous studies has revealed that some cytokines play key roles in the pathogenesis of RA and might also be valuable for evaluating therapeutic efficacies. For instance, TNF-α was shown to serve as a center of the cytokine network in the pathogenesis of RA by activating mononuclear macrophages, T-cells, synovial fibroblasts, chondrocytes and osteoclasts via related signaling pathways (NF-κB/RANKL, PI3K/Akt, JNK) [9-12] and it might also influence the secretion of some pro-inflammatory cytokines [13]. Thus, TNF-α inhibitors have been widely used as disease-modifying anti-rheumatic drugs (DMARDs) for RA treatments. MMP-3, inter leukin-6 (IL-6), and IL-8 are also involved in RA pathogenesis by precipitating bone destruction, joint inflammation, and neutrophil aggravation and have already been reported to be associated with the clinical outcome of RA treatments. [14-18].
However, how IL-34 is related to clinical diagnosis or treatment of RA is not entirely clear. Therefore, the aim of this study was to determine the value of IL-34 for diagnosis and eventually treatment of RA, and in order to clarify whether or not IL-34 could be used as a new biomarker for RA medication efficiency, we investigated the correlations among IL-34 and TNF-α, MMP-3, IL-6, IL-8 as well as clinical and radiographic data at different times before and after administration of the TNF-α inhibitor Etanercept.
Patients and methods
Patients
The study was conducted in accordance with the ethical standards formulated in the Helsinki Declaration, and written informed consent was given by all patients and volunteers. The study was approved by the Ethical Committee of China-Japan Union Hospital of Jilin University. In this retrospective study, a total of 83 patients with established RA, diagnosed between June 2012 and November 2013, according to the 2010 ACR/EULAR criteria, at the Department of Rheumatology and Immunology of China-Japan Union Hospital of Jilin University in China, were investigated and their baseline serum cytokine concentrations compared with 35 healthy volunteers.
The RA group comprised 3 males and 80 females, mean age 49 years (range 20 to 74), with mean disease duration of 51 months (range 1.5 to 420). These patients were given a TNF-α inhibitor (Etanercept) as 25 mg subcutaneous injections twice a week for at least 12 weeks. We evaluated the clinical characteristics and serum of all patients at baseline and during a 12-week follow-up period, 4 weeks, and 12 weeks after treatment. The clinical data included swollen joint count (SJC), tender joint count (TJC), morning stiffness, Disease Activity Score 28 (DAS28) [19], patient’s and physician’s global Visual Analogue Scale (VAS) scores, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), as well as X-ray radiograms of the hands. Serum samples were prepared and stored at -80°C until used. For comparing baseline cytokine serum concentrations, 35 healthy volunteers were recruited, including 1 male and 34 female, mean age 42 years (range 22 to 70).
None of the participants had any co-existing infectious diseases, tuberculosis, tumor, hematologic disease, or any other systemic diseases.
Enzyme-linked immunosorbent assays (ELISA)
The ELISA kit for human serum IL-34, TNF-α, IL-6, IL-8, MMP-3, and anti-CCP antibodies were all purchased from Xinfan Biotechnology Co., Ltd, Shanghai, China. The serum IL-34, TNF-α, IL-6, IL-8, MMP-3, and anti-CCP antibody concentrations were measured by ELISA with the specific double-antibody sandwich method, and all procedures were performed according to the manufacturer’s instructions. The OD value was measured with a microplate reader (RT6000, Rayto, Shenzhen, China) at a wavelength of 450 nm. We then drew the standard curves and calculated the concentrations of the indicated cytokines and anti-CCP antibodies according to the standard curves.
Statistical analyses
Values are given as mean ± SD. The Mann-Whitney test was used for comparison of 2 independent variables, and the Wilcoxon test was used for comparison of 2 dependent variables. The Kruskal-Wallis test was used for comparison of cytokine levels in different subgroups of radiographic progression first, and then the Mann-Whitney test was performed for comparison of each 2 subgroups. The Spearman test was used to analyze the correlation between the variables. Differences were considered significant if P values were <0.05.
Results
Baseline characteristics are shown in Table 1. There were no significant differences in age and gender between the RA and the control group. In the RA group, we subdivided the patients into 5 subgroups based on radiographic staging [20]: Normal: 33 cases; Stage I: 12 cases; Stage II: 21 cases; Stage III: 15 cases; Stage IV: 2 cases.
Table 1.
Baseline clinical characteristics of RA patients (x̅ ± SD)
| Baseline value | |
|---|---|
| Age, year | 48.06 ± 11.98 |
| Gender, female | 80 (96.3%) |
| Disease duration, months | 51.76 ± 66.44 |
| TJC, 28 joints | 8.40 ± 7.61 |
| SJC, 28 joints | 8.73 ± 6.75 |
| Patient global VAS, mm | 63.01 ± 12.76 |
| Physician global VAS, mm | 54.46 ± 12.52 |
| Morning stiffness, minute | 102.20 ± 112.86 |
TJC, tender joint count; SJC, swollen joint count; VAS, visual analogue scale; SDAI, simplified disease activity index; CDAI, clinical disease activity index.
Serum level of IL-34 in RA patients pre- and post-therapy (Table 2; Figures 1, 2)
Table 2.
Cytokine serum levels of in the RA and control groups at baseline
| RA (n = 83) | Control (n = 35) | |
|---|---|---|
| IL-34 (pg/ml) | 382.97 ± 381.12*** | 30.20 ± 15.88 |
| TNF-α (pg/ml) | 73.05 ± 50.90*** | 5.28 ± 4.44 |
| MMP-3 (ng/ml) | 274.44 ± 263.05*** | 33.06 ± 17.17 |
| IL-6 (pg/ml) | 41.38 ± 38.96*** | 9.13 ± 1.77 |
| IL-8 (pg/ml) | 29.08 ± 26.36*** | 8.33 ± 5.74 |
| Anti-CCP antibody (pg/ml) | 510.61 ± 507.32*** | 46.15 ± 11.09 |
RA, rheumatoid arthritis; IL-34, Interleukin-34; TNF-α, Tumor necrosis factor α; MMP-3, Matrix Metalloproteinase-3; IL-6, Interleukin-6; IL-8, Interleukin-8; Anti-CCP antibody, anti-cyclic citrullinated peptide antibody;
P<0.001.
Figure 1.
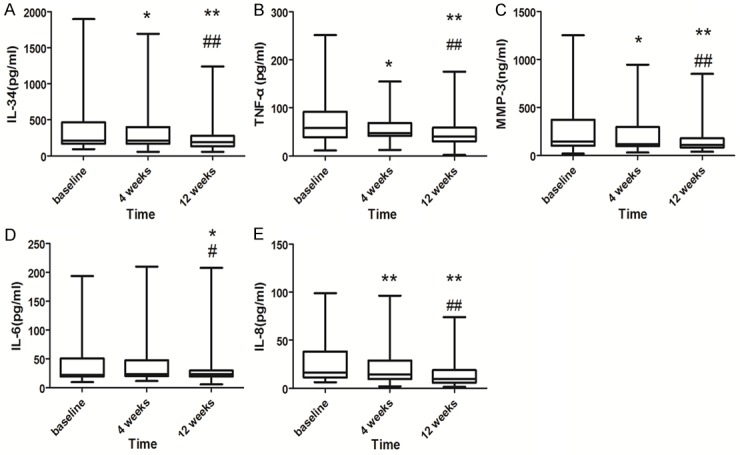
Serum cytokine levels of RA patients before and during 4 and 12 weeks therapy. A. IL-34, B. TNF-α, C. MMP-3, D. IL-6, and E. IL-8. *P<0.05 compared with baseline, **P<0.01 compared with baseline; #P<0.05 compared with 4 weeks; ##P<0.01 compared with 4 weeks. Error bars: Minimum, mean to maximum.
Figure 2.
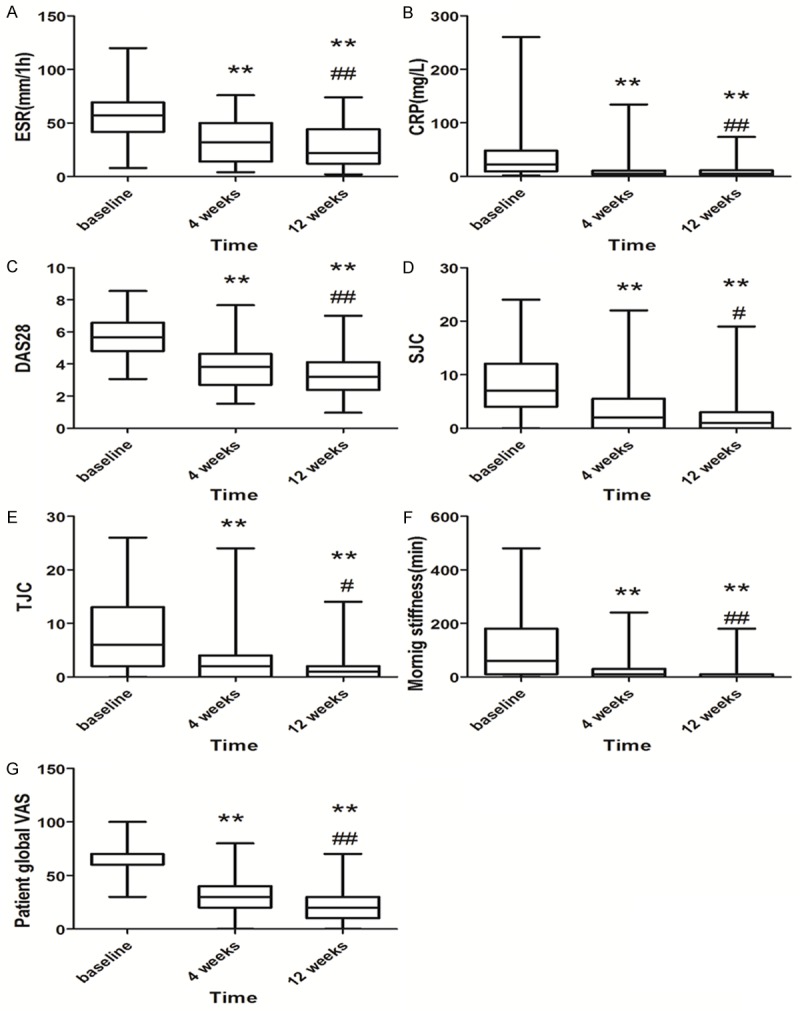
Clinical characteristics of RA patients before and 4, 8 weeks after therapy. A. ESR, B. CRP, C. DAS28, D. SJC, E. TJC, F. morning stiffness and G. patient global VAS. *P<0.05 compared with baseline; **P<0.01 compared with baseline. #P<0.05 compared with 4 weeks; ##P<0.01 compared with 4 weeks. Error bars: Minimum, mean to maximum.
The serum levels of IL-34, TNF-α, MMP-3, IL-6, IL-8, and anti-CCP antibody in the RA group and healthy controls were recorded (Table 2). IL-34, TNF-α, MMP-3, IL-6, and IL-8inthe RA group at baseline were considerably higher than those in healthy controls (P<0.001) when analyzed with the Mann-Whitney test. Meanwhile, the serum anti-CCP antibody concentration of RA patients was also significantly higher than that of the controls (P<0.001).
When analyzed with the Wilcoxon test, serum IL-34 (P<0.05), TNF-α (P<0.05), MMP-3 (P<0.05), and IL-8 (P<0.01) levels were all decreased at 4 weeks compared with baseline, while no significant difference was observed in serum IL-6 level. However, after 12 weeks of treatment, serum IL-34, MMP-3, TNF-α, and IL-8 levels decreased further (P<0.01), and IL-6 began to decrease (P<0.05). When compared with the levels at the 4-week follow-up, levels of IL-34, MMP-3, TNF-α, and IL-8 at 12 weeks were all decreased significantly (P<0.01), while IL-6 was also markedly lower (P<0.05) (Figure 1).
Regarding clinical data and disease activity, ESR, CRP, DAS28, SJC, TJC, morning stiffness and patient global VAS score were decreased within 4 weeks compared with baseline (P<0.01), and a further decrease was noticed within 12 weeks after therapy (P<0.01) (Figure 2).
Correlation between IL-34 and serum cytokines, clinical data (Table 3)
Table 3.
Correlations between IL-34 and cytokines as well as RT severity indicators before and during treatment
| IL-34 | |||
|---|---|---|---|
|
|
|||
| Baseline | 4 weeks | 12 weeks | |
|
|
|||
| r | r | r | |
| ESR | 0.275* | 0.285* | 0.317* |
| CRP | 0.320** | 0.306** | 0.364** |
| DAS28 | 0.236* | 0.215* | 0.347* |
| MMP-3 | 0.599** | 0.591** | 0.545** |
| IL-8 | 0.424** | 0.270* | 0.244* |
| IL-6 | 0.554** | 0.586** | 0.217* |
| TNF-α | 0.417** | 0.123 | 0.222 |
P<0.05;
P<0.01.
Next, we analyzed the correlations among IL-34, clinical parameters and other serum cytokines with Spearman’s correlation test. Serum IL-34 was positively correlated with ESR, CRP, and DAS28 before and after treatment. It was also positively correlated with TNF-α, IL-6, IL-8, MMP-3 (P<0.01), and anti-CCP antibody (r=0.657, P<0.01) at baseline. After TNF-α inhibitor treatment, IL-34 was still positively correlated with IL-6, IL-8, and MMP-3.In addition, serum TNF-α (r=0.244, 0.313), IL-6 (r=0.223, 0.303), IL-8 (r=0.308, 0.342), and MMP-3 (r=0.233, 0.176) were all positively correlated with ESR and CRP (P<0.05). Correlation coefficients are shown in Table 3. However, we didn’t find a direct association between any of the cytokines, SJC, TJC, morning stiffness, and patient global VAS scores (P>0.05).
Serum IL-34 in stage-IIIRA
We analyzed the 5 cytokines in the subgroup using the Kruskal-Wallis test first (stage IV was excluded because of the small sample size), and none of the subgroups showed statistically significant differences in ESR, CRP or DAS28 at baseline. Only serum IL-34 showed statistically significant differences among these subgroups (P<0.05). Furthermore, when analyzed with the Mann-Whitney test, serum IL-34 was markedly elevated in the stage III subgroup (427.90 ± 290.22 pg/ml) compared with stage II (214.93 ± 152.24 pg/ml) (P<0.05).
Discussion
Hwang and colleagues showed that IL-34 expression was elevated in RA synovial fluid (SF) and was a biologically active stimulator of osteoclast differentiation [8]. Regarding the clinical significance of IL-34, only one study has demonstrated that IL-34 correlated with disease activity, but changes of IL-34 levels during or after treatment were not determined [4]. Therefore, in this study, we investigated the effectiveness of IL-34 for RA diagnosis and treatment marker. It has been demonstrated that TNF-α can influence the expressions of pro-inflammatory cytokines such as IL-6, MMP-3, and IL-8, which triggers synovitis, chondrocyte activation, and leukocyte accumulation. Studies have also shown that TNF-α, IL-6, and MMP-3 are related to disease activity and could be used as biomarkers for evaluating RA therapy outcomes [21-23]. In this study, we found that IL-34 concentrations were significantly elevated in the sera of RA patients compared with healthy volunteers, which suggested that IL-34 is associated with RA. We found that IL-34 significantly correlated positively with inflammatory parameter changes such as ESR, CRP and disease activity, as assessed by DAS28, as well as with IL-6, IL-8, TNF-α, MMP-3 and anti-CCP antibody serum fluctuations. We suggest that IL-34 might be used to monitor the efficacy of TNF-α inhibitor therapies, as it also decreased nearly simultaneously with clinical disease characteristics (TJC, SJC, morning stiffness, patients global VAS).
Based on previous studies, using synovium biopsy, which have already demonstrated that IL-34 was related to bone erosion in RA pathogenesis [7,8], we investigated the expression of IL-34 in different stages of RA, in order to confirm the role of IL-34 in bone destruction without the use of invasive examinations. We detected that the serum level of IL-34 was much higher in the third stage of X-ray progression than in the second, which suggests that IL-34 level is also related to the severity of bone destruction. This is also supported by the finding, that IL-34 positively correlated with MMP-3 expression and anti-CCP antibody secretion, which have already been confirmed to be associated with the severity of RA bone erosion [24-26]. Considering the diagnostic significance of anti-CCP antibodies and its strong correlation with IL-34, IL-34 may play a role in the diagnostic severity evaluation of RA, although more studies are needed to confirm this.
In summary, we demonstrated for the first time that IL-34 serum concentrations correlated with clinical symptoms, inflammatory indicators, disease activity, and serum biomarkers of RA during a TNF-α inhibitor therapy. Additionally, according to X-ray radiography scores, since its serum level also correlated with RA progression and anti-CCP antibody concentrations, IL-34 might become a marker of RA severity.
Acknowledgements
This project was supported by grants from the Science & Technology Ministry ‘11th Five-Year Plan’ to support science and technology of China (No. 2008BAI59B01).
Disclosure of conflict of interest
None.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 3.Clavel G, Thiolat A, Boissier MC. Interleukin newcomers creating new numbers in rheumatology: IL-34 to IL-38. Joint Bone Spine. 2013;80:449–453. doi: 10.1016/j.jbspin.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Tian Y, Shen H, Xia L, Lu J. Elevated serum and synovial fluid levels of interleukin-34 in rheumatoid arthritis: possible association with disease progression via interleukin-17 production. J Interferon Cytokine Res. 2013;33:398–401. doi: 10.1089/jir.2012.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon SJ, Hong YS, Ju JH, Kwok SK, Park SH, Min JK. Increased levels of interleukin 34 in serum and synovial fluid are associated with rheumatoid factor and anticyclic citrullinated peptide antibody titers in patients with rheumatoid arthritis. J Rheumatol. 2013;40:1842–1849. doi: 10.3899/jrheum.130356. [DOI] [PubMed] [Google Scholar]
- 6.Chemel M, Le Goff B, Brion R, Cozic C, Berreur M, Amiaud J, Bougras G, Touchais S, Blanchard F, Heymann MF, Berthelot JM, Verrecchia F, Heymann D. Interleukin 34 expression is associated with synovitis severity in rheumatoid arthritis patients. Ann Rheum Dis. 2012;71:150–154. doi: 10.1136/annrheumdis-2011-200096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Buki K, Vaaraniemi J, Gu G, Vaananen HK. The critical role of IL-34 in osteoclastogenesis. PLoS One. 2011;6:e18689. doi: 10.1371/journal.pone.0018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang SJ, Choi B, Kang SS, Chang JH, Kim YG, Chung YH, Sohn DH, So MW, Lee CK, Robinson WH, Chang EJ. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 2012;14:R14. doi: 10.1186/ar3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee A, Qiao Y, Grigoriev G, Chen J, Park-Min KH, Park SH, Ivashkiv LB, Kalliolias GD. Tumor necrosis factor alpha induces sustained signaling and a prolonged and unremitting inflammatory response in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2013;65:928–938. doi: 10.1002/art.37853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarilina A, Xu K, Chan C, Ivashkiv LB. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum. 2012;64:3856–3866. doi: 10.1002/art.37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han W, Xiong Y, Li Y, Fang W, Ma Y, Liu L, Li F, Zhu X. Anti-arthritic effects of clematichinenoside (AR-6) on PI3K/Akt signaling pathway and TNF-alpha associated with collagen-induced arthritis. Pharm Biol. 2013;51:13–22. doi: 10.3109/13880209.2012.698287. [DOI] [PubMed] [Google Scholar]
- 12.Kanbe K, Chiba J, Nakamura A. Inhibition of JNK in synovium by treatment with golimumab in rheumatoid arthritis. Rheumatol Int. 2014;34:125–130. doi: 10.1007/s00296-012-2626-7. [DOI] [PubMed] [Google Scholar]
- 13.Macias I, Garcia-Perez S, Ruiz-Tudela M, Medina F, Chozas N, Giron-Gonzalez JA. Modification of pro-and antiinflammatory cytokines and vascular-related molecules by tumor necrosis factor-a blockade in patients with rheumatoid arthritis. J Rheumatol. 2005;32:2102–2108. [PubMed] [Google Scholar]
- 14.Sun S, Bay-Jensen AC, Karsdal MA, Siebuhr AS, Zheng Q, Maksymowych WP, Christiansen TG, Henriksen K. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet Disord. 2014;15:93. doi: 10.1186/1471-2474-15-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 16.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 17.Qu N, Xu M, Mizoguchi I, Furusawa J, Kaneko K, Watanabe K, Mizuguchi J, Itoh M, Kawakami Y, Yoshimoto T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, IL-23, in inflammatory diseases. Clin Dev Immunol. 2013;2013:968549. doi: 10.1155/2013/968549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraan MC, Patel DD, Haringman JJ, Smith MD, Weedon H, Ahern MJ, Breedveld FC, Tak PP. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synthesis of the chemokine CXCL8 (interleukin-8) Arthritis Res. 2001;3:65–71. doi: 10.1186/ar141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Mod ified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 20.Gu J. Internal Medicine. 7th edition. Beijing: People’s medical publishing house; 2008. Rheumatoid Arthritis; pp. 848–855. [Google Scholar]
- 21.Avdeeva AS, Aleksandrova EN, Novikov AA, Cherkasova MV, Panasyuk EY, Nasonov EL. [Relationship of the clinical efficiency of tocilizumab therapy to the serum level of matrix metalloproteinase-3 in patients with rheumatoid arthritis] . Ter Arkh. 2013;85:24–29. [PubMed] [Google Scholar]
- 22.Li L, Cai B, Liao J, Yang B, Huang Z, Wang L. [Clinical value of serum matrix metalloproteinase-3 in evaluating joint destruction and therapeutic effect in rheumatoid arthritis patients] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:966–969. [PubMed] [Google Scholar]
- 23.Shimamoto K, Ito T, Ozaki Y, Amuro H, Tanaka A, Nishizawa T, Son Y, Inaba M, Nomura S. Serum interleukin 6 before and after therapy with tocilizumab is a principal biomarker in patients with rheumatoid arthritis. J Rheumatol. 2013;40:1074–1081. doi: 10.3899/jrheum.121389. [DOI] [PubMed] [Google Scholar]
- 24.Alessandri C, Priori R, Modesti M, Mancini R, Valesini G. The role of anti-cyclic cytrullinate antibodies testing in rheumatoid arthritis. Clin Rev Allergy Immunol. 2008;34:45–49. doi: 10.1007/s12016-007-8023-4. [DOI] [PubMed] [Google Scholar]
- 25.Fathi NA, Ezz-Eldin AM, Mosad E, Bakry RM, Hamed HB, Ahmed S, Mahmoud M, Rashed HA, Abdullah F. Diagnostic performance and predictive value of rheumatoid factor, anti-cyclic-citrullinated peptide antibodies and HLA-DRB1 locus genes in rheumatoid arthritis. Int Arch Med. 2008;1:20. doi: 10.1186/1755-7682-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syversen SW, Goll GL, van der Heijde D, Landewe R, Gaarder PI, Odegard S, Haavardsholm EA, Kvien TK. Cartilage and bone biomarkers in rheumatoid arthritis: prediction of 10-year radiographic progression. J Rheumatol. 2009;36:266–272. doi: 10.3899/jrheum.080180. [DOI] [PubMed] [Google Scholar]


