Abstract
Background: Breast cancer (BC) deaths are a major concern worldwide, and modified radical mastectomy (MRM) still represents a primary therapeutic strategy. Post-surgery administration of interleukin (IL)-2 for BC therapy has been implemented in China recently. Although its impact on regulatory T cells (Tregs) has been documented in some cancer types, such as melanoma, the IL-2-mediated changes in the Treg composition after MRM in BC treatment remain unknown. Methods: As registered with the Chinese Clinical Trial Registry, 34 newly diagnosed BC patients, aged 20-65 years, were enrolled in this trial. Patients were randomized to the IL-2-treated group (n=15) and the untreated control group (n=19). Peripheral blood mononuclear cells were isolated at time points of pre-operation (PreOP) and post-operation Day 1 (POD1), POD3, and POD7. Cells were subjected to flow cytometric assays to identify CD4+ CD25+ Foxp3+ Tregs, as well as real-time quantitative polymerase chain reaction analysis of FOXP3 expression. Results: We found that the surgery caused a significant decrease in the percentage of Tregs on POD1, followed by a significant increase characterized by a peak value on POD7 with a more than 18% increase relative to the Pre-OP levels. We observed that the Treg percentages in the IL-2-treated group were significantly greater than those in the control group on POD3 and POD7, whereas no such statistical difference was observed on POD1. The FOXP3 expression analysis revealed consistent trends as observed by flow cytometry. Conclusions: Post-operative administration of IL-2 amplifies the surgery-induced augmentation of both Tregs and FOXP3 expression in BC therapy.
Keywords: Breast cancer, modified radical mastectomy, regulatory T cells, interleukin-2
Introduction
Breast cancer (BC) has been recognized by the world health organization (WHO) as one of the leading causes of death among women between the ages of 20 and 59 years. Although progress has been made toward better therapy and earlier diagnosis, the global BC incidence has continued to increase over the past several decades [1-4]. The most serious increase in BC-associated mortality has been observed in Asian countries, particularly in China [4]. Surgical excision is currently the mainstay of BC treatment; however, post-surgery recurrence with increased malignancies has been reported [5-7]. Circulating tumor cell (CTCs) of BC has been proposed to be a main cause of such recurrence [5,7-11]. These CTCs are derived from either shed BC cells in the peripheral blood or metastatic cells that can seed back to the locus of the removed tumor [12,13]. Favorable to CTC reseeding and growth, the local and systemic release of growth factors or cytokines [14] and suppression of cell-mediated immunity [15-18] are frequently observed. These changes in immunological conditions largely drive the host defense toward a compromised capacity of BC-specific immunity [19-21]. In support of these findings, post-surgery changes in the numbers and proportions of peripheral natural killer (NK) cells, cytotoxic T lymphocytes, dendritic cells, and T-helper cells are frequently reported [21-23]. Another cell type, regulatory T cells (Tregs), has been frequently shown to infiltrate BC tissues and partially accounts for the immunosuppressive tumor microenvironment [24-26]. Among several known types of Tregs, CD4+ CD25+ Foxp3+ T cells have been most extensively investigated and confirmed to be associated with immunosuppressive activity and thus to be physiologically relevant to the human immune status [27]. Generally, Foxp3 expression in naive T cells drives the expression of CD25 and other Treg-associated cell-surface molecules and cytokines, such as cytotoxic T cell-associated antigen-4 (CTLA-4), glucocorticoid-induced tumor necrosis factor (TNF) receptor family-related gene/protein (GITR), transforming growth factor (TGF)-β, and interleukin (IL)-10, whereas it represses the production of IL-2, interferon (IFN)-γ, and IL-4 [28]. Treg accumulation has been reported to correlate with tumor progression and poorer prognosis in numerous cancers [6,29-34]. This is explainable as cancers may try to hijack this intrinsically immunosuppressive mechanism to combat the tumor-specific immunity in the human body. Although there have been reports showing that IL-2 administration increases the systemic Treg level in melanoma and renal cancer patients [35], the effects of IL-2 on the change in Tregs in the peripheral blood after radical mastectomy has not been addressed according to our knowledge. Notably, the postoperative period has been recognized as a critical window for the minimal residual disease of BC [36]. Thus, the evaluation of Treg-related changes after IL-2 treatment is of potential clinical significance for understanding the immunological impact of current widely used BC therapeutic practices. Thus, we performed the first randomized controlled trial to observe the changes in the Treg population after modified radical mastectomy and to study the effect of recombinant human (rh) IL-2 administered postoperatively on the Treg population. We found that IL-2 administration induced a significant increase in the postoperative Treg composition in BC patients.
Material and methods
Subjects and treatments
With approval from the ethics committee of the Third Xiangya Hospital of Central South University (CSU), the current trial was registered with the Chinese Clinical Trial Registry (ChiCTR-TRC-14004716). All patients provided written informed consent for participation in the trial. The cohort inclusion criteria included treatment with modified radical mastectomy for primary BC as diagnosed by micro-invasive biopsy in the Third Xiangya Hospital of CSU and age within 20-65 years. The cohort exclusion criteria included previous BC surgery (except for diagnostic biopsy), inflammatory BC, treatment with chemotherapy and/or radiotherapy prior to surgery, an American Society of Anesthesiologists (ASA) Physical Status III-IV or greater, complication with diseases of the immune system or the endocrine system, and long-term use of medication that impacted the immune or endocrine system. Eligible patients were randomly assigned to two groups: the rhIL-2-treated group, in which patients were treated with rhIL-2 (Shandong Quangang Pharmaceutical Co., Ltd.) at 1 million international units (MIU) per day for 5 days starting from postoperative day 1 (POD1), and the control group, which received standard care without rhIL-2 administration. The surgery were performed under general anesthesia with fentanyl, propofol plasma target-controlled infusions (TCI), and vecuronium. Some patients received chemotherapy on POD7 according to the results of pathologic examination.
Peripheral blood samples were collected on the morning of surgery (Pre-OP) as well as on POD1, POD3, and POD7, between 7:00 and 9:00 A.M. Blood samples were immediately delivered to the laboratory and processed within 1 h.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated by standard Ficoll-density-gradient centrifugation and subjected to the fluorescence-activated cell sorting (FACS) analysis within 4 h post-collection using a Canto II Flow Cytometer (BD, Shanghai, China). The FACSDiva software version 6.1.3 (BD) was used to analyze the FACS data. Antibodies included fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD4 and phycoerythrin (PE)-Cy7-conjugated mouse anti-human CD25 monoclonal antibodies (mAbs), and fluorochrome-conjugated anti-mouse IgG was used as an isotype control (BD). Intracellular staining with PE-conjugated mouse anti-human Foxp3 mAb (BD) was performed according to the manufacturer’s instructions.
Real-time qPCR analysis on FOXP3 expression
Total RNA was extracted by using Trizol (Life Technologies, USA).The specific primer sequences for the target genes were as follows: FOXP3, 5’-CTGACCAAGGCTTCATCTGTG-3’ (forward), 5’-GAACTCTGGGAATGTGCTGTT-3’ (reverse); β-actin, 5’-ACCGAGCGCGGCTACAG-3’ (forward), 5’-CTTAATGTCACGCACGATTTCC-3’ (reverse).
Synthesis of cDNAs was controlled by performing fluorescence quantitative (FQ)-polymerase chain reaction (PCR) using the β-actin primers. The procedure were performed by using PrimeScript RT reagent Kit with gDNA Eraser (TAKARA) with Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific) following the manufacturer’s protocol. The values of the sample copies were obtained after quantitative amplification and normalized to β-actin levels. The relative levels of gene expression were represented as ΔCt=Ctgene-Ctreference, and the fold change in gene expression was calculated by the 2-ΔΔCt method.
Statistical analysis
Statistical analysis was performed using the SPSS statistical package (version 18.0; SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± SD. All statistical analyses were performed using repeated measures analysis of variance (ANOVA) with intro-group comparison among 4 time points by Bonferroni’s post hoc testing, and inter-group comparison by unpaired t-tests with Bonferroni’s correction. P<0.05 was considered statistically significant.
Results
Patient enrollment and clinical characteristics
A total of 40 patients met the initial inclusion and not the exclusion criteria and were enrolled into the control group (n=20) and the rhIL-2-treated group (n=20). One patient in the control group was excluded post-surgery due to dexamethasone inhalation, and five patients in the rhIL-2-treated group were excluded post-operatively, including 4 cases of rhIL-2 toxicity with fever and 1 case of hypotension. Nineteen and fifteen subjects in the control and the rhIL-2-treated group, respectively, completed the trial. Their clinical characteristics are shown in Table 1. We observed no significant differences in the listed clinical variables between the two groups (Table 1).
Table 1.
Baseline characteristics of patients
| Variable | Control group | rhIL-2-treated group | P value |
|---|---|---|---|
| Age (years) | 49.4 | 44.6 | >0.05 |
| Body surface area (m2) | 1.63 | 1.62 | >0.05 |
| Duration of operation (min) | 114.6 | 124.2 | >0.05 |
| Blood loss in operation (ml) | 78 | 84.55 | >0.05 |
Impact of surgery on PBMC Tregs and FOXP3 expression
Upon comparing PBMC samples by FACS with the Pre-OP in control groups, we observed that the Treg percentage was significantly decreased on POD1 (P<0.01; Figure 1). Such percentages longitudinally increased in the surgical control group and reached a peak value on POD7, with a more than 18% increase (from the absolute percentage of ~6.1% to ~7.2%) compared to the Pre-OP group (P<0.01; Figure 1). The PBMC FOXP3 expression analyses demonstrated a similar trend to the FACS results, with a significant decrease on POD1 (P<0.01) and significant increases until POD7, with approximately 2-fold up-regulation compared with the Pre-OP group (P<0.01; Figure 2).
Figure 1.
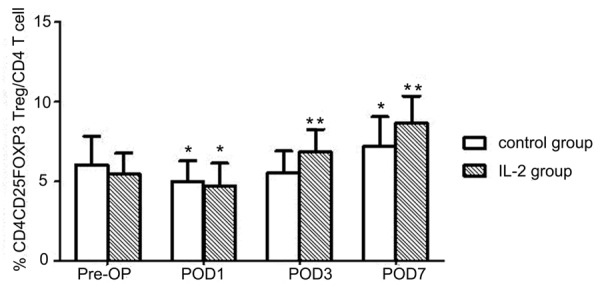
The pre- and post-surgery changes in the percentages of Tregs among PBMCs. Cells were analyzed by FACS, and data are shown as mean ± SD. *P<0.01, compared with the PreOP group; **P<0.05, compared with control group.
Figure 2.
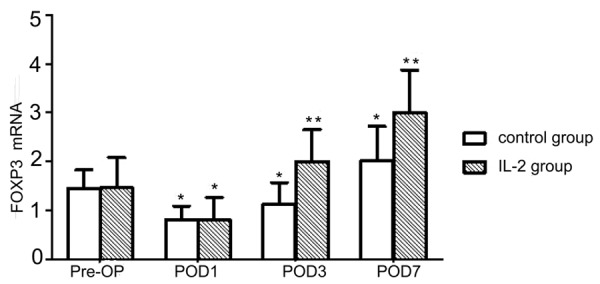
The pre- and post-surgery changes in FOXP3 gene expression. RT-qPCR results for FOXP3 expression normalized to ACTB expression. Data are shown as mean ± SD. *P<0.01, compared with the PreOP group; **P<0.01, compared with the control group.
Effect of rhIL-2 treatment on PBMC Tregs and FOXP3 expression
No significant Treg-related differences, either in PBMC Treg composition (Figure 1) or FOXP3 expression (Figure 2), were observed at POD1 between the control group and the rhIL-2-treated group. The Tregs reached a similarly low level after surgery with and without rhIL-2 treatment. However, according to the FACS results, the Treg percentage in the rhIL-2-treated group was significantly greater than that in the control group on POD3 (~20% relative increase, P<0.01) and POD7 (~19% relative increase, P<0.05; Figure 1).
Such changes in Treg percentages were consistent with the results of FOXP3 expression analysis (Figure 2). rhIL-2 treatment significantly promoted FOXP3 expression on POD3 and POD7 (P<0.01; Figure 2).
Discussion
We here report, for the first time, that post-operative administration of rhIL-2 amplifies the surgery-induced augmentation of both the Treg composition and FOXP3 expression in BC therapy with modified radical mastectomy. One of the major roles of IL-2 is to unselectively promote the proliferation of activated CD4+ T cells. These cells include both tumor antigen-specific T cells, which represent a minor T-cell population and may not be activated, and Tregs, which are found more abundantly in the peripheral blood and at an activated state. Our findings indicate that IL-2 treatment increased the Treg population significantly (a >1% absolute percentage in CD4+ T cells). It is of clinical significance that the IL-2-induced changes in Treg composition have a potentially general suppressive effect on the adaptive immunity that may compensate the gain of anti-tumor immunity, referred to as the compensation hypothesis hereafter. This is in agreement with the results of numerous reports, which have proposed that the increase in Tregs leads to cellular immunosuppression and promotes post-surgery recurrence and metastasis in numerous cancer types [5,7,8,10]. Hence, larger scale cohort studies of the correlation between IL-2 application and post-surgery prognosis for BC are warranted.
To our knowledge, information regarding how surgery influences Treg status is limited. Wicherek et al found the Treg population decreases in the peripheral blood of patients on POD5 in ovarian cancer and reached a conclusion that solid tumor removal may help to restore the immune response [37]. However, they ignored stress on Tregs due to surgical trauma and other causes. Based on the results shown in this study, we propose that prolonged observation may lead to a different conclusion. In support of this hypothesis, Saito et al showed that the Treg subpopulations are significantly higher on POD6 in patients who underwent massively invasive surgeries, including pancreatoduodenectomy, distal pancreatectomy, segmentectomy, liver resection, and gastrectomy [38]. Indeed, both studies described above provide support for our results, which showed a decrease in the Treg population on POD1 and rebound on POD3 and POD7 after surgical treatment of BC. We reason that such a trend reflects the initial pro-inflammatory response to injury on POD1 and the systemically anti-inflammatory responses evoked on POD3 and POD7 [23,39].
Numerous reports do not recommend the use of IL-2 alone, and combined treatment with famotidine, vaccines, and chemotherapy is preferable [40-43]. For example, Dillman et al reported that IL-2 administration alone does not benefit patients with distant metastatic melanomas [44], which is in agreement with our compensation hypothesis. Furthermore, the same group confirmed that high-dose IL-2, in combination with active specific immunotherapy, is associated with better survival than IL-2 treatment alone in melanoma therapy [43]. Thus, physicians should exercise caution when using rhIL-2 alone in post-surgery treatment for BC patients.
Disclosure of conflict of interest
None.
References
- 1.Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC, Martin M, Namer M, O’Shaughnessy JA, Shen ZZ, Albain KS ABREAST Investigators. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 2.Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BO, Jakesz R. Breast cancer issues in developing countries: an overview of the Breast Health Global Initiative. World J Surg. 2008;32:2578–2585. doi: 10.1007/s00268-007-9454-z. [DOI] [PubMed] [Google Scholar]
- 4.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, Sandelin K, Derossis A, Cody H, Foulkes WD. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–768. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Mimori K, Ueo H, Karimine N, Barnard GF, Sugimachi K, Akiyoshi T. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer. 1996;68:739–743. doi: 10.1002/(SICI)1097-0215(19961211)68:6<739::AID-IJC8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, Tsukada K, Saiki I. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–555. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 10.Camara O, Kavallaris A, Noschel H, Rengsberger M, Jorke C, Pachmann K. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol. 2006;4:67. doi: 10.1186/1477-7819-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boomsma MF, Garssen B, Slot E, Berbee M, Berkhof J, Meezenbroek Ede J, Slieker W, Visser A, Meijer S, Beelen RH. Breast cancer surgery-induced immunomodulation. J Surg Oncol. 2010;102:640–648. doi: 10.1002/jso.21662. [DOI] [PubMed] [Google Scholar]
- 12.Eschwege P, Dumas F, Blanchet P, Le Maire V, Benoit G, Jardin A, Lacour B, Loric S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet. 1995;346:1528–1530. doi: 10.1016/s0140-6736(95)92054-4. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25:231–243. doi: 10.1053/ejso.1998.0634. [DOI] [PubMed] [Google Scholar]
- 15.Koerner P, Westerholt A, Kessler W, Traeger T, Maier S, Heidecke CD. [Surgical trauma and postoperative immunosuppression] . Chirurg. 2008;79:290–294. doi: 10.1007/s00104-008-1465-2. [DOI] [PubMed] [Google Scholar]
- 16.Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793–808. doi: 10.1007/s00595-010-4323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menges P, Kessler W, Kloecker C, Feuerherd M, Gaubert S, Diedrich S, van der Linde J, Hegenbart A, Busemann A, Traeger T, Cziupka K, Heidecke CD, Maier S. Surgical trauma and postoperative immune dysfunction. Eur Surg Res. 2012;48:180–186. doi: 10.1159/000338196. [DOI] [PubMed] [Google Scholar]
- 19.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale F, Chinellato I, Caimmi S, Peroni DG, Franceschini F, Miraglia Del Giudice M, Bernardini R. Perioperative period: immunological modifications. Int J Immunopathol Pharmacol. 2011;24:S3–12. doi: 10.1177/03946320110240s302. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE, Flemmer M. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg. 2012;73:801–808. doi: 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- 22.Ahlers O, Nachtigall I, Lenze J, Goldmann A, Schulte E, Hohne C, Fritz G, Keh D. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101:781–787. doi: 10.1093/bja/aen287. [DOI] [PubMed] [Google Scholar]
- 23.Bartal I, Melamed R, Greenfeld K, Atzil S, Glasner A, Domankevich V, Naor R, Beilin B, Yardeni IZ, Ben-Eliyahu S. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun. 2010;24:376–386. doi: 10.1016/j.bbi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 24.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Kruijf EM, van Nes JG, Sajet A, Tummers QR, Putter H, Osanto S, Speetjens FM, Smit VT, Liefers GJ, van de Velde CJ, Kuppen PJ. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010;16:1272–1280. doi: 10.1158/1078-0432.CCR-09-1844. [DOI] [PubMed] [Google Scholar]
- 27.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhary B, Abd Al Samid M, al-Ramadi BK, Elkord E. Phenotypic alterations, clinical impact and therapeutic potential of regulatory T cells in cancer. Expert Opin Biol Ther. 2014;14:931–945. doi: 10.1517/14712598.2014.900539. [DOI] [PubMed] [Google Scholar]
- 30.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 31.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 32.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 33.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 34.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 35.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC. Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet. 1999;354:197–202. doi: 10.1016/s0140-6736(98)10175-7. [DOI] [PubMed] [Google Scholar]
- 37.Wicherek L, Jozwicki W, Windorbska W, Roszkowski K, Lukaszewska E, Wisniewski M, Brozyna AA, Basta P, Skret-Magierlo J, Koper K, Rokita W, Dutsch-Wicherek M. Analysis of Treg cell population alterations in the peripheral blood of patients treated surgically for ovarian cancer - a preliminary report. Am J Reprod Immunol. 2011;66:444–450. doi: 10.1111/j.1600-0897.2011.01024.x. [DOI] [PubMed] [Google Scholar]
- 38.Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Iwahashi S, Yamada S, Asanoma M. Regulatory T cells in the blood: a new marker of surgical stress. Surg Today. 2013;43:608–612. doi: 10.1007/s00595-013-0517-5. [DOI] [PubMed] [Google Scholar]
- 39.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman HL, Taback B, Sherman W, Kim DW, Shingler WH, Moroziewicz D, DeRaffele G, Mitcham J, Carroll MW, Harrop R, Naylor S, Kim-Schulze S. Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med. 2009;7:2. doi: 10.1186/1479-5876-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camisaschi C, Filipazzi P, Tazzari M, Casati C, Beretta V, Pilla L, Patuzzo R, Maurichi A, Cova A, Maio M, Chiarion-Sileni V, Tragni G, Santinami M, Vergani B, Villa A, Berti E, Umansky L, Beckhove P, Umansky V, Parmiani G, Rivoltini L, Castelli C. Effects of cyclophosphamide and IL-2 on regulatory CD4+ T cell frequency and function in melanoma patients vaccinated with HLA-class I peptides: impact on the antigen-specific T cell response. Cancer Immunol Immunother. 2013;62:897–908. doi: 10.1007/s00262-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan WD Jr, Quan FM. Activity of outpatient intravenous interleukin-2 and famotidine in metastatic clear cell kidney cancer. Cancer Biother Radiopharm. 2014;29:58–61. doi: 10.1089/cbr.2013.1555. [DOI] [PubMed] [Google Scholar]
- 43.Dillman RO, Depriest C, McClure SE. High-dose IL2 in metastatic melanoma: better survival in patients immunized with antigens from autologous tumor cell lines. Cancer Biother Radiopharm. 2014;29:53–57. doi: 10.1089/cbr.2013.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillman RO, O’Connor AA, Simpson L, Barth NM, VanderMolen LA, Vanderplas P. Does continuous-infusion interleukin-2 increase survival in metastatic melanoma? Am J Clin Oncol. 2003;26:141–145. doi: 10.1097/00000421-200304000-00008. [DOI] [PubMed] [Google Scholar]


