Abstract
Gastric adenocarcinoma (GA) is one of the most common cancer worldwide. ATP citrate lyase (ACLY) is generally recognized as a key enzyme of de novo fatty acid synthesis responsible for generation of oxaloacetate and cytosolic acetyl-CoA. This study aimed to investigate the expression level of ACLY in GA and evaluate the relationship between ACLY expression and the prognosis of GA patients. Paraffin archived samples from 83 GA patients were used to analyze ACLY expression by immunohistochemistry. ACLY was significantly upregulated in GA tissues compared with adjacent normal tissues (P < 0.001). High ACLY expression was correlated with advanced stages (P = 0.007) and lymph node metastasis (P = 0.022). Furthermore, patients with low ACLY expression had longer survival time than those with high ACLY expression (P = 0.031). In conclusion, these results indicate that ACLY might serve as a biomarker to predict the progression and prognosis of GA patients.
Keywords: Gastric cancer, ATP citrate lyase, prognosis, lipid synthesis
Introduction
Gastric cancer was the fourth most common cancer in the world [1]. Most cases occur in developing countries. It’s worth noting that 90% of all gastric tumors are malignant, and 95% of the total number of malignancies is comprised of gastric adenocarcinoma. Although China has made progress in reducing the incidence and mortality of gastric cancer, the overall 5-year survival rate of gastric cancer patients is still far from satisfactory. Therefore, more attentions should be given to early diagnosis and treatments.
Increasing evidence has indicated that cancer is not only a genetic disease, but also a metabolic disease [2-7]. Lipogenic pathway is one of the pathways that were reprogrammed in the development and progression of cancer. ATP citrate lyase (ACLY) catalyzes the accumulated citrate in cytoplasm to generate acetyl-CoA, a pivotal precursor of fatty acids. It is well know that fatty acid synthesis pathway is significantly upregulated in many kinds of tumors, and the dysregulation of ACLY is contributed to these alterations [8-11]. Although the expression level of ACLY expression is low in normal cells, it has been found its expression is obviously increased in various types of tumors [12-16]. Recently, researchers have found that inactivation of ACLY could suppress cancer cells viability and inhibit cancer growth [8,17-23]. Moreover, the latest study found that ACLY depletion induced cancer growth suppression with altered fatty acid composition in cancer cells [24]. Considering all these findings, ACLY might be a potential biomarker and an effective therapeutic target for cancer.
In the present study, we examined the expression of ACLY in 73 pairs of gastric adenocarcinoma (GA) and adjacent normal tissues, and evaluated the relationship between its expression and clinical pathological parameters and outcome of patients.
Materials and methods
Samples
A total of 73 pairs of GA and adjacent normal tissues were obtained from the BioBank of National Engineering Center for Biochip at Shanghai. All cases were histologically confirmed. Follow-up information of patients was collected by phone. We calculated survival time from the surgery date to the date of follow-up or death date. All patients signed an information consent form according to the protocols approved by the Ethics Committees of National Engineering Center for Biochip at Shanghai.
Tissue microarray construction
After being fixed with 4% formaldehyde, tissues were embedded in the paraffin wax. Tissue microarray was constructed according to a previously published method [7]. Representative GA regions and adjacent nonmalignant tissues were chosen from each tissue block, and a single 0.6 mm core was taken from every donor block. An automated tissue arrayer (Beecher instruments) were used to construct microarray blocks. Adjacent normal gastric tissue was used as an internal control. Tissues were then stained with H&E staining to determine the presence of tumor within each core by two independent pathologists.
Immunohistochemistry (IHC)
Tissue microarray was dewaxed in xylene, rehydrated in alcohol and immersed in 3% hydrogen peroxide for 10 min. The slides were then treated with antigen in 0.01 mol/L sodium citrate buffer for 20 minutes. After being rinsed with phosphate-buffered saline (PBS) for three times, sections were incubated with primary ACLY antibody at room temperature. Three hours later, sections were washed with PBS for three times, each for 10 minutes. The samples then were treated with horseradish peroxidase-conjugated streptomycin working solution for 30 minutes and washed with PBS for three times. Finally, envision staining was performed.
To evaluate IHC results, two independent pathologists randomly selected 5 visual fields for every spot and counted the number of total tumor cell, positive cell and recorded staining intensity. To determine the staining intensity of samples, the semi-quantitative method was used. We divided the staining intensity (I) into 4 grades 0, none; 1, poor; 2, moderate and 3 strong. The percentage of positive cells (P) was scored as: 0, 0%; 1, 1%-25%; 2, 26%-50%; 3, 50-75%; and 4, > 75%. The total score (I × P) ≤ 4 was defined as low expression, and score > 4 was defined as high expression.
Statistical analyses
X2 test and Fisher’s exact test were used to analyze the associations between ACLY and clinicopathological features. To measure the survival rates in different groups, the Log-rank test and Kaplan-Meier method were analyzed. The Cox proportional hazard model was used for multivariate analysis. All analyses were performed using the SPSS 19.0 statistical software package. A two-tailed P value ≤ 0.05 was considered statistically significant.
Results
Clinicopathological features
Histological and clinical data of 83 patients were displayed in Table 1, including 28 females and 55 males. The median age was 65 (range 37-84). According to the TNM staging system, there were 7 cases in stage I, 20 in stage II, 37 in stage III, and 9 in stage IV. Nineteen cases had no regional lymph node metastasis (LNM), and 63 cases had regional LNM. The median of tumor size was 5 cm (range, 1.2-15.0 cm) (Table 1).
Table 1.
Clinicopathological characteristics of GA patients
| Characteristics | No. of Patients | % |
|---|---|---|
| Age (years) | ||
| median | 65 | |
| range | 37-84 | |
| Sex | ||
| female | 28 | 33.7 |
| male | 55 | 66.3 |
| Histologic grade | ||
| 1 | 1 | 1.2 |
| 2 | 20 | 24.1 |
| 3 | 62 | 74.7 |
| TNM stage | ||
| I | 7 | 8.4 |
| II | 20 | 24.1 |
| III | 37 | 44.6 |
| IV | 9 | 10.8 |
| unknown | 5 | 12.0 |
| Lymph node status | ||
| negative | 19 | 22.9 |
| positive | 63 | 75.9 |
| unknown | 1 | 1.2 |
| Tumor size (cm) | ||
| median | 5.0 | |
| range | 1.2-15.0 | |
| Distant metastasis | ||
| negative | 74 | 89.2 |
| positive | 9 | 10.8 |
ACLY expression in GA and adjacent normal tissues
Ten cases had no cancer tissues were excluded from further analysis. IHC results revealed that ACLY was located predominately in the cytoplasm but rarely in nucleus. Cancer tissues showed a strongly positive ACLY expression, whereas adjacent normal tissues showed a weakly positive expression (Figure 1). The expression level of ACLY in GA tissues was significantly higher than that in adjacent normal tissues (P < 0.001).
Figure 1.
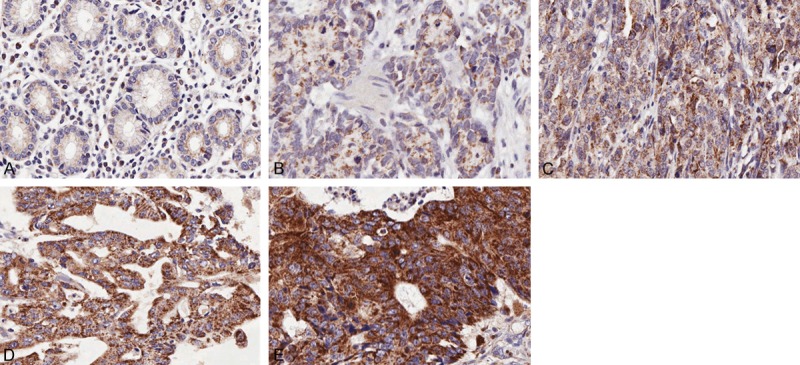
Immunohistochemical analysis of ACLY in GA and adjacent normal tissues. ACLY showed weak positive expression in normal paracancerous tissues (A) and TNM stage I cancers (B) TNM stage II cancers showed moderate ACLY expression (C) whereas stages III and IV cancers showed strong ACLY expression (D and E).
The relationship between ACLY expression and clinicopathological features of GA
We further evaluated the relationship between ACLY expression and clinicopathological characteristics. High ACLY expression was significantly related to LNM (P = 0.022) and advanced stages (P = 0.007). There was a weak but not significant difference in distant metastasis between low and high ACLY expression groups (P = 0.052). There were no significant differences between ACLY expression and age, sex, tumor size, and histologic grade (all P > 0.05) (Table 2).
Table 2.
Associations between ACLY expression and the clinicopathological features of GA patients
| Variables | ACLY expression | ||
|---|---|---|---|
|
| |||
| Low | High | P value | |
| Age (years) | |||
| ≤ 60 | 10 (47.6) | 19 (37.3) | 0.440 |
| > 60 | 11 (52.4) | 32 (62.7) | |
| Sex | |||
| male | 16 (76.2) | 32 (61.5) | 0.284 |
| female | 5 (23.8) | 20 (38.5) | |
| Tumor size (cm) | |||
| ≤ 6 | 16 (76.2) | 28 (54.9) | 0.202 |
| > 6 | 5 (23.8) | 23 (45.1) | |
| Histologic grade | |||
| 1+2 | 5 (23.8) | 14 (26.9) | 1.000 |
| 3 | 16 (76.2) | 38 (73.1) | |
| LNM | |||
| positive | 12 (60.0) | 45 (86.5) | 0.022 |
| negative | 8 (38.1) | 7 (13.5) | |
| TNM stage | |||
| I+II | 10 (58.8) | 10 (21.3) | 0.007 |
| III+IV | 7 (41.2) | 37 (78.7) | |
| Distant metastasis | |||
| negative | 21 (100.0) | 43 (82.7) | 0.052 |
| positive | 0 (0) | 9 (17.3) | |
Survival analysis
The median survival time (MST) for all the patients was 47 months (95% CI: 39.772-54.506). During follow-up period, 38 (73.1%) of 52 patients with high ACLY expression and 10 (47.6%) of 21 cases with low ACLY expression died of the disease. Patients with high ACLY expression had a shorter MST than those with low ACLY expression (23 months vs 78 months; log-rank test, P = 0.031) (Figure 2). Cox regression analyses showed that advanced TNM stages [hazard ratio (HR) = 1.985; 95% confidence interval (CI), 1.058-3.723; P = 0.033) and high ACLY expression (HR = 2.103; 95% CI, 1.047-4.226; P = 0.037) were associated with worse survival time (Table 3). However, these differences were disappeared when multivariate Cox model analysis was performed (P > 0.05).
Figure 2.
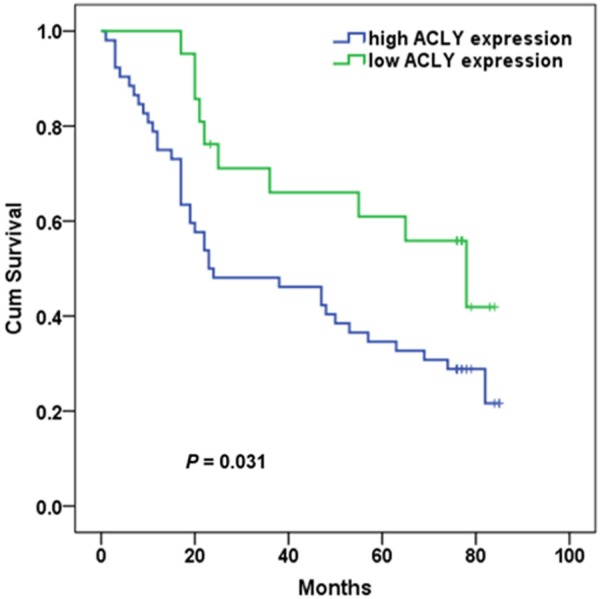
Kaplan-Meier curves of the overall survival of 73 GA patients with low and high ACLY expression.
Table 3.
Univariate Cox regression analysis of overall survival
| Features | Univariate analysis | |
|---|---|---|
|
| ||
| HR (95% CI) | P value | |
| Age (years), > 60 vs ≤ 60 | 1.656 (0.925-2.963) | 0.089 |
| Sex, female vs male | 1.419 (0.810-2.485) | 0.221 |
| Histologic grade, 3 vs 1+2 | 1.875 (0.940-3.741) | 0.074 |
| Tumor size (cm), > 6 vs ≤ 6 | 1.499 (0.868-2.586) | 0.146 |
| LNM, positive vs negative | 1.750 (0.877-3.494) | 0.112 |
| TNM, III+IV vs I+II | 1.985 (1.058-3.723) | 0.033 |
| Distant metastasis, positive vs negative | 2.105 (0.983-4.507) | 0.055 |
| ACLY expression, high vs low | 2.103 (1.047-4.226) | 0.037 |
Discussion
According to a systematic analysis based on Globocan 2008, gastric cancer is one of the most common causes of cancer death (738,000 deaths, 9.7%) [25]. It’s a devastating cancer with obscure early symptom, rapid progression and poor prognosis. The mortality of gastric cancer remains a huge health burden in China. Therefore, it’s urgent to find more potential biomarkers for early diagnosis and prognosis of gastric cancer.
Cancer cells tend to display a high rate of glucose utilization with redirecting the excess glycolytic end product pyruvate toward lipid synthesis. ACLY is one of the key enzyme that link glucose metabolism to lipid synthesis. Distinctive upregulation of ACLY expression and activity has been found in prostate, bladder, breast, liver, lung, colon, and gastric cancers [8,13,14,26]. ACLY is mainly a cytosolic enzyme. It’s regarded that ACLY is bound to endoplasmic reticulum. However, it’s also detected in nuclei of different cells [27,28]. In this study, we found ACLY was mainly located in the cytoplasm of GA cells and its expression in cancer tissues was significantly higher than that in adjacent normal tissues. High ACLY expression was associated with metastasis and advanced stages. These results indicate that higher ACLY confers growth advantage to cancer cells.
Previous study showed that p-ACLY expression was significantly associated with tumor differentiation and poorer prognosis in non-small cell lung cancer [8]. In the present study, our findings revealed that high ACLY expression predicted poor prognosis in GA. ACLY expression might be served as a biomarker for prognosis of cancer patients. Since it’s an important building block for fatty acid, cholesterol and isoprenoid biosynthesis, more and more attention was focused on cancer therapy through ACLY inhibition. Previous studies reported that inhibition of ACLY suppressed cancer cells proliferation both in vivo and in vitro [9,22]. Hanai et al. [29] reported that knockdown of ACLY promoted lung cancer cells differentiation and apoptosis, leading to inhibition of tumor growth in vivo. Although underlying molecular mechanisms of such regulation is not fully elucidated, these findings hold promise that ACLY could be a potential target for therapy.
In conclusion, we found that ACLY was upregulated in GA and its expression was associated with the prognosis of GA patient. Cytoplasmic ACLY may serve as a potential biomarker for GA. However, further studies are required to elucidate these findings.
Acknowledgements
This study was supported by the Research Project of National Health and Family Planning Commission of China (No. W201305) and by the Natural Science Foundation of Jiangsu Province of China (No. BK2008301).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: a review. Clin Cancer Res. 2009;15:431–440. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Masoudi-Nejad A, Asgari Y. Metabolic Cancer Biology: Structural-based analysis of cancer as a metabolic disease, new sights and opportunities for disease treatment. Semin Cancer Biol. 2015;30C:21–29. doi: 10.1016/j.semcancer.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Sebastian C. Tracking down the origin of cancer: metabolic reprogramming as a driver of stemness and tumorigenesis. Crit Rev Oncog. 2014;19:363–382. doi: 10.1615/critrevoncog.2014011844. [DOI] [PubMed] [Google Scholar]
- 6.Bobrovnikova-Marjon E, Hurov JB. Targeting metabolic changes in cancer: novel therapeutic approaches. Annu Rev Med. 2014;65:157–170. doi: 10.1146/annurev-med-092012-112344. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, He C, He C, Chen B, Liu Y, Kong M, Wang C, Lin L, Dong Y, Sheng H. Nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209:510–515. doi: 10.1016/j.prp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, Okumura S, Nakagawa K, Ishikawa Y. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547–8554. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- 9.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 11.Khwairakpam AD, Shyamananda M, Sailo BL, Rathnakaram SR, Padmavathi G, Kotoky J, Kunnumakkara AB. ATP Citrate Lyase (ACLY): A Promising Target for Cancer Prevention and Treatment. Curr Drug Targets. 2015;16:156–163. doi: 10.2174/1389450115666141224125117. [DOI] [PubMed] [Google Scholar]
- 12.Szutowicz A, Kwiatkowski J, Angielski S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br J Cancer. 1979;39:681–687. doi: 10.1038/bjc.1979.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turyn J, Schlichtholz B, Dettlaff-Pokora A, Presler M, Goyke E, Matuszewski M, Kmiec Z, Krajka K, Swierczynski J. Increased activity of glycerol 3-phosphate dehydrogenase and other lipogenic enzymes in human bladder cancer. Horm Metab Res. 2003;35:565–569. doi: 10.1055/s-2003-43500. [DOI] [PubMed] [Google Scholar]
- 14.Halliday KR, Fenoglio-Preiser C, Sillerud LO. Differentiation of human tumors from nonmalignant tissue by natural-abundance 13C NMR spectroscopy. Magn Reson Med. 1988;7:384–411. doi: 10.1002/mrm.1910070403. [DOI] [PubMed] [Google Scholar]
- 15.Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, Frierson H Jr, Powell SM, Knuutila S, Kallioniemi A, El-Rifai W. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–2629. [PubMed] [Google Scholar]
- 16.Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, Sekiya M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Nagai R, Ishibashi S, Kadowaki T, Makuuchi M, Ohnishi S, Osuga J, Yamada N. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41:1316–1322. doi: 10.1016/j.ejca.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Ozkaya AB, Handan AK, Atay S, Aydin HH. Targeting Mitochondrial Citrate Transport in Breast Cancer Cell Lines. Anticancer Agents Med Chem. 2015;15:374–381. doi: 10.2174/1871520615666141216150659. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Islam MS, Tian J, Lui VW, Xiao D. Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014;349:15–25. doi: 10.1016/j.canlet.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Barfeld SJ, Itkonen HM, Urbanucci A, Mills IG. Androgen-regulated metabolism and biosynthesis in prostate cancer. Endocr Relat Cancer. 2014;21:T57–66. doi: 10.1530/ERC-13-0515. [DOI] [PubMed] [Google Scholar]
- 20.Beckner ME, Fellows-Mayle W, Zhang Z, Agostino NR, Kant JA, Day BW, Pollack IF. Identification of ATP citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int J Cancer. 2010;126:2282–2295. doi: 10.1002/ijc.24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz L, Abolhassani M, Guais A, Sanders E, Steyaert JM, Campion F, Israel M. A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: preliminary results. Oncol Rep. 2010;23:1407–1416. doi: 10.3892/or_00000778. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi N, Royaux I, Swinnen JV, Smans K. ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol Cancer Ther. 2012;11:1925–1935. doi: 10.1158/1535-7163.MCT-12-0095. [DOI] [PubMed] [Google Scholar]
- 23.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 24.Migita T, Okabe S, Ikeda K, Igarashi S, Sugawara S, Tomida A, Soga T, Taguchi R, Seimiya H. Inhibition of ATP citrate lyase induces triglyceride accumulation with altered fatty acid composition in cancer cells. Int J Cancer. 2014;135:37–47. doi: 10.1002/ijc.28652. [DOI] [PubMed] [Google Scholar]
- 25.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 26.Yancy HF, Mason JA, Peters S, Thompson CE 3rd, Littleton GK, Jett M, Day AA. Metastatic progression and gene expression between breast cancer cell lines from African American and Caucasian women. J Carcinog. 2007;6:8. doi: 10.1186/1477-3163-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linn TC, Srere PA. Binding of ATP citrate lyase to the microsomal fraction of rat liver. J Biol Chem. 1984;259:13379–13384. [PubMed] [Google Scholar]
- 28.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanai J, Doro N, Sasaki AT, Kobayashi S, Cantley LC, Seth P, Sukhatme VP. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2012;227:1709–1720. doi: 10.1002/jcp.22895. [DOI] [PMC free article] [PubMed] [Google Scholar]


